Abstract
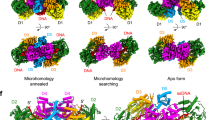
DNA double-strand breaks occur daily in all human cells and must be repaired with high fidelity to minimize genomic instability. Deficiencies in high-fidelity DNA repair by homologous recombination lead to dependence on DNA polymerase θ, which identifies DNA microhomologies in 3′ single-stranded DNA overhangs and anneals them to initiate error-prone double-strand break repair. The resulting genomic instability is associated with numerous cancers, thereby making this polymerase an attractive therapeutic target. However, despite the biomedical importance of polymerase θ, the molecular details of how it initiates DNA break repair remain unclear. Here, we present cryo-electron microscopy structures of the polymerase θ helicase ___domain bound to microhomology-containing DNA, revealing DNA-induced rearrangements of the helicase that enable DNA repair. Our structures show that DNA-bound helicase dimers facilitate a microhomology search that positions 3′ single-stranded DNA ends in proximity to align complementary bases and anneal DNA microhomology. We characterize the molecular determinants that enable the helicase ___domain of polymerase θ to identify and pair DNA microhomologies to initiate mutagenic DNA repair, thereby providing insight into potentially targetable interactions for therapeutic interventions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Cryo-EM maps and associated atomic models were deposited to the EMDB and the PDB, respectively, with the following accession codes: apo PolθH tetramer, EMD-44534, PDB 9BH6; apo PolθH dimer, EMD-44535, PDB 9BH7; PolθH–DNA microhomology searching, EMD-44536, PDB 9BH8; PolθH–DNA microhomology aligning, EMD-44537, PDB 9BH9; PolθH–DNA microhomology annealed, EMD-44538, PDB 9BHA. The D2 symmetric tetramer map and the locally refined tetramer protomer map are available as additional maps under EMD-44534. The C2 symmetric dimer map and the locally refined dimer protomer map are available as additional maps under EMD-44535. Source data are provided with this paper.
References
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Scully, R., Panday, A., Elango, R. & Willis, N. A. DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat. Rev. Mol. Cell Biol. 20, 698–714 (2019).
Ceccaldi, R., Rondinelli, B. & D’Andrea, A. D. Repair pathway choices and consequences at the double-strand break. Trends Cell Biol. 26, 52–64 (2016).
Ramsden, D. A., Carvajal-Garcia, J. & Gupta, G. P. Mechanism, cellular functions and cancer roles of polymerase-θ-mediated DNA end joining. Nat. Rev. Mol. Cell Biol. 23, 125–140 (2022).
Wood, R. D. & Doublié, S. DNA polymerase θ (POLQ), double-strand break repair, and cancer. DNA Repair 44, 22–32 (2016).
Chan, S. H., Yu, A. M. & McVey, M. Dual roles for DNA polymerase θ in alternative end-joining repair of double-strand breaks in Drosophila. PLoS Genet. 6, e1001005 (2010).
Yousefzadeh, M. J. et al. Mechanism of suppression of chromosomal instability by DNA polymerase POLQ. PLoS Genet. 10, e1004654 (2014).
Mateos-Gomez, P. A. et al. Mammalian polymerase θ promotes alternative NHEJ and suppresses recombination. Nature 518, 254–257 (2015).
Ceccaldi, R. et al. Homologous-recombination-deficient tumours are dependent on Polθ-mediated repair. Nature 518, 258–262 (2015).
Wyatt, D. W. et al. Essential roles for polymerase θ-mediated end joining in the repair of chromosome breaks. Mol. Cell 63, 662–673 (2016).
Zatreanu, D. et al. Polθ inhibitors elicit BRCA-gene synthetic lethality and target PARP inhibitor resistance. Nat. Commun. 12, 3636 (2021).
Zhou, J. et al. A first-in-class polymerase θ inhibitor selectively targets homologous-recombination-deficient tumors. Nat. Cancer 2, 598–610 (2021).
Study of orally administered MOMA-313 in participants with advanced or metastatic solid tumors (NCT06545942). https://clinicaltrials.gov/study/NCT06545942 (2024).
A study of ART4215 for the treatment of advanced or metastatic solid tumors (NCT04991480). https://clinicaltrials.gov/study/NCT04991480 (2021).
A study to investigate the safety, tolerability, pharmacokinetics (PK), and preliminary anticancer activity of GSK4524101 alone or with niraparib in participants with solid tumors (NCT06077877). https://clinicaltrials.gov/study/NCT06077877 (2023).
Seki, M., Marini, F. & Wood, R. D. POLQ (Pol θ), a DNA polymerase and DNA-dependent ATPase in human cells. Nucleic Acids Res. 31, 6117–6126 (2003).
Carvajal-Garcia, J. et al. Mechanistic basis for microhomology identification and genome scarring by polymerase θ. Proc. Natl Acad. Sci. USA 117, 8476–8485 (2020).
He, P. & Yang, W. Template and primer requirements for DNA Pol θ-mediated end joining. Proc. Natl Acad. Sci. USA 115, 7747–7752 (2018).
Luedeman, M. E. et al. Poly(ADP) ribose polymerase promotes DNA polymerase θ-mediated end joining by activation of end resection. Nat. Commun. 13, 4547 (2022).
Kent, T., Chandramouly, G., McDevitt, S. M., Ozdemir, A. Y. & Pomerantz, R. T. Mechanism of microhomology-mediated end-joining promoted by human DNA polymerase θ. Nat. Struct. Mol. Biol. 22, 230–237 (2015).
Fijen, C. et al. Sequential requirements for distinct Polθ domains during theta-mediated end joining. Mol. Cell 84, 1460–1474 (2024).
Black, S. J. et al. Molecular basis of microhomology-mediated end-joining by purified full-length Polθ. Nat. Commun. 10, 4423 (2019).
Mateos-Gomez, P. A. et al. The helicase ___domain of Polθ counteracts RPA to promote alt-NHEJ. Nat. Struct. Mol. Biol. 24, 1116–1123 (2017).
Schaub, J. M., Soniat, M. M. & Finkelstein, I. J. Polymerase θ-helicase promotes end joining by stripping single-stranded DNA-binding proteins and bridging DNA ends. Nucleic Acids Res. 50, 3911–3921 (2022).
Newman, J. A., Cooper, C. D. O., Aitkenhead, H. & Gileadi, O. Structure of the helicase ___domain of DNA polymerase θ reveals a possible role in the microhomology-mediated end-joining pathway. Structure 23, 2319–2330 (2015).
Ozdemir, A. Y., Rusanov, T., Kent, T., Siddique, L. A. & Pomerantz, R. T. Polymerase θ-helicase efficiently unwinds DNA and RNA–DNA hybrids. J. Biol. Chem. 293, 5259–5269 (2018).
Guo, H. et al. Cryo-EM structure of DNA polymerase θ helicase ___domain in complex with inhibitor novobiocin. Preprint at bioRxiv https://doi.org/10.1101/2023.01.20.524915 (2023).
Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 630, 493–500 (2024).
Büttner, K., Nehring, S. & Hopfner, K.-P. Structural basis for DNA duplex separation by a superfamily-2 helicase. Nat. Struct. Mol. Biol. 14, 647–652 (2007).
Richards, J. D. et al. Structure of the DNA repair helicase Hel308 reveals DNA binding and autoinhibitory domains. J. Biol. Chem. 283, 5118–5126 (2008).
Gyimesi, M., Sarlós, K. & Kovács, M. Processive translocation mechanism of the human Bloom’s syndrome helicase along single-stranded DNA. Nucleic Acids Res. 38, 4404–4414 (2010).
Schrempf, A., Slyskova, J. & Loizou, J. I. Targeting the DNA repair enzyme polymerase θ in cancer therapy. Trends Cancer 7, 98–111 (2021).
Stroik, S. et al. Stepwise requirements for polymerases δ and θ in theta-mediated end joining. Nature 623, 836–841 (2023).
Zahn, K. E., Averill, A. M., Aller, P., Wood, R. D. & Doublié, S. Human DNA polymerase θ grasps the primer terminus to mediate DNA repair. Nat. Struct. Mol. Biol. 22, 304–311 (2015).
Cheng, A. et al. Leginon: new features and applications. Protein Sci. 30, 136–150 (2021).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Punjani, A. Real-time cryo-EM structure determination. Microsc. Microanal. 27, 1156–1157 (2021).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A.cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Kleywegt, G. J. et al. Community recommendations on cryoEM data archiving and validation. IUCrJ 11, 140–151 (2024).
Tan, Y. Z. et al. Addressing preferred specimen orientation in single-particle cryo-EM through tilting. Nat. Methods 14, 793–796 (2017).
Afonine, P. V. et al. New tools for the analysis and validation of cryo-EM maps and atomic models. Acta Crystallogr. D 74, 814–840 (2018).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Croll, T. I. ISOLDE: a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr. D 74, 519–530 (2018).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D 74, 531–544 (2018).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Barad, B. A. et al. EMRinger: side chain-directed model and map validation for 3D cryo-electron microscopy. Nat. Methods 12, 943–946 (2015).
Pintilie, G. et al. Measurement of atom resolvability in cryo-EM maps with Q-scores. Nat. Methods 17, 328–334 (2020).
Acknowledgements
We thank J. C. Ducom at Scripps Research high-performance computing and C. Bowman at Scripps Research for computational support, as well as W. Lessin at the Scripps Research EM facility for microscopy support. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health (NIH) under award number F32CA288144 (C.J.Z.). G.C.L. is supported by NIH grant GM14305 and the work used equipment supported by NIH grant S10OD032467.
Author information
Authors and Affiliations
Contributions
C.J.Z. prepared all the cryo-EM samples, collected the data, produced the high-resolution structures, built all the atomic models, performed all the biochemical experiments, and wrote the paper. Y.B., B.A.S.-A., and T.G. provided initial support on the PolθH–DNA binding studies and the native PAGE assay. C.J.Z. and G.C.L. designed all the experiments and performed all the mechanistic interpretation. G.C.L. provided guidance in the cryo-EM data collection and analyses and edited the paper.
Corresponding author
Ethics declarations
Competing interests
Y.B., B.A.S.-A. and T.G. are employees of MOMA Therapeutics. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Dimitris Typas, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Comparison of apo PolθH tetramer cryo-EM and crystal structures.
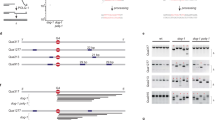
a, Coomassie-stained SDS-PAGE of proteins used in this study. This experiment was independently repeated three times with similar results. WT: PolθH amino acids 2–894. ΔLH: PolθH with amino acids 838–860 deleted. ΔD5: PolθH amino acids 2–789. 2 µg of protein was loaded in each well. b, In the PolθH tetramer cryo-EM structure, two dimeric PolθH subunits are rotated about 4º with respect to the crystal structure (PDB: 5A9J). Residues 35–66 are omitted from the crystal structure representation.
Extended Data Fig. 2 Native PAGE screening for suitable substrates for PolθH-DNA cryo-EM studies.
a, An example gel from Native PAGE screening for DNA substrates that bind PolθH, consecutively stained with Diamond DNA stain (top) and Coomassie protein stain (bottom). This experiment was independently repeated three times with similar results. Gel migration of each species is labeled on the left, and the key is on the right. MH = microhomology. b, The stem-loop DNA species pursued for structural studies, with 6 bases of self-complementary microhomology (green) at the end of the 3′ overhang.
Extended Data Fig. 3 3′ ssDNA encounters a negatively charged patch on the C-terminal helix of D5 at the PolθH DNA tunnel exit.
DNA from the PolθH-DNA searching model is merged with the apo PolθH dimer model. The D5 surface of one PolθH protomer is colored by electrostatic potential, and the ratchet helix is colored pink.
Extended Data Fig. 4 DNA binding to PolθH causes two protomers to hinge apart, destabilizing the PolθH tetramer.
a, Surface representations of the atomic models of the apo and DNA-bound PolθH dimers are shown after aligning the lower subunit (colored red) of each. The upper subunits are colored blue, with the apo subunit semi-transparent. Upon DNA-binding, the two PolθH protomers hinge 14° apart from each other. For clarity, DNA and D5 (residues 790–894) are omitted from each surface representation. b, On the left, the apo tetramer is shown as a gray tube representation, with one dimer colored as in a and highlighted with a semi-transparent molecular surface. On the right, a DNA-bound dimer (colored and highlighted with DNA omitted) is overlaid on an apo tetramer (gray), aligned to the lower red protomer. The overlay demonstrates that upon DNA binding, the hinging and the D5 conformational change introduce steric clashes with the adjacent and diagonal tetramer protomers (clashing residues colored magenta), destabilizing the PolθH tetramer. Atoms less than 2 Å apart are considered to be clashing.
Extended Data Fig. 5 Representative cryo-EM density of PolθH.
Cryo-EM map (DNA microhomology annealed state) and model of one DNA-bound PolθH protomer with domains colored, surrounded by representative map density from alpha helices in each PolθH ___domain. Domain 1 (D1): residues 147–161. Domain 2 (D2): residues 347–360. Domain 3 (D3): residues 529–540. Domain 4 (D4): residues 745–769. Domain 5 (D5): residues 872–890. ssDNA: bases -2 to +8. dsDNA is in the same pose as in the central protomer.
Extended Data Fig. 6 Comparison of DNA-bound structures of PolθH and A. fulgidus Hel308.
a, PolθH-DNA structure (red and yellow) overlaid with A. fulgidus Hel308-DNA structure (PDB: 2P6R, purple and green). b, The unwinding loop of A. fulgidus Hel308 (purple, with residues shown) is larger than the equivalent PolθH loop (red). In the A. fulgidus Hel308 unwinding loop, F351 and Y354 are positioned to form pi-stacking interactions with DNA bases. Some bases in the DNA duplex have been hidden for clarity. c, R592 and W599 in the A. fulgidus Hel308 ratchet helix are positioned to stack with DNA. d, V757 and M761 in the PolθH ratchet helix wedge between DNA bases +5 and +6.
Extended Data Fig. 7 Apo cryo-EM data collection and initial processing.
Data collection and initial processing described for apo PolθH. CTF correction was performed in cryoSPARC live, and micrographs were exported to cryoSPARC for particle picking, extraction, and further processing based on particle presence in 2D and map homogeneity in 3D as described in the methods. One representative micrograph is shown with picked particles in red. For 2D sorting jobs, representative classes are shown. Initial models were created Ab initio from particles.
Extended Data Fig. 8 Apo cryo-EM image analysis workflow.
Representative processing workflow described for apo PolθH. For 3D sorting jobs, representative classes are shown. In this scheme, Ab initio models were utilized as volume inputs for iterative heterogeneous refinement, which separated tetrameric and dimeric PolθH particles. Non-uniform refinement was performed with symmetry imposed for both species to produce symmetric reconstructions. In addition, one protomer from each species was masked and symmetry expanded, subject to 3D variability analysis and/or 3D classification without alignment, and a consensus protomer of both species was locally refined. This yielded final protomer reconstructions both shown adjacent to an angular distribution plot and a 3DFSC histogram with the map-to-model FSC overlaid. To generate each final ‘combined’ map, the locally refined protomer was multiplied by its protomer mask, copied, and fit to the appropriate symmetric map.
Extended Data Fig. 9 PolθH-DNA cryo-EM data collection and initial processing.
Data collection and initial processing described for DNA-bound PolθH. CTF correction was performed in cryoSPARC live, and micrographs were exported to cryoSPARC for particle picking, extraction, and further processing based on particle presence in 2D and map homogeneity in 3D as described in the methods. One representative micrograph is shown with picked particles in red. For 2D sorting jobs, representative classes are shown. The initial model was created Ab initio from particles.
Extended Data Fig. 10 PolθH-DNA cryo-EM image analysis workflow.
Representative processing workflow described for DNA-bound PolθH. For 3D sorting jobs, representative classes are shown. In this scheme, the Ab initio model was utilized as a volume input for iterative heterogeneous refinement. Particles with apparent DNA density were subject to iterative 3D variability analysis with a mask encompassing the most heterogeneous regions to eliminate particles with no D5 density and poor DNA density. Particles were sorted into three groups based on DNA conformation (colored) and subject to non-uniform refinement to produce final reconstructions of PolθH in three different DNA pairing states, each above an angular distribution plot and a 3DFSC histogram with the map-to-model FSC overlaid. As some DNA density disappears upon map sharpening, a locally filtered map is also shown for each reconstruction.
Supplementary information
Supplementary Information
Supplementary Table 1 and Figs. 1 and 2.
Supplementary Video 1
PolθH undergoes conformational rearrangements to facilitate microhomology annealing.
Source data
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed native PAGE gels.
Source Data Extended Data Fig. 1
Unprocessed SDS–PAGE protein gel.
Source Data Extended Data Fig. 2
Unprocessed native PAGE gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zerio, C.J., Bai, Y., Sosa-Alvarado, B.A. et al. Human polymerase θ helicase positions DNA microhomologies for double-strand break repair. Nat Struct Mol Biol 32, 1061–1068 (2025). https://doi.org/10.1038/s41594-025-01514-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-025-01514-8
This article is cited by
-
Making 3â² ends meet
Nature Structural & Molecular Biology (2025)