Abstract
Molecular chaperone heat shock protein 90 (Hsp90) is a ubiquitous regulator that fine-tunes and remodels diverse client proteins, exerting profound effects on normal biology and diseases. Unraveling the mechanistic details of Hsp90’s function requires atomic-level insights into its client interactions throughout the adenosine triphosphate-coupled functional cycle. However, the structural details of the initial encounter complex in the chaperone cycle, wherein Hsp90 adopts an open conformation while engaging with the client, remain elusive. Here, using nuclear magnetic resonance spectroscopy, we determined the solution structure of Hsp90 in its open state, bound to a disordered client. Our findings reveal that Hsp90 uses two distinct binding sites, collaborating synergistically to capture discrete hydrophobic segments within client proteins. This bipartite interaction generates a versatile complex that facilitates rapid conformational sampling. Moreover, our investigations spanning various clients and Hsp90 orthologs demonstrate a pervasive mechanism used by Hsp90 orthologs to accommodate the vast array of client proteins. Collectively, our work contributes to establish a unified conceptual and mechanistic framework, elucidating the intricate interplay between Hsp90 and its clients.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The atomic coordinates for Hsp90M–Δ131Δa, Hsp90M–Δ131Δb and Hsp90–Δ131Δ were deposited to the PDB with accession codes 8K2S, 8K2R and 8K2T, respectively. The NMR assignments for Δ131Δ, FtsZC, Hsp90N, Hsp90C, Hsp90M–Δ131Δa, Hsp90M–Δ131Δb and Hsp90–Δ131Δ were deposited to the Biological Magnetic Resonance Data Bank (BMRB) under accession codes 52367, 52368, 52364, 52366, 36581, 36580 and 36582, respectively. Additionally, previously published models of Hsp90 or Hsp90–client complexes essential for building the Hsp90–Δ131Δ structure and describing the bipartite binding mode were included in this study with corresponding PDB accession codes 2IOQ, 5FWK, 7KRJ and 7KW7 and references. Other data are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Zhao, R. et al. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the Hsp90 chaperone. Cell 120, 715–727 (2005).
Taipale, M., Jarosz, D. F. & Lindquist, S. Hsp90 at the hub of protein homeostasis: emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 11, 515–528 (2010).
Taipale, M. et al. Quantitative analysis of Hsp90–client interactions reveals principles of substrate recognition. Cell 150, 987–1001 (2012).
Lackie, R. E. et al. The Hsp70/Hsp90 chaperone machinery in neurodegenerative diseases. Front Neurosci. 11, 254 (2017).
Kolhe, J. A., Babu, N. L. & Freeman, B. C. The Hsp90 molecular chaperone governs client proteins by targeting intrinsically disordered regions. Mol. Cell 83, 2035–2044 (2023).
Whitesell, L. & Lindquist, S. L. Hsp90 and the chaperoning of cancer. Nat. Rev. Cancer 5, 761–772 (2005).
Trepel, J., Mollapour, M., Giaccone, G. & Neckers, L. Targeting the dynamic Hsp90 complex in cancer. Nat. Rev. Cancer 10, 537–549 (2010).
Jaeger, A. M. & Whitesell, L. Hsp90: enabler of cancer adaptation. Annu. Rev. Cancer Biol. 3, 275–297 (2019).
Shiau, A. K., Harris, S. F., Southworth, D. R. & Agard, D. A. Structural analysis of E. coli Hsp90 reveals dramatic nucleotide-dependent conformational rearrangements. Cell 127, 329–340 (2006).
Wickner, S., Nguyen, T. L. & Genest, O. The bacterial Hsp90 chaperone: cellular functions and mechanism of action. Annu Rev. Microbiol 75, 719–739 (2021).
Krukenberg, K. A., Forster, F., Rice, L. M., Sali, A. & Agard, D. A. Multiple conformations of E. coli Hsp90 in solution: insights into the conformational dynamics of Hsp90. Structure 16, 755–765 (2008).
Southworth, D. R. & Agard, D. A. Species-dependent ensembles of conserved conformational states define the Hsp90 chaperone ATPase cycle. Mol. Cell 32, 631–640 (2008).
Ali, M. M. et al. Crystal structure of an Hsp90–nucleotide–p23/Sba1 closed chaperone complex. Nature 440, 1013–1017 (2006).
Kirschke, E., Goswami, D., Southworth, D., Griffin, P. R. & Agard, D. A. Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell 157, 1685–1697 (2014).
Verba, K. A. et al. Atomic structure of Hsp90–Cdc37–Cdk4 reveals that Hsp90 traps and stabilizes an unfolded kinase. Science 352, 1542–1547 (2016).
Wang, R. Y. et al. Structure of Hsp90–Hsp70–Hop–GR reveals the Hsp90 client-loading mechanism. Nature 601, 460–464 (2022).
Noddings, C. M., Wang, R. Y., Johnson, J. L. & Agard, D. A. Structure of Hsp90–p23–GR reveals the Hsp90 client-remodelling mechanism. Nature 601, 465–469 (2022).
Hellenkamp, B., Wortmann, P., Kandzia, F., Zacharias, M. & Hugel, T. Multidomain structure and correlated dynamics determined by self-consistent FRET networks. Nat. Methods 14, 174–180 (2017).
Mickler, M., Hessling, M., Ratzke, C., Buchner, J. & Hugel, T. The large conformational changes of Hsp90 are only weakly coupled to ATP hydrolysis. Nat. Struct. Mol. Biol. 16, 281–286 (2009).
Saio, T., Guan, X., Rossi, P., Economou, A. & Kalodimos, C. G. Structural basis for protein antiaggregation activity of the trigger factor chaperone. Science 344, 1250494 (2014).
Karagoz, G. E. et al. Hsp90–tau complex reveals molecular basis for specificity in chaperone action. Cell 156, 963–974 (2014).
Huang, C., Rossi, P., Saio, T. & Kalodimos, C. G. Structural basis for the antifolding activity of a molecular chaperone. Nature 537, 202–206 (2016).
Huang, C. & Kalodimos, C. G. Structures of large protein complexes determined by nuclear magnetic resonance spectroscopy. Annu. Rev. Biophys. 46, 317–336 (2017).
Jiang, Y., Rossi, P. & Kalodimos, C. G. Structural basis for client recognition and activity of Hsp40 chaperones. Science 365, 1313–1319 (2019).
Rosenzweig, R. & Kay, L. E. Bringing dynamic molecular machines into focus by methyl-TROSY NMR. Annu. Rev. Biochem. 83, 291–315 (2014).
Burmann, B. M. et al. Regulation of α-synuclein by chaperones in mammalian cells. Nature 577, 127–132 (2020).
Faust, O. et al. Hsp40 proteins use class-specific regulation to drive Hsp70 functional diversity. Nature 587, 489–494 (2020).
Street, T. O., Lavery, L. A. & Agard, D. A. Substrate binding drives large-scale conformational changes in the Hsp90 molecular chaperone. Mol. Cell 42, 96–105 (2011).
Balasubramanian, A., Markovski, M., Hoskins, J. R., Doyle, S. M. & Wickner, S. Hsp90 of E. coli modulates assembly of FtsZ, the bacterial tubulin homolog. Proc. Natl Acad. Sci. USA 116, 12285–12294 (2019).
Takeuchi, K. & Wagner, G. NMR studies of protein interactions. Curr. Opin. Struct. Biol. 16, 109–117 (2006).
Fenton, W. A., Kashi, Y., Furtak, K. & Horwich, A. L. Residues in chaperonin GroEL required for polypeptide binding and release. Nature 371, 614–619 (1994).
Stirling, P. C., Bakhoum, S. F., Feigl, A. B. & Leroux, M. R. Convergent evolution of clamp-like binding sites in diverse chaperones. Nat. Struct. Mol. Biol. 13, 865–870 (2006).
Karagoz, G. E. et al. N-terminal ___domain of human Hsp90 triggers binding to the cochaperone p23. Proc. Natl Acad. Sci. USA 108, 580–585 (2011).
Bohen, S. P. & Yamamoto, K. R. Isolation of Hsp90 mutants by screening for decreased steroid receptor function. Proc. Natl Acad. Sci. USA 90, 11424–11428 (1993).
Genest, O. et al. Uncovering a region of heat shock protein 90 important for client binding in E. coli and chaperone function in yeast. Mol. Cell 49, 464–473 (2013).
Park, S. J., Kostic, M. & Dyson, H. J. Dynamic interaction of Hsp90 with its client protein p53. J. Mol. Biol. 411, 158–173 (2011).
Wayne, N. & Bolon, D. N. Dimerization of Hsp90 is required for in vivo function. Design and analysis of monomers and dimers. J. Biol. Chem. 282, 35386–35395 (2007).
O’Shea, E. K., Klemm, J. D., Kim, P. S. & Alber, T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science 254, 539–544 (1991).
Lopez, A. et al. Client binding shifts the populations of dynamic Hsp90 conformations through an allosteric network. Sci. Adv. 7, eabl7295 (2021).
Krois, A. S., Ferreon, J. C., Martinez-Yamout, M. A., Dyson, H. J. & Wright, P. E. Recognition of the disordered p53 transactivation ___domain by the transcriptional adapter zinc finger domains of CREB-binding protein. Proc. Natl Acad. Sci. USA 113, E1853–E1862 (2016).
Khanra, N., Rossi, P., Economou, A. & Kalodimos, C. G. Recognition and targeting mechanisms by chaperones in flagellum assembly and operation. Proc. Natl Acad. Sci. USA 113, 9798–9803 (2016).
Park, S. J., Borin, B. N., Martinez-Yamout, M. A. & Dyson, H. J. The client protein p53 adopts a molten globule-like state in the presence of Hsp90. Nat. Struct. Mol. Biol. 18, 537–541 (2011).
McLaughlin, S. H., Smith, H. W. & Jackson, S. E. Stimulation of the weak ATPase activity of human Hsp90 by a client protein. J. Mol. Biol. 315, 787–798 (2002).
Motojima-Miyazaki, Y., Yoshida, M. & Motojima, F. Ribosomal protein L2 associates with E. coli HtpG and activates its ATPase activity. Biochem. Biophys. Res. Commun. 400, 241–245 (2010).
Street, T. O. et al. Cross-monomer substrate contacts reposition the Hsp90 N-terminal ___domain and prime the chaperone activity. J. Mol. Biol. 415, 3–15 (2012).
Weickert, S., Wawrzyniuk, M., John, L. H., Rudiger, S. G. D. & Drescher, M. The mechanism of Hsp90-induced oligomerizaton of tau. Sci. Adv. 6, eaax6999 (2020).
Canadillas, J. M. et al. Solution structure of p53 core ___domain: structural basis for its instability. Proc. Natl Acad. Sci. USA 103, 2109–2114 (2006).
Keramisanou, D., Vasantha Kumar, M. V., Boose, N., Abzalimov, R. R. & Gelis, I. Assembly mechanism of early Hsp90–Cdc37–kinase complexes. Sci. Adv. 8, eabm9294 (2022).
Delaglio, F. et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 (1995).
Marsh, J. A., Singh, V. K., Jia, Z. & Forman-Kay, J. D. Sensitivity of secondary structure propensities to sequence differences between α- and γ-synuclein: implications for fibrillation. Protein Sci. 15, 2795–2804 (2006).
Conicella, A. E. et al. An intrinsically disordered motif regulates the interaction between the p47 adaptor and the p97 AAA+ ATPase. Proc. Natl Acad. Sci. USA 117, 26226–26236 (2020).
Bieri, M. & Gooley, P. R. Automated NMR relaxation dispersion data analysis using NESSY. BMC Bioinformatics 12, 421 (2011).
Chen, Y. et al. Assembly status transition offers an avenue for activity modulation of a supramolecular enzyme. eLife 10, e72535 (2021).
Shammas, S. L. et al. A mechanistic model of tau amyloid aggregation based on direct observation of oligomers. Nat. Commun. 6, 7025 (2015).
Acknowledgements
We gratefully acknowledge I. Chen, Y. Xia and T. Xie for insightful discussions and the Bionmr Laboratory of the Division of Life Sciences and Medicine at the University of Science and Technology of China for NMR data collection. A portion of this work was performed at the Steady High Magnetic Field Facility (High Magnetic Field Laboratory, Chinese Academy of Sciences). This study was supported by the National Natural Science Foundation of China (31770807, 31971144 and T2221005) to C.H., the National Key R&D Program of China (2023YFC3403102) to C.H. and the Anhui Provincial Natural Science Foundation (2208085MC47) to C. Wan.
Author information
Authors and Affiliations
Contributions
C.H. and W.X. designed the research. X.Q., S.Z., C. Wan, T.J. and W.X. prepared the protein samples and conducted biochemical experiments. X.Q., S.Z., C. Wan, L.Z. and C.H. performed the NMR experiments. C.H., X.Q., S.Z., C. Wan, P.R., J.W., C.G.K., C. Wang and W.X. analyzed the data. X.Q., P.R. and C.H. performed the structure calculations. C.H., X.Q., P.R. and C.G.K. drafted the manuscript. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Helen Dyson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Sara Osman, in collaboration with the Nature Structural and Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 NMR characterization of clients.
a, 1H–15N HSQC spectrum of full-length Δ131Δ. b, Overlay of 1H–15N HSQC spectrum of full-length Δ131Δ and the fragments (Δ131ΔN and Δ131ΔC, corresponding to the sites a and b, respectively). The excellent resonance correspondence indicates that the conformational properties of isolated fragments recapitulate those of the full-length protein. c,d, Secondary structure propensity (SSP) values of Δ131Δ (c) and FtsZC (d) plotted as a function of the amino acid sequence. A SSP score at a given residue of 1 or −1 reflects a fully formed α-helical or β-structure (extended), respectively, whereas a score of, for example, 0.5 indicates that 50% of the conformers in the native-state ensemble of the protein are helical at that position. The Hsp90-binding sites are highlighted. The data from panels c and d collectively suggest that the recognition sites of Hsp90 within clients are independent of their secondary structure patterns.
Extended Data Fig. 2 Interactions between the client and various nucleotide bound states of Hsp90.
a,b, 1H–15N HSQC spectrum of Δ131Δ fragments and FtsZC in the absence and presence of AMP-PNP-bound (a) and ADP-bound (b) Hsp90. c, ITC analysis of Δ131Δ and Δ131ΔC binding to different nucleotide bound states of Hsp90. d,e, NMR titration profiles using two-state exchange models to determine the affinities of Apo form of Hsp90 and different nucleotide bound state of Hsp90 to Δ131Δ (d) and FtsZC (e). The titration profiles for selected residues are displayed on the top. These data indicate the binding affinities throughout the chaperone cycle remain largely unaltered.
Extended Data Fig. 3 Interactions of clients with Hsp90 domains.
a-c, Overlay of 1H–15N HSQC spectrum of Δ131Δ fragments and FtsZC in the absence and presence of the NTD (a), CTD (b) and MD (c) of Hsp90. These data collectively suggest that the middle ___domain alone is responsible for client recognition, with neither Hsp90N nor Hsp90C involved in binding.
Extended Data Fig. 4 Synergistic binding of the MDs of Hsp90 to clients.
a-d, Overlay of 1H–15N HSQC spectra of Δ131Δ fragments and FtsZC in the absence and presence of Hsp90NM (a), Hsp90MC (b), Hsp90MCLC (c) and the dimerized Hsp90MM (d). These data collectively demonstrate a significant binding synergy between Hsp90M and clients.
Extended Data Fig. 5 NMR characterizations and assignments of Hsp90 domains.
a, Superimposed methyl-TROSY spectra of the full-length Hsp90 with its individual domains: NTD, MD, CTD, their combinations- NM domains and MC domains. Analysis of these spectra reveals a lack of extensive contacts between the individual domains. b, Representative strips extracted from the non-uniformly sampled HNCACB experiments of the individual domains, displaying the backbone resonance assignment.
Extended Data Fig. 6 Identifications of the client-binding sites on Hsp90.
a, Overlay of the methyl-TROSY spectra of Hsp90 and the Hsp90-Δ131Δ complex. b, Overlay of 1H–15N TROSY HSQC spectra of Hsp90M and in complex with Δ131Δa, Δ131Δb and FtsZC. c, Overlay of methyl-TROSY spectra of Hsp90M in complex with Δ131Δa, Δ131Δb and FtsZC. d, Inter-molecular NOEs between Hsp90 and the Δ131Δ protein. Representative strips extracted from the 13C-edited HMQC–NOESY–HMQC NMR experiments. The NOE cross-peaks between Hsp90 and residues of Δ131Δ fragments are designated by a dashed-line red circle.
Extended Data Fig. 7 Strategy for structure determination of the Hsp90-Δ131Δ complex.
More details can be found in Methods.
Extended Data Fig. 8 Detailed contacts between Hsp90 and the Δ131Δ protein.
Inter-molecular contacts between Hsp90 and site a (a) and site b (b) generated by Ligplot.
Extended Data Fig. 9 Interactions between the clients and different Hsp90s.
a, 1H–15N HSQC spectrum of Δ131Δ fragments and FtsZC in the absence and presence of yeast Hsp90 (Hsc82). b, 1H–15N HSQC spectrum of Δ131Δ fragments and FtsZC in the absence and presence of human Hsp90 (Hsp90β). The recognition sites in Δ131Δ fragments and FtsZC are indicated. The data reveal significant overlap in the regions of the client proteins bound by different Hsp90 species, suggesting a conserved binding mechanism.
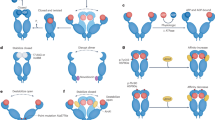
Extended Data Fig. 10 A simplified scheme illustrating the functional cycles of client chaperoning by Hsp90.
Initially, Hsp90 captures the client in an unfolded conformation in its open state, with two stretches engaged simultaneously by one hydrophobic groove on each protomer. ATP binding, assisted by client engagement and/or cochaperone associations, shifts the conformation equilibrium of Hsp90 towards clamp closure. ATP hydrolysis triggers a significant conformational change in Hsp90, transitioning it to its ADP state, and subsequent ADP release leads to the complex returning to its nucleotide free state.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4.
Supplementary Data 1
Primer sequences used in this study.
Source data
Source Data Fig. 1
Source data for NMR titrations in Fig. 1f,g.
Source Data Fig. 2
Source data for NMR titrations in Fig. 2.
Source Data Fig. 6
Raw readings for the Fig. 6b,c.
Source Data Extended Data Fig. 2
Source data for NMR titrations in Extended Data Fig. 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qu, X., Zhao, S., Wan, C. et al. Structural basis for the dynamic chaperoning of disordered clients by Hsp90. Nat Struct Mol Biol 31, 1482–1491 (2024). https://doi.org/10.1038/s41594-024-01337-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-024-01337-z
This article is cited by
-
The dynamic triage interplay of Hsp90 with its chaperone cycle and client binding
Nature Communications (2024)