Abstract
Altered cholesterol metabolism has been linked to a poor prognosis in various types of cancer. Cholesterol oxidation can lead to lipid peroxidation, membrane damage, and cell death. Ferroptosis is a regulated form of cell death characterized by the accumulation of lipid peroxides, which significantly inhibits the growth of ovarian cancer cells. SQLE is the primary enzyme responsible for catalyzing cholesterol lipid synthesis and is notably expressed in ovarian cancer tissues and cells. This study aims to investigate the role of squalene monooxygenase (SQLE) in ferroptosis in ovarian cancer. The protein and mRNA expression of SQLE was assessed using qRT-PCR, Western Blot, and immunohistochemistry. The association between SQLE and ferroptosis was demonstrated through analysis of TCGA and GTEx databases, TMT protein sequencing, as well as validation by qRT-PCR, Western Blot, immunofluorescence, ROS detection, and lipid peroxide detection. Animal experiments further confirmed the relationship between SQLE and ferroptosis in ovarian cancer. The protein and mRNA expression of SQLE was found to be upregulated in both ovarian cancer tissues and cell lines. Decreased SQLE expression led to ferroptosis in ovarian cancer cells, thereby increasing their sensitivity to ferroptosis inducers. Our research demonstrates that SQLE is significantly upregulated in both ovarian cancer tissues and cells. The overexpression of SQLE in ovarian cancer may facilitate tumorigenesis by conferring resistance to ferroptosis, thus shedding light on potential novel therapeutic strategies for ovarian cancer.
Similar content being viewed by others
Introduction
Ovarian cancer (OC) ranks as the third most common gynecological malignancy worldwide, with the highest mortality rate among these cancers1. Patients with OC often lack awareness of the disease and therefore delay seeking treatment until symptoms appear in advanced or metastatic stages2,3. There are 8 histological types of OC, including serous, mucinous, endometrioid, clear cell, transitional cell, undifferentiated, unclassified, and mixed ovarian surface epithelial malignant tumors4. Epithelial OC (EOC) is the most prevalent type, with a 5-year survival rate of 45.6%, while patients with High-grade serous ovarian carcinoma (HGSOC) have a 5-year survival rate of only 30%5. Due to the absence of specific symptom detection methods, many ovarian cancer patients are diagnosed at an advanced stage with metastasis already present6. To address this issue, novel approaches utilizing next-generation sequencing have been developed.
Ferroptosis is a novel form of regulated cell death, induced by chronic lipid peroxidation due to the production of reactive oxygen species (ROS) and iron overload. Unlike other forms of regulated cell death (such as autophagy, apoptosis, necrosis, and cytokinesis), ferroptosis displays distinct morphological, biochemical, and genetic characteristics. Key features of ferroptosis include condensed mitochondrial membranes, reduced or absent mitochondrial cristae, smaller mitochondria, and disrupted outer membranes7,8,9. Moreover, ferroptosis is primarily driven by lipid peroxidation (LPO), leading to accumulation of lethal lipid peroxides9. ROS are byproducts of aerobic processes that are continuously generated, transformed, and utilized by all organisms. Superoxide (O− 2), hydrogen peroxide (H2O2), hydroxyl radical (HO–), and lipid peroxide (ROOH) are common ROS found in living organisms 8. In cancer cells, endogenous ROS generation is enhanced by specific enzymes such as lipoxygenases (LOXs), NADPH oxidases (NOXs), and cyclooxygenase (COXs)9,10.
Acsl4, a member of the acyl-coenzyme long-chain family (Acsl), plays a crucial role in ferroptosis. Therefore, cellular lipids are considered pivotal factors in determining sensitivity and resistance to ferroptosis10. Acsl4 regulates the esterification of CoA into free fatty acids through ATP utilization. The formed Acyl-CoA then promotes the oxidation or synthesis of corresponding fatty acids11. Inhibition of Glutathione Peroxidase 4 (GPX4) weakens the transition from Glutathione (GSH) to GSH/oxidized glutathione (GSSH), leading to lipid peroxidation accumulation and subsequent ferroptosis in tumor cells12.
Ferritin is composed of ferritin light chain (FTL) and ferritin heavy chain 1 (FTH1), playing a crucial role in maintaining cellular iron homeostasis. Suppression of iron responsive element binding protein 2 (IREB2) expression, a key transcription factor involved in iron metabolism, significantly upregulates the expression of FTL and FTH1, thereby inhibiting erastin-induced ferroptosis13,14.
Cholesterol, as the primary sterol in mammalian cell membranes, plays a crucial role in maintaining cell integrity, fluidity, and intracellular homeostasis15. Shimada et al. demonstrated that dysregulation of cholesterol metabolism is linked to ferroptosis16. In the context of breast cancer, disruption of cholesterol homeostasis contributes to resistance to ferroptosis, thereby enhancing carcinogenicity and metastasis17 Inhibition of cholesterol uptake in cholesterol-dependent lymphoma cells can impede the metabolism of oxidized lipids and induce ferroptosis18. Squalene monooxygenase (SQLE) catalyzes squalene conversion to 2,3-epoxy squalene as the second rate-limiting enzyme in cholesterol biosynthesis. SQLE is encoded by a gene on chromosome 3p16 and has been implicated in cell cycle progression19,20, apoptosis, and differentiation. Furthermore, SQLE participates in cholesterol synthesis21,22. And has been associated with prognosis, disease progression, immune environment regulation as well as serving as a regulator for ferroptosis in breast cancer23,24. Reduced expression of SQLE sensitizes ALK + large cell lymphoma cells to GPX4 inhibitors through decreased squalene levels. This inhibition also suppresses tumor growth in vivo and enhances sensitivity to ferroptosis induced by ML162 and RSL3 (GPX4 inhibitors) through squalene accumulation25.
Results
We confirmed the upregulation of SQLE in ovarian cancer and observed that silencing SQLE led to elevated generation of reactive oxygen species and lipid peroxides in ovarian cancer cells, resulting in ferroptosis. In vivo experiments demonstrated that SQLE knockdown attenuated subcutaneous tumor growth and induced ferroptosis in ovarian cancer cells in nude mice. Furthermore, downregulating SQLE increased the production of lipid peroxides, thereby enhancing the efficacy of ferroptosis inducers.
SQLE is a ferroptosis-associated gene and is highly expressed in ovarian cancer
Publicly available datasets were utilized for the analysis of gene expression data, which was obtained from the Cancer Genome Atlas (TCGA) and GTEx databases. Due to the absence of control tissue samples in TCGA-OV, normal ovarian cancer samples were sourced from the GTEx database to assess differential ferroptosis gene expression in ovarian cancer and normal ovarian samples (Fig. 1A). Subsequently, the Robust Multi-array average method was employed for normalization of raw expression data (Fig. 1B)26.
SQLE is a ferroptosis-associated gene and is highly expressed in ovarian cancer. (A) Cancer genome atlas (TCGA) and GTEx databases via Robust Multi-Array Average to normalize raw expression data for ferroptosis-associated genes (adjusted p-value < 0.05). (B) Gene ontology (GO) and Kyoto Encyclopedia of genes and genomes (KEGG) results of ferroptosis-associated genes via the R language “ggplot2” and “org.Hs.eg.db” package (adjust p value < 0.05).
Squalene monooxygenase (SQLE) is an aberrant metabolic gene overexpressed in ovarian cancer
Western blot analysis of fresh tissues revealed significantly higher levels of SQLE in ovarian cancer (OC) tissues compared to normal tissues (Fig. 2A,B). Furthermore, immunohistochemistry analysis of 149 ovarian cancers, 14 junctional ovarian tumors, and 6 benign ovarian lesions demonstrated high expression of SQLE in OC, moderate expression in junctional ovarian tumors, and low expression in benign ovarian lesions (Fig. 2C). Additionally, the protein and mRNA expression of SQLE in the IOSE-80 ovarian epithelial cell line was low, while it varied among different OC cell lines including SKOV3, HEY, A2780, ES-2, HO8910, and OVCAR3 as confirmed by protein immunoblot and Real-Time PCR (Fig. 2D–E). Lentivirus-mediated knockdown of SQLE resulted in decreased expression in SKOV3 and Hey cell lines with high SQLE levels. Conversely, overexpression of SQLE using lentiviral vectors led to increased expression specifically in OVCAR3 cells with low baseline levels. The efficacy of knockdown and overexpression was validated through protein immunoblotting, Quantum Real-Time PCR analysis, and cellular immunofluorescence assays (Fig. 2F–H). The CCK8 assay was employed to assess the proliferative capacity of SKOV3 and HEY cells following SQLE knockdown, as well as the proliferation ability of OVCAR3 cells post-SQLE overexpression. The findings demonstrated that downregulation of SQLE resulted in decreased proliferation capability of ovarian cancer cells (Fig. 2I).
Squalene monooxygenase (SQLE) is an aberrant metabolic gene overexpressed in ovarian cancer (A) SQLE protein expression levels were assessed by western blot in normal and cancer ovarian tissues and quantified by ImageJ software with grayscale values. (B) There was a statistically difference of p < 0.05 in SQLE between normal and cancer ovarian tissues. (C) The expression of SQLE in benign ovarian lesions, ovarian junctional tumors, and OC was detected by immunohistochemistry. (D, E) in human normal ovarian epithelial cell line (IOSE-80) and 6 OC cell lines by qRT-PCR and western blot. SQLE levels in the six OC cell lines. *P < 0.05, **P < 0.01, #P < 0.001. (F, G, H) The efficiency of knockdown and overexpression of SQLE in three ovarian cancer cells was verified by qRT-PCR 、western blot and immunofluorescence methods.
Effect of SQLE on proteomics of SKOV3 ovarian cancer cells
We utilized lentivirus to silence SQLE in the ovarian cancer cell line SKOV3, followed by TMT proteomics sequencing. The quality specification analysis was conducted thrice, and the raw data was retrieved from the database. Subsequently, reliable proteins were filtered based on the criteria of Score Sequence HT > 0 and unique peptide ≥ 1, with blank values excluded. Statistical analysis and visualization of trustworthy protein results revealed 191 up-regulated proteins and 185 down-regulated proteins after SQLE knockdown (Fig. 3A), along with heat maps depicting protein abundance levels across different groups. Biological replicates (repeats 1, 2, 3) were included (Fig. 3B). KEGG database was employed for Pathway-based analysis of differential proteins, using hypergeometric distribution test to calculate significance of enrichment in each pathway entry expressed as p value. Top20 KEGG enrichment analysis results are presented in descending order based on log10Pvalue for each entry (Fig. 3C)27,28,29.
Effect of SQLE on proteomics of SKOV3 ovarian cancer cells. (A) A volcanic map drawn from all identified genes. The dotted line indicates the threshold value: |log2FoldChange|≥ 2 (vertical dotted line) and p < 0.05 (horizontal dotted line). Red represents a significant increase, blue represents a significant decrease, and gray represents genes that do not meet the set criteria. (B) Clustering is carried out according to the amount of protein expression. Red represents high expression protein, blue represents low expression protein, and each line represents the amount of expression of each protein in different groups. (C) Based on DEGs, the first 20 significantly enriched the KEGG pathway. The Y-axis on the left represents the KEGG pathway, the X-axis represents the "enrichment factor", and the pathway is represented by the ratio of the number of DEGs to the total number of annotated genes of each gene. The lower the p value, the more significant the enrichment. The low p value (p < 0.01) was displayed in the red circle, and the high p value (p > 0.01) was displayed in the blue circle. The area of the circle represents the DEGs number. (Explanation of colors in the legend of this figure).
SQLE knockdown resulted in ferroptosis and its overexpression prevented ferroptosis in ovarian cancer cells
To investigate the impact of SQLE knockdown on ferroptosis, alterations in ROS generation and lipid peroxides in OC cell lines SKOV3 were assessed. The expression of ferroptosis-associated molecular markers COX2, NOX1, ACSL4, GPX4, and FTH1 was confirmed through protein immunoblotting, quantitative real-time PCR, and cellular immunofluorescence (Fig. 4A–C). Additionally, a marginal reduction in ROS levels and lipid peroxides was observed in Hey and OVCAR3 cells following SQLE knockdown. It is well-established that ROS-induced lipid peroxidation plays a crucial role in cell ferroptosis16 (Fig. 4D).
SQLE knockdown resulted in ferroptosis and its overexpression prevented ferroptosis in ovarian cancer cells. (A) protein immunoblot analysis of ferroptosis associated molecular markers proteins COX2, NOX1, ACSL4, GPX4, and FTH1 expression. (B) qRT-PCR analysis of ferroptosis associated molecular markers molecules COX2, NOX1, ACSL4, GPX4, and FTH1 expression, ns P > 0.05,*P < 0.05, **P < 0.01, #P < 0.001. (C) Cell immunofluorescence analysis of ferroptosis associated molecular markers molecules COX2, NOX1, ACSL4, GPX4, and FTH1 expression. (D) Differences in ROS and lipid peroxides generation were analyzed by flow cytometry.
SQLE knockdown increased the sensitivity of ovarian cancer cells to ferroptosis inducers, whereas SQLE overexpression decreased the sensitivity of ovarian cancer cells to ferroptosis inducers
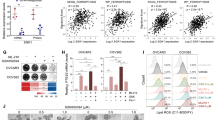
To further investigate the impact of SQLE intervention on the sensitivity of OC cells to ferroptosis inducers, intracellular lipid peroxide levels were measured by flow cytometry for 30 min following treatment with 10uM ML210 (a ferroptosis inducer) in SKOV3 and Hey cells with SQLE knockdown, as well as OVCAR3 cells with SQLE overexpression. The expression levels of GPX4 were confirmed through protein immunoblot and cell immunofluorescence assay after C11-BODIPY staining for 1 h (Fig. 5A). It was observed that SQLE treatment post-knockdown in SKOV3 and Hey led to increased intracellular lipid peroxide accumulation and decreased GPX4 levels, while SQLE treatment post-overexpression in OVCAR3 resulted in a relative decrease in intracellular lipid peroxide accumulation and less depletion of GPX4 (Fig. 5B–C).
SQLE knockdown increased the sensitivity of ovarian cancer cells to ferroptosis inducers, whereas SQLE overexpression decreased the sensitivity of ovarian cancer cells to ferroptosis inducers. (A) flow cytometric analysis detected changes in lipid peroxide levels in OC cells treated with ferroptosis inducer, (B) protein imprint assay detected GPX4 protein expression in OC cells treated with ferroptosis inducer, and (C) cell immunofluorescence assay detected protein expression levels of GPX4 in OC cells treated with ferroptosis inducer.
Expression of COX2, NOX1, ACSL4, GPX4, FTH1 in subcutaneous tumors of nude mouse ovarian cancer tissues
In the initial investigation, it was observed that knockdown of SQLE inhibited the growth of OC xenografts in nude mice25 (Fig. 6A–B) . Subsequent investigations were conducted to assess whether SQLE induces ferroptosis in SKOV3 cells in vivo. Immunohistochemistry results revealed elevated protein levels of GPX4, COX2, NOX1, ACSL4, and FTH1 when SQLE is downregulated in vivo. The results showed an increase in GPX4, COX2, NOX1, ACSL4, and FTH1 after SQLE knockdown (Fig. 6C–D). Tumor size was measured every 5 days and based on the growth status of the tumor; it was observed that the growth rate slowed down after SQLE knockdown. At the end of the experiment, tumors were isolated and weighed. The weight of tumors in the SQLE knockdown group was significantly lower than that of the control group (Fig. 6E–F).
Expression of COX2, NOX1, ACSL4, GPX4, FTH1 in subcutaneous tumors of nude mouse ovarian cancer tissues. (A–B) Differential tumor growth in nude mice harboring SKOV3, an OC cell line with knockdown and normal SQLE. (C) HE staining of tumor body. (D) Immunohistochemistry staining of COX2, NOX1, ACSL4, GPX4, and FTH1 in each nude murine homozygous tumor of SKOV3 an OC cell line with knockdown and normal SQLE.
Discussion
In this study, we observed a significant upregulation of SQLE (squalene monooxygenase) in ovarian cancer tissues compared to normal tissues. Furthermore, the knockdown of squalene monooxygenase resulted in increased production of reactive oxygen species and lipid peroxides, ultimately leading to ferroptosis.
Ovarian cancer is a disease with a poor prognosis, and there have been minimal advancements in treatment procedures2. TThere are three primary types of ovarian tumors: epithelial tumors (60%), germline tumors (30%), and ovarian sex cord stromal tumor (8%). The majority of malignant ovarian cancers (80–85%) are epithelial in nature30. Squalene Monooxygenase (SQLE) is a key enzyme in the biosynthesis of cholesterol, and its overexpression has been implicated in various cancers with oncogenic effects31. Studies have demonstrated that SQLE-mediated reprogramming of lipid metabolism contributes to the progression of prostate, pancreatic, and bladder cancers32,33,34. The activation of SQLE promotes the proliferation of cancer cells and expedites the progression of colorectal cancer35,36. Additionally, SQLE plays a role in cholesterol lipid synthesis, promoting malignant behavior in hepatocytes associated with non-alcoholic fatty liver disease-induced liver cancer and serving as a potential target for antifungal therapy37. Furthermore, it may be linked to the clinical prognosis of cervical and gastric cancers36,38. Moreover, as a gene related to ferroptosis, SQLE contributes to glioblastoma resistance against temozolomide treatment39. In vitro studies have indicated that inhibition of SQLE can enhance the sensitivity of pancreatic cancer cells to chemotherapeutic agents40. Lastly, terbinafine acts as an inhibitor of SQLE and enhances colorectal cancer sensitivity to 5-fluorouracil (5-FU)41.
During this investigation, we explored the high expression of SQLE as a gene associated with ferroptosis in ovarian cancer patients' tissues. Knocking down SQLE led to the accumulation of ROS and lipid peroxides in OC cells, along with increased expression of enzymes related to endogenous ROS such as NOX1 and COX2. Additionally, upregulation of ACSL4, a key factor associated with ferroptosis, promoted an increase in lipid peroxides. While GPX4 can prevent lipid peroxidation 42,43, it has been reported that knockout of FTH1 in the gut of mice results in excessive iron absorption and promotes ferroptosis; FTH1 has an anti-ferroptosis effect44,45,46. Knocking down SQLE did not decrease the expression of FTH1 and GPX4 but instead increased their expression, possibly due to the protective mechanism against ferroptosis47,48. Cholesterol is essential for membrane components and various biological metabolites involved in cancer 31. Recent studies have indicated a positive correlation between elevated serum cholesterol levels and the risk of certain types of cancer such as prostate cancer. Statin-mediated reduction in cholesterol levels is associated with decreased risk of melanoma, non-Hodgkin's lymphoma, endometrial cancer, and breast cancer49,50,51,52. In this study, knockdown of SQLE sensitized OC cells to the GPX4 inhibitor (ML210) for inducing ferroptosis pathway enhancement as potential drug targets for ovarian cancer treatment.
In our study, we acknowledge the limitations of using only SKOV3 for protein sequencing without considering HEY. When evaluating the sensitivity of ML210 to ferroptosis, GPX4 was chosen as the sole indicator. Platinum chemotherapy drugs have been shown to induce DNA damage and apoptosis in cancer cells by increasing ROS levels53. We hypothesize that reducing cholesterol metabolism may elevate ROS production in cancer cells of patients undergoing chemotherapy, leading to improved therapeutic outcomes.
Conclusion
In conclusion, our study demonstrates that SQLE is significantly upregulated in both ovarian cancer tissues and cells. The overexpression of SQLE in ovarian cancer may facilitate tumorigenesis by conferring resistance to ferroptosis, thus offering novel insights into potential therapeutic targets for ovarian cancer treatment.
Materials and methods
Data acquisition and processing
All the data utilized in this investigation have public access. Gene expression data and clinical history were acquired from the Cancer Genome Atlas (TCGA) and the GTEx databases. The data of ferroptosis related genes come from the FerrDb database (http://www.zhounan.org/ferrdb/Current/). Then, to normalize raw expression data the Robust Multiarray Average was applied, and 58 genes associated with ferroptosis were selected.
Immunohistochemical staining
SQLE staining was carried out on 149 OC tissues, 14 ovarian junctional ovarian tumors, and 6 benign ovarian lesions via the ABC Immunostaining program (Solebro, China). These organizations are all from postoperative specimens of past patients in the Pathology Department of the First Affiliated Hospital of Bengbu Medical College.According to the blind randomized staining intensity assessments and stained cells percentage, microarrays were scored by 2 independent researchers. On a scale of 0 to 3; 0 = negative, 1 = weak, 2 = moderate, and 3 = strong. The percentage of positive staining cells ranged from 0 to 4: 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%). Final product scores were 0 to 12, and if the final SQLE protein expression score was ≥ 6 it was declared positive. SQLE Antibody Information: proteintech Cat No. 12544-1-AP,1:100.
Cell culture
Normal human ovarian epithelial cell lines (IOSE-80) and human OC cell lines SKOV-3, OVCAR3, ES2, A2780, HEY, and HO8910 were utilized in this investigation. A2780 and HO8910 cells were propagated in RPMI-1640 media. SKOV-3 in McCoy's 5A media, and ES2 and HEY cells in DMEM. All the mediums were augmented with 10% fetal bovine serum. All cultures were put in the incubator with a moist atmosphere at 37 °C and 5% CO2. The above cell lines were purchased from Pricella, Wuhan, China (https://www.procell.com.cn) All of them passed STR identification and mycoplasma detection.
ROS detection
ROS levels in OC cells were evaluated by probe 2′, 7′-DCFH-DA (Beyotime), this probe is oxidized to dichlorofluorescein (highly fluorescent) by intracellular oxygen. The cells were kept in dark augmented with a 10 μM DCFH-DA for 30 min in the aforementioned incubator, followed by ice-cold PBS rinsing, thrice. Then the cells were re-dissolved in PBS (300μL) and their fluorescence intensity was determined by the flow cytometer (BD Biosciences), using FlowJo X 10.0. 7 Software.
Immunofluorescence staining
A 24-pore plate was propagated with cells by a prearranged cell clamp, rinsed thrice with PBS, followed by fixation using 4% paraformaldehyde for 15 min, and then transfused for 5 min in 0.1% Triton X, followed by overnight (4 °C) incubation with an anti-incubation regimen targeting SQLE (diluted 1: 200), GPX4 (diluted 1: 200), ACSL4 (diluted 1: 200), COX2 (diluted 1: 200), NOX1 (diluted 1: 200), and for 1 h with FTH1 (diluted 1: 200) at room temperature. Then, cells were kept in Alexa Fluor® 488 conjugated dizumab (dilution 1: 1,000) with 4′, 6-diamidine-2-phenylindole (DAPI) at environmental temperature for 2 h. Images were acquired via the fluorescence microscope.
shRNA knockdown of SQLE
A lentiviral shRNA vector was constructed, directed at the SQLE coding region sequence (GCATTGCCACTTTCACCTA) and this was used to establish SQLE-knockdown in SKOV3 and HEY cells. Successfully established knockdown cells were separated by puromycin resistance selection.
Quantitative proteomics based on tandem mass spectrometry tags
All analyses were performed by a Q Exactive mass spectrometer (Thermo, USA) equi -pped with a Nanospray Flex source (Thermo, USA). Samples were loaded and sepa-rated by a C18 column (25 cm × 75 µm) on an EASY-nLTM 1200 system (Thermo, USA). The flow rate was 300 nL/min and total run was 75 min (0 ~ 40 min, 5–28% B; 40 ~ 60 min, 28–42% B; 60 ~ 65 min, 42–90%B; 65 ~ 75 min, 90%B; mobile phase A = 0.1% FA in water and B = 0.1% FA in ACN). Full MS scans were acquired in the mass range of 350–1500 m/z with a mass resolution of 60,000 and the AGC target value was set at 1e6. The ten most intense peaks in MS were fragmented with higher-energy collisional dissociation (HCD) with NCE of 36. MS/MS spectra were 13obtained with a resolution of 30,000 with an AGC target of 1e5 and a max injection time of 80 ms. The dynamic exclusion was set for 30.0 s and run under positive mode.
Quantitative real-time PCR (qRT-PCR)
qRT-PCR was performed as previously described (14) Primers used were:
human SQLE forward: TGA CAA TTC TCA TCT GAG GTC CA; human SQLE reverse: GAG GGA TAA CCC TTT AGC AGT TTT; human GPX4forward: GAGGCAAGACCGAAGTAAACTAC; human GPX4 reverse: CCGAACTGGTTACACGGGAA; human ACSL4 forward: CATCCCTGGAGCAGATACTCT; human ACSL4 reverse: TCACTTAGGATTTCCCTGGTCC; human COX2 forward: CTGGCGCTCAGCCATACAG; human COX2reverse: CGCACTTATACTGGTCAAATCCC; human NOX1forward: TTGTTTGGTTAGGGCTGAATGT; human NOX1 reverse: GCCAATGTTGACCCAAGGATTTT; human FTH1forward: CCCCCATTTGTGTGACTTCAT; human FTH1 reverse: GCCCGAGGCTTAGCTTTCATT; human GAPDH forward: ACCACAGTCCATGCCATCAC; human GAPDH reverse: TCCACCACCCTGTTGCTGTA.
Western blot analysis
This analysis was carried out and quantified by the protocol described previously54. The following antibodies were utilized for probing the membranes; SQLE (proteintech Cat No. 12544-1-AP 1:1000), GAPDH (proteintech Cat No. 60004–1-Ig 1:5000), GPX4 (Affinity, catalog: DF6701 1:1000), ACSL4 (Affinity, catalog: DF12141 1:1000), COX2 (Affinity, catalog: AF7003 1:1000), NOX1 (Affinity, catalog: DF8684 1:1000), and FTH1 (Affinity, catalog: DF6278 1:1000). The secondary antibody was HRP-conjugated goat anti-mouse (proteintech Cat No. SA00001-1 1:5000) and goat anti-rabbit (proteintech, Cat No. SA00001-2 1:5000).
C11-BODIPY staining
Cells were propagated in 6-well plates, followed by 30 min of 10 μM C11-BODIPY (Thermo Fisher Scientific) treatment. The fluorescence intensity of C11-bodipy staining was assessed via the BD LSRII flow cytometer (Becton Dickinson).
CCK-8 detection of cell viability
Inoculate 2000 cells from each group into a 96 well plate. After the cells adhere to the wall, add 10% CCK8 solution to the culture medium and incubate at 37 °C for 30 min. Measure the absorbance of the liquid at a wavelength of 450 nm to calculate the initial absorbance of the cells. Measure the absorbance using the same method at the following 24, 48, and 72 h to detect the proliferation status of the cells.
Animal experiments
Female BALB/c nude mice were selected for this investigation and categorized as sh-ctrl group (administered with SKOV3 cells) and sh-SQLES1 group (administered with SQLE knockdown SKOV3 cells). In the right dorsum of each mouse ̴ 0.2 mL of a cell suspension (1000 × 104 cells) was administered. After the experiment ended, digital calipers were used to determine the size of the tumor in each mouse. The tumor volume was assessed every 5 days by calculating the width and length. 30 days later, each mouse was executed and the tumor tissue was removed and weighed. The nude mice used in this experiment were anesthetized with isoflurane gas using a small animal gas anesthesia machine in the injection of ovarian cancer cells and the dissection of tumor body. When the experiment was terminated, euthanize nude mice with pentobarbital sodium.Animal handling was performed according to institutional guidelines. The experimental animals were raised in a sterile environment and fed normally. and this experiment was approved by the Ethics Committee of Bengbu Medical College (2,020,162) (Supplementary material).
Immunohistochemistry in animals
Isolated tumors were stained with SQLE, GPX4, ACSL4, FTH1, COX2, and NOX1 using the ABC immunostaining program (Solebro, China). Antibody information was as follows: SQLE (proteintech Cat No. 12544-1-AP 1: 100), GPX4 (Affinity, Catalog: DF6701: 100), ACSL4 (Affinity, COX2: 100), FX (AFX: 100), and FX: 100 (AFX: 100).
Hematoxylin–eosin staining in animals
The prepared animal tumor tissue slices were dewaxed and rewatered, 5 min of soyuzu extract staining was followed by running water for 5 min, 30 s in 1% hydrochloric acid ethanol, 30 s in distilled water for 0.5% iridescent solution staining for 3 min, 80% ethanol rinsing was followed by dehydration and transparent resin sealing.
Statistical analysis of cell culture experiments
Excel and Prism 9 (Graphpad software) were utilized for performing statistics. All experiments were repeated thrice minimum of three replicates per condition. Intergroup comparison was assessed and statistical significance was evaluated via two-tailed unpaired Student’s t-tests. Data are presented as mean ± standard deviation.
Data availability
All data needed to evaluate the conclusions of the paper are in the paper and/or supplementary materials. Figure 1A–B analysed used clinical data publicly available in the following online databases: TCGA (https://portal.gdc.cancer.gov/), GTEx (Genotype-Tissue Expression (GTEx) (https://gtexportal.org/home/)), and Fig. 3 proteomic sequencing analysis data is added to the supplementary materials.
References
Webb, P. M. & Jordan, S. J. Global epidemiology of epithelial ovarian cancer. Nat. Rev. Clin. Oncol. 21(5), 389–400 (2024).
Kuroki, L. & Guntupalli, S. R. Treatment of epithelial ovarian cancer. Bmj 371, m3773 (2020).
Doubeni, C. A., Doubeni, A. R. & Myers, A. E. Diagnosis and management of ovarian cancer. Am. Fam. Physician 93(11), 937–944 (2016).
Peres, L. C. et al. Histotype classification of ovarian carcinoma: A comparison of approaches. Gynecol. Oncol. 151(1), 53–60 (2018).
Hollis, R. L. et al. Multiomic characterization of high-grade serous ovarian carcinoma enables high-resolution patient stratification. Clin. Cancer Res. 28(16), 3546–3556 (2022).
Wang, C. K. et al. MEX3A mediates p53 degradation to suppress ferroptosis and facilitate ovarian cancer tumorigenesis. Cancer Res. 83(2), 251–263 (2023).
Wang, H., Liu, C., Zhao, Y. & Gao, G. Mitochondria regulation in ferroptosis. Eur. J. Cell Biol. 99(1), 151058 (2020).
Chen, X., Kang, R., Kroemer, G. & Tang, D. Broadening horizons: the role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 18(5), 280–296 (2021).
Li, D. & Li, Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct. Target Ther. 5(1), 108 (2020).
Klasson, T. D. et al. ACSL3 regulates lipid droplet biogenesis and ferroptosis sensitivity in clear cell renal cell carcinoma. Cancer Metab. 10(1), 14 (2022).
Ma, Y. et al. Long-chain acyl-CoA synthetase 4-mediated fatty acid metabolism sustains androgen receptor pathway-independent prostate cancer. Mol. Cancer Res. 19(1), 124–135 (2021).
Chen, P. et al. Combinative treatment of β-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics 10(11), 5107–5119 (2020).
Arosio, P., Elia, L. & Poli, M. Ferritin, cellular iron storage and regulation. IUBMB Life 69(6), 414–422 (2017).
Fang, Y. et al. Inhibiting ferroptosis through disrupting the NCOA4-FTH1 interaction: A New mechanism of action. ACS Cent. Sci. 7(6), 980–989 (2021).
Espinosa, G., López-Montero, I., Monroy, F. & Langevin, D. Shear rheology of lipid monolayers and insights on membrane fluidity. Proc. Natl. Acad. Sci. U S A 108(15), 6008–6013 (2011).
Shimada, K. et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat. Chem. Biol. 12(7), 497–503 (2016).
Liu, W. et al. Dysregulated cholesterol homeostasis results in resistance to ferroptosis increasing tumorigenicity and metastasis in cancer. Nat. Commun. 12(1), 5103 (2021).
Rink, J. S. et al. Targeted reduction of cholesterol uptake in cholesterol-addicted lymphoma cells blocks turnover of oxidized lipids to cause ferroptosis. J. Biol. Chem. 296, 100100 (2021).
Zou, Y., Zhang, H., Bi, F., Tang, Q. & Xu, H. Targeting the key cholesterol biosynthesis enzyme squalene monooxygenasefor cancer therapy. Front. Oncol. 12, 938502 (2022).
Nagai, M. et al. Localization of the squalene epoxidase gene (SQLE) to human chromosome region 8q24.1. Genomics 44(1), 141–143 (1997).
Gill, S., Stevenson, J., Kristiana, I. & Brown, A. J. Cholesterol-dependent degradation of squalene monooxygenase, a control point in cholesterol synthesis beyond HMG-CoA reductase. Cell Metab. 13(3), 260–273 (2011).
Zelcer, N. et al. The E3 ubiquitin ligase MARCH6 degrades squalene monooxygenase and affects 3-hydroxy-3-methyl-glutaryl coenzyme A reductase and the cholesterol synthesis pathway. Mol. Cell Biol. 34(7), 1262–1270 (2014).
Tang, W., Xu, F., Zhao, M. & Zhang, S. Ferroptosis regulators, especially SQLE, play an important role in prognosis, progression and immune environment of breast cancer. BMC Cancer 21(1), 1160 (2021).
Brown, D. N. et al. Squalene epoxidase is a bona fide oncogene by amplification with clinical relevance in breast cancer. Sci. Rep. 6, 19435 (2016).
Garcia-Bermudez, J. et al. Squalene accumulation in cholesterol auxotrophic lymphomas prevents oxidative cell death. Nature 567(7746), 118–122 (2019).
Irizarry, R. A. et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4(2), 249–264 (2003).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28(1), 27–30 (2000).
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28(11), 1947–1951 (2019).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51(D1), D587-d592 (2023).
Devouassoux-Shisheboran, M. & Genestie, C. Pathobiology of ovarian carcinomas. Chin. J. Cancer 34(1), 50–55 (2015).
Xu, H., Zhou, S., Tang, Q., Xia, H. & Bi, F. Cholesterol metabolism: New functions and therapeutic approaches in cancer. Biochim. Biophys. Acta. Rev. Cancer 1874(1), 188394 (2020).
Xu, Z. et al. SQLE mediates metabolic reprogramming to promote LN metastasis in castration-resistant prostate cancer. Onco Targets Ther 14, 4285–4295 (2021).
Xu, R. et al. SQLE promotes pancreatic cancer growth by attenuating ER stress and activating lipid rafts-regulated Src/PI3K/Akt signaling pathway. Cell Death Dis. 14(8), 497 (2023).
Zou, F. et al. SQLE Knockdown inhibits bladder cancer progression by regulating the PTEN/AKT/GSK3β signaling pathway through P53. Cancer Cell Int. 23(1), 221 (2023).
Li, C. et al. Squalene epoxidase drives cancer cell proliferation and promotes gut dysbiosis to accelerate colorectal carcinogenesis. Gut 71(11), 2253–2265 (2022).
Zhang, Y., Qin, Y., Li, D. & Yang, Y. A risk prediction model mediated by genes of APOD/APOC1/SQLE associates with prognosis in cervical cancer. BMC Womens Health 22(1), 534 (2022).
Liu, D. et al. Squalene epoxidase drives NAFLD-induced hepatocellular carcinoma and is a pharmaceutical target. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aap9840 (2018).
Ma, Y. C., Jin, S. J., Gu, G. J., Zhao, L. F. & Xu, S. T. The expression of squalene epoxidase in human gastric cancer and its clinical significance. Indian J. Pathol. Microbiol. 66(4), 799–803 (2023).
Yao, L., Li, J., Zhang, X., Zhou, L. & Hu, K. Downregulated ferroptosis-related gene SQLE facilitates temozolomide chemoresistance, and invasion and affects immune regulation in glioblastoma. CNS Neurosci. Ther. 28(12), 2104–2115 (2022).
Zhao, F. et al. SQLE inhibition suppresses the development of pancreatic ductal adenocarcinoma and enhances its sensitivity to chemotherapeutic agents in vitro. Mol. Biol. Rep. 49(7), 6613–6621 (2022).
Liu, Q. et al. Squalene epoxidase promotes the chemoresistance of colorectal cancer via (S)-2,3-epoxysqualene-activated NF-κB. Cell Commun. Signal 22(1), 278 (2024).
Stockwell, B. R., Jiang, X. & Gu, W. Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol. 30(6), 478–490 (2020).
Xu, C. et al. The glutathione peroxidase Gpx4 prevents lipid peroxidation and ferroptosis to sustain Treg cell activation and suppression of antitumor immunity. Cell Rep. 35(11), 109235 (2021).
Tao, Y. et al. MBD5 regulates iron metabolism via methylation-independent genomic targeting of Fth1 through KAT2A in mice. Br. J. Haematol. 166(2), 279–291 (2014).
Kong, N. et al. Baicalin induces ferroptosis in bladder cancer cells by downregulating FTH1. Acta Pharm. Sin B 11(12), 4045–4054 (2021).
Luo, T., Wang, Y. & Wang, J. Ferroptosis assassinates tumor. J Nanobiotechnology 20(1), 467 (2022).
Mi, Y. et al. Melatonin inhibits ferroptosis and delays age-related cataract by regulating SIRT6/p-Nrf2/GPX4 and SIRT6/NCOA4/FTH1 pathways. Biomed. Pharmacother. 157, 114048 (2023).
Huang, J., Chen, G., Wang, J., Liu, S. & Su, J. Platycodin D regulates high glucose-induced ferroptosis of HK-2 cells through glutathione peroxidase 4 (GPX4). Bioengineered 13(3), 6627–6637 (2022).
Shafique, K. et al. Cholesterol and the risk of grade-specific prostate cancer incidence: evidence from two large prospective cohort studies with up to 37 years’ follow up. BMC Cancer 12, 25 (2012).
Pelton, K., Freeman, M. R. & Solomon, K. R. Cholesterol and prostate cancer. Curr. Opin. Pharmacol. 12(6), 751–759 (2012).
Allott, E. H. et al. Serum lipid profile and risk of prostate cancer recurrence: Results from the SEARCH database. Cancer Epidemiol. Biomark. Prev. 23(11), 2349–2356 (2014).
Cardwell, C. R., Hicks, B. M., Hughes, C. & Murray, L. J. Statin use after colorectal cancer diagnosis and survival: a population-based cohort study. J. Clin. Oncol. 32(28), 3177–3183 (2014).
Zhang, Q. et al. ACSL1-induced ferroptosis and platinum resistance in ovarian cancer by increasing FSP1 N-myristylation and stability. Cell Death Discov. 9(1), 83 (2023).
Chai, W., Ye, F., Zeng, L., Li, Y. & Yang, L. HMGB1-mediated autophagy regulates sodium/iodide symporter protein degradation in thyroid cancer cells. J. Exp. Clin. Cancer Res. 38(1), 325 (2019).
Acknowledgements
We thank all the participants in this study for their valuable input. We also thank the Central Laboratory of Bengbu Medical College for providing research equipment and technical support for this study.
Funding
The present study was supported by the Foundation of Educational Department in Anhui province, (No.KJ2021A0720), and the Bengbu Medical College Postgraduate Innovation Plan, (No.Byycx21092).
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and and consent to participate
This study was conducted in accordance with the relevant guidelines and regulations (Declaration of Helsinki) and in accordance with ARRIVE guidelines (https://arriveguidelines.org). The human tissues in the study were all postoperative specimens from previous patients in the Pathology Department of the First Affiliated Hospital of Bengbu Medical College.Obtain written informed consent from all participants prior to publication of this study.The manuscript was ethically approved by the Research Ethics Committee of Bengbu Medical College (approval No. S2022281).
Consent for publication
Not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, R., Zhang, L., Fan, S. et al. Squalene monooxygenase (SQLE) protects ovarian cancer cells from ferroptosis. Sci Rep 14, 22646 (2024). https://doi.org/10.1038/s41598-024-72506-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72506-9
Keywords
This article is cited by
-
Advances in understanding the role of squalene epoxidase in cancer prognosis and resistance
Molecular Biology Reports (2025)