Abstract
Light-based gamma entrainment using sensory stimuli (GENUS) shows considerable potential for the treatment of Alzheimer’s disease (AD) in both animal and human models. While the clinical efficacy of GENUS for AD is paramount, its effectiveness will eventually also rely on the barrier to treatment adherence imposed by the discomfort of gazing at luminance flickering (LF) light. Currently, there have been few attempts to improve the comfort of GENUS. Here we investigate if Invisible spectral flicker (ISF), a novel type of light-based 40 Hz GENUS for which the flicker is almost imperceptible, can be used as a more comfortable option. We found that whereas ISF, LF, and chromatic flicker (CF) all produce a 40 Hz steady-state visually evoked potential (SSVEP), ISF scores significantly better on measures of comfort and perceived flicker. We also demonstrate that, while there is a trend towards a lower SSVEP response, reducing the stimulation brightness has no significant effect on the 40 Hz SSVEP or perceived flicker, though it significantly improves comfort. Finally, there is a slight decrease in the 40 Hz SSVEP response when stimulating with ISF from increasingly peripheral angles. This may ease the discomfort of GENUS treatment by freeing patients from gazing directly at the light.
Similar content being viewed by others
Introduction
The global prevalence of dementia has more than doubled since 19901, with a new diagnosis occurring every three seconds worldwide2. Alzheimer’s disease (AD) accounts for 60 to 80% of dementia cases, making it the most prevalent cause3. Given the escalating burden of AD, there is a pressing need for novel disease-modifying therapies4. The exact cause of AD is yet unknown. Biomarkers A\(\beta\) and tau, established indicators in AD, disrupt axonal transport, leading to cell death and cognitive deficits4,5. Neuroinflammation has also been proposed as a mechanism in AD6,7,8, with an extensive immune response contributing to neuronal damage. However, the lack of consensus9 on these interactions underscores the complexity of AD pathology.
Despite substantial research in disease-modifying therapies10, progress has been limited in the past decade, with notable exceptions such as the recently approved anti-amyloid monoclonal antibodies (MABs) Aducanumab11 and Lecanemab. The restricted licensing of Aducanumab in the United States, criticized by many due to efficacy and safety concerns12,13,14, has not been followed by the European Medicines Agency. Lecanemab15 shows promising results, exhibiting a significant reduction in amyloid markers and less cognitive decline in the active group compared to placebo. While anti-amyloid MABs therapy has shown positive effects on amyloid load, it is associated with side effects16, including amyloid-related imaging abnormalities (ARIA) and infusion reactions, and its clinical impact remains limited17. Therefore, further exploration of non-pharmacological interventions beyond amyloid-focused approaches18,19, including novel approaches for neurodegenerative disorders16 remains imperative.
Recently, alternative approaches such as Electrophysiological alterations have shown promising insight into the neuropathological aspects of AD20. AD is among a group of neurological and psychiatric disorders in which a decrease in the power of gamma (40 Hz) oscillations and brain network dysfunction (BND)21,22 are observed. Despite this decrease in gamma, studies of the electroencephalography (EEG) spectrum in AD have focused mostly on the low-frequency bands (\(<35\) Hz)22,23,24,25,26,27 and have shown a general tendency toward slowing of oscillatory activity primarily with a decrease in the alpha band. Alpha-band and gamma-band activity28,29 both play an active role in information processing, with alpha-band activity serving dual functions in inhibition and timing of attention30. While spectral changes also occur in the normal aging brain, including slowing of activity mainly by a decrease in alpha power and frequency31, this is more prominent in AD32,33,34. Evidence even suggests a correlation between the slowing of the oscillatory activity and the progression of AD exists23,35. The exact link between gamma activity and AD is challenging to establish due to the high inter-subject variability in gamma activity that is observed even among healthy subjects36. Furthermore, contradicting findings of the resting state EEG in patients with AD report both reduced36 and increased37 gamma activity, the latter being explained by a compensatory increase in mental exertion by the AD group. The interneurons38,39 may have a key role for the electrophysiological changes and cognitive symptoms that occur with AD. Particularly parvalbumin-positive interneurons40,41, which are responsible for cortical communication and play a role for cognitive behavior42. Especially 40 Hz activity resonates with the inhibitory interneurons and generates oscillatory rhythms during higher brain functions like attention43. It is unknown whether the decrease in gamma power in AD patients is a cause or an effect. This leads to the hypothesis that the progression of AD might be slowed if gamma power could be increased.
New evidence has shown that activation of gamma activity using 40 Hz stimulation has great potential for the treatment of AD with clear positive effects in mouse models44,45,46,47. Stimulation of AD mouse models with 40 Hz light-based stimulation resulted in decreased A\(\beta\) and tau load, an increase in microglia recruitment, and additional neuroprotective effects46,47,48. While clinical benefits are not yet certain, 40 Hz can be induced in human participants as measured with EEG and has shown promising therapeutic potential49,50,51,52. Recent independent studies on patients with AD showed a significant reduction in white matter atrophy and a decrease in cognitive decline after administering 40 Hz light stimulation one hour daily51,53,54. Inducing 40 Hz activity in the brain using transcranial magnetic stimulation has also been shown to improve cognitive function for up to 8 weeks after treatment in patients with probable AD55. Gamma stimulation therapy might have considerably fewer side effects than pharmacological interventions56,57, and may be a supplement to pharmacological therapy. Various 40 Hz stimulation methods exist, such as sensory stimulation (tactile58, visual51,59, auditory60, or a combination61), transcranial magnetic stimulation (TMS)55, transcranial alternating current stimulation (tACS)62,63, and Transcranial photobiomodulation (tPBM)64. Each method has distinct advantages and disadvantages impacting both efficacy and usability. These include the power of the evoked response but also the user experience in terms of tolerability, safety, and possible side effects.
The term GENUS (Gamma Entrainment Using Sensory Stimuli) has been adopted to describe stimulation involving sensory inputs. Most studied is the visual and auditory, or a combination of the two. Especially, 40 Hz audiovisual stimulus has been used in mice trials65 and clinical trials52,57,61 as multi-sensory stimulation has the potential to reach wide brain regions66. Studies have found that audiovisual 40 Hz stimulation can reach subcortical structures beyond the cortex including the hippocampus61, better than visual or auditory stimulation alone67. However, visual 40 Hz stimulus alone has also demonstrated the ability to propagate to the hippocampus, particularly in combination with a cognitive task68. Due to its non-invasiveness, stimulation using the visual pathway alone can have greater potential for success in terms of feasibility and real-life application.
Light-based stimulations are generally considered safe, primarily due to their non-invasive nature involving the application of light. However, visual stimulation includes flickering light, hence lies a risk. Exposure to flickering light has mild risks which may include eye strain and fatigue, while severe risks include migraines and seizures in patients with photosensitivity. Currently, no studies have reported any severe adverse events related to the use of GENUS treatment57,61. Guidelines from authorities like the IEEE and CIE should always be considered in the design of visual stimulation devices. For commercially sold LED appliances, the current IEEE guideline recommends a maximum 5% modulation for flicker frequencies below 90 Hz77,78.
Visual 40 Hz stimulation uses temporal light modulation (TLM), modulating a light source at 40 Hz. The light can be modulated in either luminance (i.e. brightness) resulting in luminance flicker (LF) or chromaticity (i.e. color composition) resulting in chromatic flicker (CF) or a combination of the two. White luminance flicker and red-green chromatic flicker have been found to evoke the highest cortical response69,70,71,72,73,74,75,76.
The perception and sensitivity to visual flicker are highly subjective and vary depending on the stimulus. The critical flicker-fusion frequency (CFF)79 is the threshold frequency above which flicker is not perceived. For luminance flicker with a 100% modulation depth, the CFF threshold lies in the range of 60-100 Hz78,80. In patients with AD, the CFF is found to be significantly lower81. For chromatic flicker, the CFF depends on the colors used (as well as brightness, field-of-view, and waveform) but lies in the range of 25–50 Hz82,83,84,85,86. Studies have shown that CF light can induce a cortical response with a minimum sensation of flicker87 and that a neural response can be measured with flicker stimulus even beyond the CFF88,89. Therefore, CF may be a more appropriate implementation of visual 40 Hz stimulation to improve comfort.
According to the IEEE, flicker is defined as modulated light that is perceived as flickering78. The IEEE78 furthermore defines Invisible flicker as light where the modulation can be sensed but not perceived. Invisible Spectral Flicker (ISF) is a novel technique to mitigate the perception and minimize the sensation of flicker90. ISF alternates between two phases of white light that are of similar color temperature but spectrally different. Stimulation with 40 Hz ISF also evokes a strong 40 Hz cortical response91.
In previous pre-clinical and clinical literature, there has been a high degree of focus on the ability to evoke a (strong) cortical 40 Hz response rather than the user experience associated with the treatment implementation. A recent study on the safety and feasibility of GENUS with combined 40 Hz flicker and audiostimulation 1-hour daily for 6 months (n = 74 included) resulted in a 28.38% dropout rate92. These results highlight the importance of treatment feasibility to reduce the dropout rate, increase the duration of correct usage, and ultimately optimize efficacy and effectiveness. In comparison, a similar study using 1-hour daily visual stimulation for 6 weeks (n = 11 included) reported no dropouts: Although, the study had a shorter duration and a smaller sample size51. Importantly, the treatments were implemented differently in the two studies. Hajós et al.92 used wearable glasses and earpieces, whereas Agger et al.51 used a standalone table-top light stimulation device. The designs of the treatment may play a vital role in participant retention and adherence as interventions demanding active engagement may face neglect. Adherence is key to any treatment and reliable measures are needed to truly evaluate the efficacy of a treatment.
In this study, we aim to understand how LF, CF, and ISF impact gamma entrainment and user experience (see Fig. 1). The study contains three experiments. In experiment A, we measure the steady-state visual evoked potential (SSVEP) response of ISF, LF, and CF at different levels of brightness to investigate the effects of brightness and stimulus. In experiment C we investigate how the brightness and stimulus affect the perceived flicker and experienced discomfort. In experiment B we measure the SSVEP response from ISF to compare the effect of stimulating the peripheral, rather than the central, field of vision.
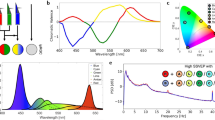
Graphical illustration of the neural response during 40 Hz visual stimulation. (a) Administration of exogenous 40 Hz temporally modulated light evokes an electrical neural response oscillating at the same frequency as the flickering light stimulation. ISF evokes a lower response than LF but is rated more comfortable. (b) ISF will evoke a response from direct exposure but also by stimulating at a peripheral angle.
We hypothesize that ISF, while inducing a lower acute response91, has a lower level of perceived flicker and results in higher comfort compared to LF and CF, thereby, increasing its potential for real-world application. We propose that better visual stimulation options can be achieved by rendering the flicker experience from TLM near imperceptible and freeing the user from active involvement.
Methods
Participants and ethics
Twenty-five healthy participants between the ages of 20 to 44 (15 males and 10 females) without vision impairment were recruited through the Technical University of Denmark (DTU) (Anker Engelunds Vej 1, Bygning 101A, 2800 Kongens Lyngby, Denmark). Participants were selected based on exclusion criteria, excluding those above the age of 50 and individuals with a history of light sensitivity, neurological or psychiatric diseases, epilepsy, or familial history of epilepsy. Participants underwent a self-reported assessment guided by questions from the investigator. This approach identified individuals with potential photosensitivity or known neurological or psychiatric disorders. One participant was excluded based on their self-reported photosensitivity.
All participants completed every experiment, but due to recording issues and missing data, only a subset of 20 participants (7 females) could be used in the analysis (see further details in supplementary materials). Before inclusion, written and verbal information was provided to the participants, and written informed consent was obtained. The recruitment and experimental procedures adhered to the Declaration of Helsinki, the General Data Protection Regulation (GDPR), and were approved by the Institutional Review Board at DTU Compute, Technical University of Denmark (application number: COMP-IRB-2020-01, approved 14-02-2020).
Visual stimulation system
During all three experiments (A, B and C) the Visual Stimulation System (VSS1.0) developed by OptoCeutics ApS was used. The device includes a flat light diffuser screen of width 25 cm and height 18 cm (see Fig. 3b). The device is equipped with two sets of six LEDs (blue, cyan, green, lime, amber, red) that can be customized for various light settings. The LEDs are a mix of direct and phosphor-converted emitters. A specialized LED controller is employed to facilitate precise adjustment of the brightness for each of the six independent channels. This ensures precise calibration of the 40 Hz stimulation.
A total of four different types of light were designed for the experimental stimuli. A modified version of the VSS device using only the blue, red, green, lime, and red LEDs was used for the experiment. Each LED channel was independently controlled, to produce the different types of light output needed. The visual stimuli included a 40 Hz luminance flicker (LF), a 40 Hz invisible spectral flicker stimulus (ISF), a 40 Hz color-fused red/green stimulus (CF), and a non-flickering control light (CON). Modulation depth of 100%, 5%, 25%, and 0% respectively. The stimulation frequency was calibrated to 40 Hz and manually measured using an oscilloscope before and after the data collection to 40.01 Hz, corresponding to an acceptable 0.25% deviation. All the 40 Hz modulated stimuli had a 50% duty cycle. Except for the color-fused stimulus, the light-based stimuli were designed to have similar white appearance and spectral composition (see Fig. 3d and Supplementary Material Fig. S3). The color temperature of the LF, CON and ISF lights were matched at 2572 K and CF at 1717 K. In experiments, A and C, the brightness of the four types of light varied in intensity by three levels - approximately 5,200, 9,100, and 10,400 lux - measured at the surface of the device. This corresponds to luminance values of 1577 \({\mathrm{cd/m^2}},\) 2589 \({\mathrm{cd/m^2}}\) and 3019 \({\mathrm{cd/m^2}}\). Stimuli were selected based on existing literature for entrainment from chromatic and luminance flicker stimuli69,70,71,72,73,74,75,76. We addressed a gap in the literature by comparing brightness, discomfort, and flicker perception. Following prior and ongoing studies (NCT04574921, NCT05260177), we selected the lowest brightness and explored higher levels at 75% and 100% increases.
To ensure a similar experience of brightness between the four types of stimuli, the brightness was visually matched. The total lux value was not just integrated over a full cycle (Supplementary Material Fig. S2). Instead, the energy was preserved over half a cycle (12.5 ms) as both the SSVEP measure of interest and the brightness are perceived on a millisecond level. For experiment B, only a single light stimulus (ISF) was used at the highest level of brightness. Marked fixation points were used to help the participants focus76, and participants were carefully instructed before each experiment on where to look.
Experimental paradigm
The study included three experiments; two successive EEG experiments (A and B) followed by a separate assessment of the different lights (experiment C). Experiments A and C measured the EEG response and subjective discomfort rating for four types of light stimuli (LF, CON, ISF, CF) at three different levels of brightness. Experiment B measured the EEG response for ISF under varying exposure angles. The three experiments are considered independent and participants were reminded about the conditions before each experiment. The data were collected during a single experimental visit and the total time of the experiment visit was no longer than 90 minutes.
Experiment A
A within-subject design was used with four types of light stimuli and three light intensities, crossed to obtain a total of 12 stimuli. Each of these was repeated six times. The order of the 72 stimuli presentations was randomized individually for each test participant. The order was validated using graphical methods post hoc. Repetitions in random order were incorporated into the experimental design and analysis to account for potential effects arising from the perceiver’s adaptation to the light stimulus, including aspects such as attention fluctuations and neural adaptation. Each stimulus was presented for 20 seconds with an interstimulus interval (ISI) of 5 seconds. Before and after the sequence of stimuli, a 60 second eyes-open baseline was recorded. The total experiment duration was 32 minutes. The participants were asked to rest their gaze at a fixation point at the center of the light source (Fig. 3b).
Experiment B
A within-subject study design with two blocks was used. Four different fixation points were used for each subject and repeated only once in each of the two blocks. The order within each block was random (Fig. 2). Like experiment A, repetitions were included to account for attention and adaption. The order of gazing points was randomized for each individual before the experiment for the investigator to instruct the participant to move their gaze. The order was validated before the experiment and post hoc. During this experiment, 40 Hz ISF was presented for 60 seconds, and during a 20 second ISI, the participant was instructed to look at a new fixation point placed on or around the stimulation device. These included two fixation points within the perimeter of the device; at the center and 10° to the right, and three fixation points outside this perimeter; at angles 20° to the right, 30° to the right, and 20° above, see Fig. 3a, b. The total experiment duration was 10 minutes. Participants were stratified into two groups. The first 10 participants were instructed to gaze at points along the horizontal angles, while the remaining 10 participants, were instructed to gaze at fixation points outside of the device. The center gaze was included in both groups as a reference.
Experimental Designs: Experiment A (top) included four different types of light stimulus (ISF, CF, LF, and CON) at three levels of brightness. (Brightness is not illustrated in the figure). Each stimulus was presented six times and the order was randomized. An interstimulus interval (ISI) of five seconds was introduced between each stimulus. 60 seconds baseline with eyes-open and no light was also recorded at the beginning and the end of the experiment. EEG was recorded during the experiment. Experiment B (middle) included light stimulation with ISF. Participants were asked to gaze at different fixation points only once in each of the two blocks. The order within each block was random. The light stimulus was turned on for 60 seconds, followed by 20 second ISI to allow for verbal instructions on the next fixation point. EEG was recorded during the experiment. Experiment C (bottom) included the same 12 light stimuli as experiment A, however, participants would rate each of the stimuli in terms of perceived discomfort and flicker. Each stimulus was presented four times in random order. The stimulus would change when rating was completed, and pace of the experiment was thereby controlled by the participant.
Experimental setup. Participants were placed approximately 60 cm from the light device and instructed to rest their gaze at different fixation points according to the experiment. During experiment A, participants were instructed to gaze at the center fixation point (indicated with no angles). During experiment B, participants were instructed during the ISI about which fixation point to gaze at during the following stimulation period. Four light settings at three different levels of brightness were used in experiment A; a non-flickering control light (CON), a 40 Hz luminance flickering light, a 40 Hz invisible spectral flicker (ISF) light, and a 40 Hz red/green color-fused flickering light (CF). Light settings are designed as given in (c) and (d). LF, CON, and ISF are all positioned at the same coordinates (0.41, 0.34) in the CIE diagram and therefore appear visually similar to the observer. CF does not have a similar appearance and is therefore positioned at different coordinates (0.58, 0.40). The coordinates of the two half-cycles of ISF are (0.52, 0.36) and (0.3, 0.32).
Experiment C
A within-subject design was used with four repetitions of each stimulus and complete randomization of stimulus order. In the experiment, participants were exposed to the same 12 lights as experiment A while simultaneously rating their experienced discomfort and perceived flicker using a PC as described in the following section. The duration of the experiment was 10 to 15 minutes but varied according to each participant. The same randomization as in experiment A was applied.
Electroencephalography recording
All experiments were carried out in a purposely designed EEG laboratory with semi-blinded windows. Electrical interference was minimized by disconnecting all non-essential electronic devices and ambient light. The stimulus system, VSS 1.0, being the only powered device, was supplied using a shielded cable to minimize noise. To further reduce noise and artifacts from the participants, they were seated in a comfortable chair approximately 60 cm from the stimulus device. Participants were instructed on the importance of remaining still and relaxing throughout the EEG recording.
EEG data was collected using a FDA-approved wireless 19-channel headset (Zeto Inc. medical device manufacturer, Santa Clara, CA.). The system uses dry active electrodes, it has a bandwidth of 0.003– 250 Hz and noise levels are \(<1\,\upmu{\text{V}}\) RMS. Electrodes were placed according to the international 10-20 system and the following 12 channels were used for data analysis: Fp1, Fp2, Fz, F3, F4, T5, T6, P3, P4, Pz, O1 and O2. Fpz was used as ground and linked mastoids as reference. Data was sampled using dedicated software at a sampling rate of 500 Hz via Bluetooth connection.
Subjective light assessment
An assessment scale was adapted from the visual analogue scale (VAS), including the following three measures: 1) Discomfort (“How much discomfort do you experience?”), 2) flicker (“How much flicker do you experience?”), and 3) color (“How do you like the color?”). Participants were instructed to evaluate each stimulation based on the three specified assessments using an 11-point scale ranging from zero to ten. Ten being maximum discomfort, maximum flicker, and very pleasant, respectively. The assessments were conducted utilizing a PC, with the paradigm programmed in Python. Participants had unsupervised control over the pace of the assessment, initiating each evaluation by interacting with the program. The resulting data were stored in a CSV format. It is noteworthy that the light remained illuminated during the assessment and underwent changes upon interaction. By instructing participants to independently rate various factors, we employ the method of controlled comparisons to ensure an isolated assessment of discomfort irrespective of color and flicker influences. Controlled comparison facilitates a more transparent and interpretable analysis of the direct correlation between comfort ratings and underscores the immediate influence of comfort ratings on the overall lighting experience. Supplementary materials include further elaboration of the experimental setup.
EEG data pre-processing
At the conclusion of the experiments, EEG data was processed with Python using the MNE toolbox (version 0.24.1)93. Data was filtered using a zero-phase finite impulse response (FIR) notch filter at 50 Hz using a hamming window and transition band of 0.5 Hz, followed by a 1 Hz high pass filter of the same type but with a transition bandwidth of 1 Hz. Data was manually inspected to reject bad channels and mark bad segments of data. One recording from experiment A and one recording from experiment B were excluded completely based on the manual inspection of the data, due to inadequate signal quality from excessive movement and insufficient electrode connection. The process of manual inspection involved a visual examination of the data to identify and reject bad channels and mark segments unsuitable for analysis. While no specific predefined criteria or thresholds were utilized, the decision was based on a careful visual assessment of the data quality. Re-referencing to the common average was also applied along with Independent component analysis (ICA) to remove ocular artifacts. ICA was applied using the Picard algorithm to remove ocular artifacts. Ocular artifacts were discovered by visual inspection of the raw components along with their source ___location. The number of components that contain ocular artifacts varied depending on the recording. However, in general, we excluded one or two components dominated by ocular artifacts. Eye movements and blinks generate strong, non-Gaussian signals that can easily be separated from the brain activity by ICA. In a few cases, the ocular artifacts were particularly strong and we found more than two ICs.
Response quantification
It is challenging to quantify EEG spectral power and SSVEP peaks as the signals are inherently noisy due to various sources, such as muscle activity, eye movements, and electrical interference with a high subject variability. Multiple methods have been proposed in the literature including, absolute power, relative power, and baseline comparisons. The study aims to assess how 40 Hz stimulation enhances brain activity. Thus, we require a method to quantify the peak relative to the baseline. However, due to the experiment’s design and duration, obtaining a valid baseline recording was deemed impractical. This is mainly because of potential temporal fluctuations caused by motion and changes in electrode impedance thereof. The following method of estimating the SNR was chosen for its simplicity and available documentation94,95. However, the method exhibits high sensitivity to noise, and alternative approaches, such as contrast-to-noise ratio or more sophisticated techniques like FoooF (fitting oscillations and one over f), may offer improved accuracy by considering the variance in noise.
The power spectral density (PSD) was estimated by the Welch method with a Hann window of 10 seconds and 50% overlap. Segments were zero-padded to obtain a frequency resolution of 0.05 Hz. For the best estimation of the 40 Hz SSVEP peak, the frequency resolution was chosen as an integer multiple of the flicker cycle. The Welch method for PSD estimation was chosen due to its widespread use and established balance between frequency resolution and variance reduction. The Hann window was chosen for its smooth, bell-shaped shape that tapers off gradually at the edges, reducing spectral leakage. The larger window size and high overlap percentage blur the temporal resolution. However, it reduces noise or transient fluctuations in the signal which is favorable for our analysis. The selection of frequency resolution was based on a balance between capturing all details in the spectral ___domain and minimizing computational demands. The frequency resolution of 0.05 Hz was achieved through zero-padding. Opting for a lower frequency resolution was avoided to prevent excessive smoothing, which could result in the loss of finer structure in the spectrum. This resolution was deemed adequate based on the characteristics of the SSVEP under investigation.
For each frequency bin in the power spectrum, the signal-to-noise ratio (SNR) was computed relative to the average of the nearest two adjacent integer frequencies skipping the nearest integer frequency on both sides, corresponding to a frequency band from \(\pm 1\) Hz to \(\pm 2\) Hz around each target frequency. The averaging was computed by convolving with a square kernel,94,95. The SNR in the frequency bin closest to 40 Hz was extracted, averaged over channels and used in the statistical analysis.
Statistical analysis
To analyze the SNR of the SSVEP, a linear mixed effect model (a within-subject design) with a significance level of 0.05 was used. Participants and repetitions were included as random effects, whereas stimulus conditions (type of light, brightness, or exposure angle) were included as fixed effect(s). In experiment B, block was added as a random factor. Interaction effects between fixed and random effects were included. With this model it is assumed that there is a linear relationship between the independent and dependent variables and both residuals and random factors are independent, normally distributed, and homoscedastic. These assumptions are checked by Q-Q plots and scatter plots of the fitted- versus residual values to determine that there is no residual correlation structure unaccounted for. The discomfort measures were analyzed using a cumulative mixed effect96 model due to the ordinal nature of the Likert-item type data. The model included the type of light and brightness as fixed effects along with the subject as random effects. Model reductions were applied and the final models are presented here. The cumulative model accommodates the nested structure of repeated measurements within participants, and its flexibility in incorporating fixed and random effects is advantageous for capturing both population-level trends and individual variability. However, it’s crucial to note that the model assumes proportional odds and that the outcomes are less straightforward to interpret. The proportional odds assumption states that the effect of the independent variables on the odds of moving from one rating category to the next is the same across all levels of rating. The assumption was tested using graphical methods by visually inspecting the estimated distance between the odds of each rating score.
The final models, where the dependent variable, Y, is either the SNR values, experienced discomfort, or flicker, are expressed in Eqs. (1–3). For experiment A:
where \(Y_i\) is the 40 Hz SNR for the ith trial averaged across electrodes, \(c\sim N(0, \sigma _{{\text{repetition}}}^{2}),\)\(d \sim N(0,\sigma _{{\text{subject}}}^{2}),\)\(m\sim N(0,\sigma _{{\text{stimulus,brightness,subject}}}^{2}),\) and \(\epsilon \sim N(0,\sigma ^2).\)
For experiment B:
where \(Y_i\) is the 40 Hz SNR for the ith trial averaged across electrodes, \(g\sim N(0, \sigma _{{\text{angle, subject}}}^{2}),\) and \(\epsilon \sim N(0,\sigma ^2).\)
For experiment C with both outcome measures:
where \(Y_i\) is the outcome for the ith trial, \(d\sim N(0,\sigma _{{\text{subject}}}^{2}),\) and \(\epsilon \sim N(0,\sigma ^2 ).\)
Post-hoc comparisons with Šidák corrections for multiple comparisons were applied to compare the different stimulus conditions. All statistical analyses were done in R. Specifically, the validated functions lmer (lmerTest/lme4)97 and ordinal (clmm)98 were used for estimating the mixed effect models. The emmeans package was used for correction of multiple comparisons. Additionally, Bayesian ANOVA was applied, using JASP, to the mean values estimated by the models to compare the peripheral angle conditions in experiment B with the control stimulation in experiment A.
Results
Experiment A: stimulus type and brightness
The results of experiment A show that all three types of TLM evoke a significantly higher 40 Hz cortical response than the control (see Figs. 4 and 6a). On average, the distribution of the 40 Hz power across electrodes is greatest for LF and lowest for ISF, and CF is between the two, as evident from the topographic map in Fig. 4a. While the highest 40 Hz power was detected by electrodes in the occipital-parietal region, source reconstruction was not attempted, and thus volume conduction limits information from the spatial distribution of the 40 Hz signal. Instead, the average global 40 Hz power is shown for each condition as grand-averaged spectrograms in Fig. 4c and grand-averaged power spectra in Fig. 4b. The estimated global average 40 Hz power was significantly higher than the control for all TLM conditions after correction for multiple comparisons using the Sidak method for control of the family-wise error (FWE) (see Table 2). The mean difference (MD) in SNR between LF and control was 9.74 dB (95% confidence interval (CI): 8.79 to 10.69 dB, \(P<.0001\)), while the MD between CF and control was 7.32 dB (95% CI: 6.36 to 8.27 dB, \(P<.0001\)), and the MD between ISF and control was 4.31 dB (95% CI: 3.36 to 5.26 dB, \(P<.0001\)).
Comparing stimulus intensity, the highest 40 Hz cortical response was achieved by the highest intensity, followed by the middle intensity, and finally the lowest intensity, though the effect was not significant (see Table 2). The MD in SNR between the middle and lowest intensity was 0.3 dB (95% CI: -0.5 to 1.0, P = 0.67), while the MD between the highest and lowest intensity was 0.4 dB (95% CI: -0.4 to 1.2, P = 0.44).
Additionally, there were significant subject- and repetition effects, suggesting both inter-subject variability in the strength of cortical 40 Hz response and a high degree of test-retest variability over time (explained variance: \(\hat{\sigma }_{{\text{subject}}}^{2} = 1.75\) and \(\hat{\sigma }_{{\text{repetition}}}^{2} = 0.12\)). Finally, the three-way interaction effect between subject, stimulus, and intensity was significant (\(P<.0001\)) and explained most of the variance (\(\hat{\sigma }_{{\text{stimulus,brightness,subject}}}^{2} = 3.49\)), which indicates a subject preference for certain combinations of TLM type and intensity.
These results indicate that LF, CF, and ISF evoke significant 40 Hz power but at varying magnitude, and that the intensity of the light has less of an impact than the choice TLM.
Topographic maps, spectrogram, and average Power Spectral Density plot for the different stimulus types in experiment A. (a) The topographic maps show the spatial distributions of the SNR values across channels. (b) PSD shows the SSVEP response for each of the 12 stimulus conditions. (c) Spectrograms show the presence of the 40 Hz response during stimulation. Stimuli at the highest brightness levels are used for the topographic maps and spectrograms. PSD and spectrograms have been averaged over channels. Shaded areas of the PSD indicate the standard deviation. Figures are included to visualize the results. Interpolation of noisy channels was applied here for illustration purposes but not in the statistical analysis. Topographic maps for all combinations of stimulus and brightness combinations are available in the Supplementary Material Fig. S6 and S7.
Experiment B: angles of exposure
In experiment B, a 40 Hz cortical response is observed for all angles of exposure (see Fig. 5) similarly to the ISF condition in experiment A. The effect of exposure angle on SNR was significant, see Table 1. Only significant contrast was found between the 30 degrees horizontal and central conditions after correction for multiple comparisons (MD: − 1.58 dB, 95% CI: − 2.93 to − 0.23 dB, P = .02). Differences between the other angles and center were: For 10° horizontal (MD: − 0.66 dB, 95% CI: − 2.33 to 1.02 dB, P = .78), 20° horizontal (MD: − 0.91 dB, 95% CI: − 2.32 to 0.494 dB, P = .34), 30° horizontal (MD: − 1.58 dB, 95% CI: − 2.93 to − 0.23 dB, P = .02), 20° vertical (MD: − 1.54 dB, 95% CI: − 3.47 to 0.38 dB, P = .16). The distributions of SNR between stimulation angles presented in Fig. 6b shows the trend for a decrease in 40 Hz SNR with increased exposure angle.
The topographic maps in Fig. 5a show similar distributions of 40 Hz power across the electrodes between stimulation angles. While a change in the spatial pattern of cortical activation is possible as a consequence of altering which part of the field of view is stimulated, such nuances are not expected to be evident at the sensor level. Spectrograms in Fig. 5c display only a slight variation in peak 40 Hz power between angles, but also a varying degree of broadband aperiodic noise around the peak. Power spectra in Fig. 5b indicate a similarity of global 40 Hz power between angles above the aperiodic noise. The variation in broadband noise may be an artifact from moving the head position between trials, a matter of test-retest variability and low number of repetitions, and/or between subjects variability from having each subgroup of 10 subjects only be exposed at two of the four non-center angles.
There was a significant subject effect which explained most of the variance (\(\hat{\sigma }_{{\text{subject}}}^{2} = 4.33\)) suggesting a high degree of inter-subject variability. Additionally, the interaction effect between subject and angle was significant (explained variance: \(\hat{\sigma }_{{\text{subject,angle}}}^{2} = 2.17\)), indicating that subjects were affected differently by the change in exposure angle, perhaps by an uncontrolled confound of modulated visual attention.
Furthermore, Bayesian ANOVA showed extreme evidence that the cortical 40 Hz power from all angles of exposure was significantly higher than the control stimulation (BF \(\gg\) 100, see Supplementary Material Table S4).
The decline in SNR is expected both from the reduced flux of light through the pupil at increased angles and potentially reduced visual attention to the stimulus. The result implies a trade-off between the 40 Hz SNR and the geometric position of the light source, and disengaging from gazing directly at the stimulus comes at a cost of slightly lower evoked 40 Hz cortical power. However, the presence of a cortical 40 Hz response even at high exposure angles indicates that it is a viable alternative to direct stimulation.
Topographic maps, spectrogram, and average Power Spectral Density (PSD) plot for the different angles of exposure in experiment B. (a) The topographic maps show the distribution of the SNR values in each channel. (b) PSD shows the SSVEP response for each of the exposure angles. (c) Spectrograms show the presence of the 40 Hz response at all angles of exposure. PSD and spectrograms have been averaged over channels. Shaded areas of the PSD indicate the standard deviation. Figures are included to visualize the results. Interpolation of noisy channels was applied here for illustration purposes but not in the statistical analysis.
Experiment C: discomfort and flicker
In experiment C, the discomfort rating was significantly increased for all three types of TLM compared to control (see Table 2). Figure 6c shows the probability of each discomfort rating for each of the conditions. Of the three TLMs, ISF had the lowest discomfort score with an increase from control of 1.9 (95% CI: 1.4 to 2.4, \(P<.001\)), while CF scored 5.1 higher discomfort than control (95% CI: 4.7 to 5.6, \(P<.001\)), and LF scored 6.2 higher than control (95% CI: 5.8 to 6.6, \(P<.001\)). These results suggest that all types of TLM are more uncomfortable than static light, though not to the same degree. The CF was significantly more uncomfortable than ISF (MD: 3.2, 95% CI: 2.7 to 3.7, \(P<.001\)), and so was LF (MD: 4.3, 95% CI: 3.9 to 4.8, \(P<.001\)). Finally, LF was significantly more uncomfortable than CF (MD: 1.1, 95% CI: 0.7 to 1.6, \(P<.001\)). As such, the most comfortable choice among the three is ISF whose score was closer to that of the control than both CF and LF. The discomfort rating was also significantly affected by brightness though to a lower degree than the type of TLM (see Table 2). As expected, the lowest discomfort was reported for the lowest brightness and the highest for the highest brightness setting. The middle brightness was significantly more uncomfortable than the lowest (MD: 0.5, 95% CI: 0.2 to 0.8, \(P<.01\)), and so was the highest brightness with a difference to the lowest brightness of 0.6 (95% CI: 0.2 to 0.8, \(P<.001\)). The middle and highest brightness were not significantly different (MD: 0.1, 95% CI: − 0.2 to 0.5, P = 0.65).
In terms of perceived flicker, all three types of TLM scored significantly higher than the control (see Table 2). Figure 6d shows the probability of each flicker rating for each of the conditions. Of the three TLMs, ISF scored the lowest on perceived flicker at 3.4 higher than control (95% CI: 2.7 to 4.1, \(P<.001\)), while CF scored 7.2 higher than control (95% CI: 6.2 to 7.6, \(P<.001\)), and LF scored 9.1 higher than control (95% CI: 8.8 to 9.4, \(P<.001\)). These results suggest that all types of TLM are perceived to be more flickering than static light, though at vastly different degrees. Both CF and LF scored significantly higher than ISF in terms of perceived flicker. The flicker score was 3.9 higher for CF than ISF (95% CI: 3.4.8 to 4.4, \(P<.001\)) and 5.7 higher for LF than ISF (95% CI: 5.2 to 6.2, \(P<.001\)). The brightness did not significantly impact the degree of perceived flicker (see Table 2 and Fig. 6d).
Estimated mean SNR values from experiment A-B and estimated probability of each rating on perceived discomfort and flicker in experiment C. Barplot (a) shows the estimated mean SNR values of each stimulus in experiment A and estimated mean difference from CON averaged over the levels of brightness. All light-based gamma stimuli are found to be significantly different from the CON light. The level of brightness was found to be non-significant for the SNR values. Barplot (b) shows the estimated mean SNR values of each angle of exposure in experiment B and the estimated mean difference from direct exposure. Exposure from an angle of 30° to the right of the center was significantly different from the direct exposure. A trend of increasing response with increasing brightness and a trend of decreasing response with increasing exposure angle is observed. Mean differences are estimated from the linear mixed effects models after adjustment for multiple comparisons using the Sidak method. Only significant differences are shown in the barplots. The probability of each rating on perceived discomfort (c) and flicker (d) is estimated from the cumulative linked mixed effects model.
Correlation between flicker, discomfort, and entrainment
When evaluating the results of experiments A, B, and C, there appears to be a correlation between the SNR for the 40 Hz cortical response to stimulation, the degree of perceived flicker from the stimuli, and the amount of experienced discomfort from the stimuli. To further foster the discussion on the feasibility of gamma stimulation, an exploratory analysis of the correlation structure was conducted using the non-parametric Spearman’s correlation. Figure 7 shows the pairwise correlations between perceived discomfort, flicker, and the 40 Hz SSVEP SNR. A significant (\(P<.001\)) positive correlation exists between both perceived flicker and perceived discomfort (r = 0.88) and between the 40 Hz SNR and the perceived flicker (r = 0.83) and the perceived discomfort (r = 0.77). This indicates that higher flicker is significantly related to higher discomfort and that a higher perception of flicker and discomfort also is related to higher acute gamma entrainment. In practice, this means that there might be a trade-off between comfort and efficacy and that a balance must be struck to find the optimal visual stimulation implementation.
When the correlations are estimated individually for each type of stimulus, the results are more nuanced. The individual slopes for the correlation between perceived flicker and experienced discomfort are almost identical to the aggregate correlation slope (see Fig. 7c). This is supported by a cumulative mixed model indicating that experienced discomfort is explained only by the perceived flicker. However, the individual slopes for the correlation between SNR and discomfort are different from the aggregated correlation (see Fig. 7a). The cumulative mixed model also indicates a significant main effect of stimulus and an interaction between stimulus and SNR. Analysis of the perceived flicker (Fig. 7b) shows similar results. These findings imply that within a given choice of TLM, an increase in SNR is associated with a slight increase in perceived flicker or experienced discomfort.
Correlation between neural entrainment and visual perception. Scatterplot and Spearman’s correlation coefficient between (a) the 40 Hz SNR and experienced discomfort, (b) the 40 Hz SNR and the experienced flicker, and ( c) the experienced flicker and experienced discomfort for each type of stimulus. r denotes Spearman’s correlation coefficient for the aggregated data, and P is the P-value of the significance of the correlation. A significant positive correlation is found for all pairwise correlations. The scatterplots show observations from experiments A and C for each subject averaged over repetitions and EEG channels and averaged over repetitions, respectively. Linear regression models are fitted to the entire data and to each stimulus type (ISF, CF, IF) to estimate the best fit, and these lines are included in the scatterplots.
Discussion
In this study, we compared various visual GENUS options for entraining a 40 Hz brain response to assess their acute neural response and feasibility. This was done by measuring the acute 40 Hz brain entrainment and visual perception scores (perceived flicker and visual discomfort). Additionally, for further exploration of real-world application, we measured the acute 40 Hz brain entrainment under peripheral exposure by facilitating an experiment with different gazes increasing further from the center of the stimulation device.
The results showed a clear correlation between the 40 Hz entrainment and the subjective perception of discomfort and flicker. Higher entrainment is followed by higher discomfort and perception of flicker (see Fig. 7). Luminance and chromatic flicker result in the highest 40 Hz entrainment power but are also perceived as the most flickering and experienced as the least comfortable. In contrast to this, ISF stimulation results in the lowest entrainment power, though significant compared to control stimulation, and with significantly better ratings in perceived flicker and comfort than LF and CF. We propose that ISF stimulation is a more feasible option for 40 Hz visual stimulation as a potential treatment because of its tolerability during 40 Hz entrainment. This is supported by the comparatively lower drop-out frequency and higher treatment adherence in clinical trials using ISF99 than combined audiovisual stimulation53.
We find that lowering stimulation brightness significantly lowers the discomfort but not the perceived level of flicker. Stimulation at brightness levels, from 5,200 to 10,400 lux, entrain a significant 40 Hz response, though a trend towards decreased entrainment is observed with lower brightness. This trend agrees with previous findings49,70. Given that discomfort glare is affected by intensity but also highly subject-dependent100,101, these results suggest that users may benefit from an option to individually select the stimulation intensity for improved tolerability. It is important to note the effect sizes are relatively small and therefore higher sample size is required to confirm these results.
Increasing the stimulation angle emulates a treatment setting in which the user can partake in other activities while receiving stimulation passively. Despite a decrease in response with increased stimulation offset, the difference is non-significant up to 20 degrees horizontal and vertical offsets, compared to no offset. The immediate benefit of this is the prospect of users introducing the treatment into their existing habits with minimal effort. An additional analysis comparing the estimated mean values of the control stimulation in experiment A and the estimated mean values for each angle of exposure in experiment B shows evidence that there is a significant response at all angles.
Together, these findings present several trade-offs. The first is a trade-off between comfort and entrainment power. The second is a trade-off between perceived flicker and entrainment power. The third is a trade-off between brightness and entrainment power. Finally, we show a trade-off between stimulation angle and entrainment power. These trade-offs give rise to optimization problems whose Pareto fronts will be valuable to explore.
It is not yet clear how the acute entrainment power affects the medical response in AD, or if other markers are more relevant for the neuroprotective effects presented in39,46,47,48. Such markers could include the duration of entrainment above a certain power threshold, the spatial distribution of the entrainment, or a complex combination of either. Under the conservative assumption that the medical benefit is highly correlated with the entrainment power, it may still be favorable to choose a more comfortable treatment option if it leads to better adherence. Adherence pertains both to the selection of stimulus type and stimulation intensity, duration, and exposure angle parameters. By adjusting factors like brightness and angle of exposure, and even frequency or color settings, while measuring the neural entrainment it will be possible to design personalized treatment options for each individual patient. Results indicate general trends that highlight the complexity and high inter-subject variability of visual brain stimulation. Optimization schemes for the AD population should be designed not only by the acute neural response alone but also based on user feedback, attention scores, and disease progression. A possible approach could be to employ Bayesian optimization and neurofeedback techniques.
Across several medical subfields, including AD, increased treatment side effects are related to lower adherence102,103. While several light-based GENUS paradigms are suggested for AD treatment, no study has (to the author’s knowledge at the time of submission) compared them in terms of tolerability. Our results indicate that the potential treatment barriers to GENUS for AD may be overcome by carefully engineered light technologies designed for higher comfort and feasibility. Considering the traits of patients with AD, a 1-hour daily treatment session might be infeasible due to the impaired attention associated with this population104. Our findings contribute to the design and use of new treatment options by providing evidence of the effect of different stimulation, brightness, and angles of exposure. Results suggest that visual brain stimulation treatment can be administered passively, potentially during other daily activities, and that alternative methods than luminance flicker exist. It is important to note that these results should be confirmed in longitudinal studies ideally by tracking adherence followed by a stratified analysis of different groups. Agger et al.51 propose gaze-tracking as a measure of adherence and classification of direct and indirect exposure. Other approaches could include complementary EEG recordings that validate the neural entrainment during stimulation.
We recognize that this study’s participants were limited to healthy young volunteers and may not accurately reflect the AD population for which this type of treatment is intended. Findings from this experiment, however, are in agreement with those of Agger et al.91. Their main EEG findings showed a significant response from both ISF and LF (STROBE) compared to CON with the highest response achieved by LF. However, further research is warranted within this field. Both the elderly and AD populations may differ from the healthy young population in terms of retinal sensitivity to light, attention levels, neural adaptations, and synchronization with the stimulation frequency. These are all critical factors to consider when evaluating the feasibility of new treatments. Individual variance even occurs in young and healthy populations in terms of sensitivity to light, attention, and general cognitive readiness. Our findings support this as we see a significant effect and variability between the participants. Individuals with light sensitivity may exhibit lower SSVEP responses due to discomfort-induced physiological behaviors, such as squinting or blinking, potentially decreasing signal amplitudes. However, their responses might also be higher overall, influenced by a lower threshold for visual stimulation. Similarly, visual stimulation combined with a simultaneous visual attention task or cognitive load68,105 has proven to positively modulate the SSVEP response in the gamma range, both in terms of power and spatial propagation. Such findings should be considered when evaluating the effectiveness of stimulation, as some types of stimulation can more easily be combined with additional tasks than others. Confunding factors of attention and cognitive load would be interesting to investigate in further research by designing experiments and analyses that include attention or cognitive paradigms.
Future research should expand the study to include a more diverse population, including elderly participants and those with AD, to better understand the generalizability of the findings and assess the potential impact of individual differences in light sensitivity, attention levels, and cognitive function.
This study provides further evidence to support the potential of gamma entrainment therapies for AD treatment. We have shown that ISF stimulation can effectively entrain 40 Hz brain activity while being significantly more comfortable and tolerable than other methods. Additionally, we have demonstrated the feasibility of achieving entrainment from passive exposure, suggesting that ISF stimulation could be a convenient and practical treatment option for AD.
Data availability
Data can be shared upon request for scientific purposes that do not violate the data processing agreement. Contact the authors for more information.
References
Nichols, E. et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 88–106. https://doi.org/10.1016/S1474-4422(18)30403-4 (2019).
WHO. Dementia (2022).
Patients, C. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 18, 700–789. https://doi.org/10.1002/alz.12638 (2022).
Long, J. M. & Holtzman, D. M. Alzheimer disease: An update on pathobiology and treatment strategies. Cell 179, 312–339. https://doi.org/10.1016/J.CELL.2019.09.001 (2019).
Bloom, G. S. Amyloid-\(\beta\) and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 71, 505–508. https://doi.org/10.1001/jamaneurol.2013.5847 (2014).
Kinney, J. W. et al. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Trans. Res. Clin. Interv. 4, 575–590. https://doi.org/10.1016/j.trci.2018.06.014 (2018).
Wyss-Coray, T. & Rogers, J. Inflammation in Alzheimer disease-A brief review of the basic science and clinical literature. Cold Spring Harbor Perspect. Med. https://doi.org/10.1101/cshperspect.a006346 (2012).
Zotova, E., Nicoll, J. A., Kalaria, R., Holmes, C. & Boche, D. Inflammation in Alzheimer’s disease: Relevance to pathogenesis and therapy. Alzheimers Res. Ther. https://doi.org/10.1186/alzrt24 (2010).
Kocahan, S. & Doǧan, Z. Mechanisms of Alzheimer’s disease pathogenesis and prevention: The brain, neural pathology, N-methyl-D-aspartate receptors, tau protein and other risk factors. Clin. Psychopharmacol. Neurosci. 15, 1. https://doi.org/10.9758/CPN.2017.15.1.1 (2017).
Cummings, J., Lee, G., Zhong, K., Fonseca, J. & Taghva, K. Alzheimer’s disease drug development pipeline: 2021. Alzheimer’s Dement. Trans. Res. Clin. Interv. 7, e12179. https://doi.org/10.1002/trc2.12179 (2021).
Sevigny, J. et al. The antibody aducanumab reduces A\(\beta\) plaques in Alzheimer’s disease. Nature 537, 50–56. https://doi.org/10.1038/nature19323 (2016).
Dunn, B., Stein, P. & Cavazzoni, P. Approval of aducanumab for alzheimer disease-the FDA’s perspective. JAMA Intern. Med. 181, 1277–1278. https://doi.org/10.1001/jamainternmed.2021.4607 (2021).
Alexander, G. C., Emerson, S. & Kesselheim, A. S. Evaluation of aducanumab for Alzheimer Disease: Scientific evidence and regulatory review involving efficacy, safety, and futility. J. Am. Med. Assoc. 325, 1717–1718. https://doi.org/10.1001/jama.2021.3854 (2021).
Mahase, E. Aducanumab: European agency rejects Alzheimer’s drug over efficacy and safety concerns. BMJ (Clinical research ed.) 375, n3127. https://doi.org/10.1136/bmj.n3127 (2021).
van Dyck, C. H. et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. https://doi.org/10.1056/NEJMOA2212948/SUPPL_FILE/NEJMOA2212948_APPENDIX.PDF (2022).
Cummings, J. Anti-amyloid monoclonal antibodies are transformative treatments that redefine Alzheimer’s Disease therapeutics. Drugs 83, 569–576. https://doi.org/10.1007/s40265-023-01858-9 (2023).
Cummings, J. et al. Alzheimer’s disease drug development pipeline: 2023. Alzheimer’s Dement. (New York, N. Y.) https://doi.org/10.1002/TRC2.12385 (2023).
Makin, S. The amyloid hypothesis on trial. Nature 559, S4–S7. https://doi.org/10.1038/d41586-018-05719-4 (2018).
Selkoe, D. J. & Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 8, 595–608, https://doi.org/10.15252/emmm.201606210 (2016).
Babiloni, C. et al. What electrophysiology tells us about Alzheimer’s disease: A window into the synchronization and connectivity of brain neurons. Neurobiol. Aging 85, 58–73. https://doi.org/10.1016/j.neurobiolaging.2019.09.008 (2020).
Güntekin, B. et al. Alterations of resting-state Gamma frequency characteristics in aging and Alzheimer’s disease. Cogn. Neurodyn. https://doi.org/10.1007/s11571-022-09873-4 (2022).
Jeong, J. EEG dynamics in patients with Alzheimer’s disease. Clin. Neurophysiol. 115, 1490–1505 (2004).
Soininen, H. et al. Longitudinal EEG spectral analysis in early stage of Alzheimer’s disease. Electroencephalogr. Clin. Neurophysiol. 72, 290–297. https://doi.org/10.1016/0013-4694(89)90064-3 (1989).
Coben, L. A., Danziger, W. L. & Berg, L. Frequency analysis of the resting awake EEG in mild senile dementia of Alzheimer type. Electroencephalogr. Clin. Neurophysiol. 55, 372–380. https://doi.org/10.1016/0013-4694(83)90124-4 (1983).
Stigsby, B., Jóhannesson, G. & Ingvar, D. H. Regional EEG analysis and regional cerebral blood flow in Alzheimer’s and Pick’s diseases. Electroencephalogr. Clin. Neurophysiol. 51, 537–547. https://doi.org/10.1016/0013-4694(81)90230-3 (1981).
Soininen, H., Partanen, J., Pääkkonen, A., Koivisto, E. & Riekkinen, P. J. Changes in absolute power values of EEG spectra in the follow-up of Alzheimer’s disease. Acta Neurol. Scand. 83, 133–136. https://doi.org/10.1111/j.1600-0404.1991.tb04662.x (1991).
Penttilä, M., Partanen, J. V., Soininen, H. & Riekkinen, P. J. Quantitative analysis of occipital EEG in different stages of Alzheimer’s disease. Electroencephalogr. Clin. Neurophysiol. 60, 1–6. https://doi.org/10.1016/0013-4694(85)90942-3 (1985).
Miltner, W. H., Braun, C., Arnold, M., Witte, H. & Taub, E. Coherence of gamma-band EEG activity as a basis for associative learning. Nature 397, 434–436. https://doi.org/10.1038/17126 (1999).
Buzsáki, G. & Schomburg, E. W. What does gamma coherence tell us about inter-regional neural communication?. Nat. Neurosci. 18, 484–489. https://doi.org/10.1038/nn.3952 (2015).
Klimesch, W. \(\alpha\)-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 16, 606–17. https://doi.org/10.1016/j.tics.2012.10.007 (2012).
Vysata, O., Kukal, J., Prochazka, A., Pazdera, L. & Valis, M. Age-related changes in the energy and spectral composition of EEG. Neurophysiology 44, 63–67. https://doi.org/10.1007/s11062-012-9268-y (2012).
Fröhlich, S., Kutz, D. F., Müller, K. & Voelcker-Rehage, C. Characteristics of resting state EEG power in 80+-year-olds of different cognitive status. Front. Aging Neurosci. 13, 469. https://doi.org/10.3389/fnagi.2021.675689 (2021).
Babiloni, C. et al. Measures of resting state EEG rhythms for clinical trials in Alzheimer’s disease: Recommendations of an expert panel. Alzheimers Dement. 17, 1528–1553. https://doi.org/10.1002/alz.12311 (2021).
Benwell, C. S. et al. EEG spectral power abnormalities and their relationship with cognitive dysfunction in patients with Alzheimer’s disease and type 2 diabetes. Neurobiol. Aging 85, 83–95. https://doi.org/10.1016/j.neurobiolaging.2019.10.004 (2020).
Cassani, R., Estarellas, M., San-Martin, R., Fraga, F. J. & Falk, T. H. Systematic review on resting-state EEG for Alzheimer’s disease diagnosis and progression assessment. Dis. Mark. https://doi.org/10.1155/2018/5174815 (2018).
Herrmann, C. S. & Demiralp, T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin. Neurophys. https://doi.org/10.1016/j.clinph.2005.07.007 (2005).
Wang, J. et al. Enhanced gamma activity and cross-frequency interaction of resting-state electroencephalographic oscillations in patients with Alzheimer’s disease. Front. Aging Neurosci. 9, 243. https://doi.org/10.3389/fnagi.2017.00243 (2017).
Buzśaki, G. & Wang, X. J. Mechanisms of gamma oscillations. Ann. Rev. Neurosci. https://doi.org/10.1146/annurev-neuro-062111-150444 (2012).
Adaikkan, C. & Tsai, L. H. Gamma entrainment: Impact on neurocircuits, glia, and therapeutic opportunities. Trends Neurosci. 43, 24–41. https://doi.org/10.1016/j.tins.2019.11.001 (2020).
Cardin, J. A. et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667. https://doi.org/10.1038/nature08002 (2009).
Sohal, V. S., Zhang, F., Yizhar, O. & Deisseroth, K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702. https://doi.org/10.1038/nature07991 (2009).
Nahar, L., Delacroix, B. M. & Nam, H. W. The role of parvalbumin interneurons in neurotransmitter balance and neurological disease. Front. Psychiatry https://doi.org/10.3389/fpsyt.2021.679960 (2021).
Jefferys, J. G., Traub, R. D. & Whittington, M. A. Neuronal networks for induced “40 Hz” rhythms. Trends Neurosci. https://doi.org/10.1016/S0166-2236(96)10023-0 (1996).
Traikapi, A. & Konstantinou, N. Gamma oscillations in Alzheimer’s disease and their potential therapeutic role. Front. Syst. Neurosci. https://doi.org/10.3389/fnsys.2021.782399 (2021).
Tian, T., Qin, X., Wang, Y., Shi, Y. & Yang, X. 40 Hz light flicker promotes learning and memory via long term depression in wild-type mice. J. Alzheimers Dis. 84, 983–993. https://doi.org/10.3233/JAD-215212 (2021).
Martorell, A. J. et al. Multi-sensory gamma stimulation ameliorates alzheimer’s-associated pathology and improves cognition. Cell 177, 256–271. https://doi.org/10.1016/J.CELL.2019.02.014 (2019).
Adaikkan, C. et al. Gamma entrainment binds higher-order brain regions and offers neuroprotection. Neuron 102, 929–943. https://doi.org/10.1016/J.NEURON.2019.04.011 (2019).
Iaccarino, H. F. et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 540, 230–235. https://doi.org/10.1038/nature20587 (2016).
Jones, M. et al. Gamma band light stimulation in human case studies: Groundwork for potential Alzheimer’s disease treatment. J. Alzheimers Dis. 70, 171–185. https://doi.org/10.3233/JAD-190299 (2019).
Cimenser, A. et al. Sensory-Evoked 40-Hz gamma oscillation improves sleep and daily living activities in Alzheimer’s disease patients. Front. Syst. Neurosci. https://doi.org/10.3389/fnsys.2021.746859 (2021).
Agger, M. P. et al. Safety, feasibility, and potential clinical efficacy of 40 hz invisible spectral flicker versus placebo in patients with mild-to-moderate Alzheimer’s disease: A randomized, placebo-controlled, double-blinded, pilot study. J. Alzheimer’s Dis. https://doi.org/10.3233/JAD-221238 (2023).
Blanco-Duque, C., Chan, D., Kahn, M. C., Murdock, M. H. & Tsai, L.-H. Audiovisual gamma stimulation for the treatment of neurodegeneration. J. Intern. Med. 295, 146–170. https://doi.org/10.1111/joim.13755 (2024).
Megerian, J. et al. Feasibility, safety, and efficacy of gamma sensory stimulation as a novel therapeutic intervention for Alzheimer’s disease (N1. 001). Neurology 98, 1936 (2022).
Da, X. et al. Noninvasive gamma sensory stimulation may reduce white matter and myelin loss in Alzheimer’s disease. J. Alzheimers Dis. 97, 359–372. https://doi.org/10.3233/JAD-230506 (2024).
Liu, C. et al. Modulating gamma oscillations promotes brain connectivity to improve cognitive impairment. Cereb. Cortex 32, 2644–2656. https://doi.org/10.1093/cercor/bhab371 (2022).
Williams, M. et al. Safety, feasibility, and adherence of a daily, in-home gamma sensory stimulation therapy with the Cognito Sensory Stimulation System in Alzheimer’s subjects. Alzheimer’s Dement. 17, e056480. https://doi.org/10.1002/alz.056480 (2021).
He, Q. et al. A feasibility trial of gamma sensory flicker for patients with prodromal Alzheimer’s disease. Alzheimer’s Dement. Trans. Res. Clin. Interv. 7, e12178. https://doi.org/10.1002/trc2.12178 (2021).
Suk, H.-J. et al. Vibrotactile stimulation at gamma frequency mitigates pathology related to neurodegeneration and improves motor function. Front. Aging Neurosci. 15, 1129510 (2023).
Ismail, R. et al. The effect of 40-Hz light therapy on amyloid load in patients with prodromal and clinical Alzheimer’s disease. Int. J. Alzheimer’s Dis. https://doi.org/10.1155/2018/6852303 (2018).
Han, C. et al. Enhancement of the neural response during 40 Hz auditory entrainment in closed-eye state in human prefrontal region. Cogn. Neurodyn. 17, 399–410. https://doi.org/10.1007/s11571-022-09834-x (2023).
Chan, D. et al. Gamma frequency sensory stimulation in mild probable Alzheimer’s dementia patients: Results of feasibility and pilot studies. PLoS ONE 17, e0278412. https://doi.org/10.1371/journal.pone.0278412 (2022).
Zhou, D. et al. Effects of 40 Hz transcranial alternating current stimulation (tACS) on cognitive functions of patients with Alzheimer’s disease: A randomised, double-blind, sham-controlled clinical trial. J. Neurol. Neurosurg. Psychiatry 93, 568–570. https://doi.org/10.1136/JNNP-2021-326885 (2022).
Benussi, A. et al. Increasing brain gamma activity improves episodic memory and restores cholinergic dysfunction in Alzheimer’s disease. Ann. Neurol. 92, 322–334. https://doi.org/10.1002/ANA.26411 (2022).
Zomorrodi, R., Loheswaran, G., Pushparaj, A. & Lim, L. Pulsed near infrared transcranial and intranasal photobiomodulation significantly modulates neural oscillations: A pilot exploratory study. Sci. Rep. https://doi.org/10.1038/s41598-019-42693-x (2019).
Chan, D. et al. Induction of specific brain oscillations may restore neural circuits and be used for the treatment of Alzheimer’s disease. J. Intern. Med. 290, 993–1009. https://doi.org/10.1111/joim.13329 (2021).
Chen, X. et al. Gamma oscillations and application of 40-Hz audiovisual stimulation to improve brain function. Brain and behavior 12, e2811. https://doi.org/10.1002/brb3.2811 (2022).
Suk, H.-J. et al. Sensory gamma frequency stimulation in cognitively healthy and AD individuals safely induces highly coordinated 40 hz neural oscillation: A preliminary study of non-invasive sensory stimulation for treating Alzheimer’s disease. Alzheimer’s Dement. 16, e041146. https://doi.org/10.1002/alz.041146 (2020).
Khachatryan, E. et al. Cognitive tasks propagate the neural entrainment in response to a visual 40 Hz stimulation in humans. Front. Aging Neurosci. https://doi.org/10.3389/fnagi.2022.1010765 (2022).
Duart, X. et al. Evaluating the effect of stimuli color and frequency on SSVEP. Sensors (Basel) 21, 1–19. https://doi.org/10.3390/s21010117 (2021).
Lee, K. et al. Optimal flickering light stimulation for entraining gamma waves in the human brain. Sci. Rep. 11, 1–10. https://doi.org/10.1038/s41598-021-95550-1 (2021).
Godinez Tello, R. J. M., Müller, S. M. T., Ferreira, A. & Bastos, T. F. Comparison of the influence of stimuli color on steady-state visual evoked potentials. Revista Brasileira de Engenharia Biomedica 31, 218–231, https://doi.org/10.1590/2446-4740.0739 (2015).
Chen, J., Valsecchi, M. & Gegenfurtner, K. R. Enhanced brain responses to color during smooth-pursuit eye movements. J. Neurophysiol. 118, 749–754. https://doi.org/10.1152/jn.00208.2017 (2017).
Nezamfar, H., Salehi, S. S. M. & Erdogmus, D. Stimuli with opponent colors and higher bit rate enable higher accuracy for C-VEP BCI. In 2015 IEEE Signal Processing in Medicine and Biology Symposium - Proceedings, https://doi.org/10.1109/SPMB.2015.7405476 (Institute of Electrical and Electronics Engineers Inc., 2016).
Cao, T. et al. Flashing color on the performance of SSVEP-based brain-computer interfaces. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS Vol. 2012, 1819–1822 (Annu Int Conf IEEE Eng Med Biol Soc, 2012). https://doi.org/10.1109/EMBC.2012.6346304.
Huang, Y. P. et al. Brain-display interactive system by using steady-state visual evoke potential(SSVEP) stimulation. In 2014 13th Workshop on Information Optics, WIO 2014 (Institute of Electrical and Electronics Engineers Inc., 2014). https://doi.org/10.1109/WIO.2014.6933275.
Duszyk, A. et al. Towards an optimization of stimulus parameters for brain-computer interfaces based on steady state visual evoked potentials. PLoS ONE https://doi.org/10.1371/journal.pone.0112099 (2014).
Lehman, B. & Wilkins, A. J. Designing to Mitigate Effects of Flicker in LED Lighting: Reducing risks to health and safety. IEEE Power Electron. Mag. 1, 18–26. https://doi.org/10.1109/MPEL.2014.2330442 (2014).
IEEE. Recommended Practices of Modulating Current in High Brightness LEDs for Mitigating Health Risks to Viewers (IEEE, 2008).
Simonson, E. & Brozek, J. Flicker fusion frequency; background and applications. Physiol. Rev. 32, 349–378. https://doi.org/10.1152/physrev.1952.32.3.349 (1952).
Kelly, D. H. Visual response to time-dependent stimuli. I. Amplitude sensitivity measurements. J. Opt. Soc. Am. 51, 422–429. https://doi.org/10.1364/josa.51.000422 (1961).
Curran, S. & Wattis, J. Critical flicker fusion threshold: A potentially useful measure for the early detection of Alzheimer’s disease. Hum. Psychopharmacol. 15, 103–112 (2000).
Kaiser, P. K., Ayama, M. & Vimal, R. L. P. Flicker photometry: Residual minimum flicker. J. Opt. Soc. Am. A 3, 1989. https://doi.org/10.1364/josaa.3.001989 (1986).
Bimler, D. Flicker between equal-luminance colors examined with multidimensional scaling. J. Opt. Soc. Am. A 27, 523. https://doi.org/10.1364/josaa.27.000523 (2010).
Truss, C. V. Chromatic flicker fusion frequency as a function of chromaticity difference. J. Opt. Soc. Am. 47, 1130–1134. https://doi.org/10.1364/JOSA.47.001130 (1957).
Hecht, S. & Verrijp, C. D. The influence of intensity, color and retinal ___location on the fusion frequency of intermittent illumination. Proc. Natl. Acad. Sci. 19, 522–535. https://doi.org/10.1073/pnas.19.5.522 (1933).
Troland, L. T. Notes on flicker photometry: Flicker-photometer frequency as a function of the color of the standard, and of the measured, light. J. Franklin Inst. 181, 853–855. https://doi.org/10.1016/S0016-0032(16)90161-3 (1916).
Chien, Y. Y. et al. Polychromatic SSVEP stimuli with subtle flickering adapted to brain-display interactions. J. Neural Eng. https://doi.org/10.1088/1741-2552/aa550d (2017).
Sakurada, T., Kawase, T., Komatsu, T. & Kansaku, K. Use of high-frequency visual stimuli above the critical flicker frequency in a SSVEP-based BMI. Clin. Neurophysiol. 126, 1972–1978. https://doi.org/10.1016/j.clinph.2014.12.010 (2015).
Jiang, Y., Zhou, K. & He, S. Human visual cortex responds to invisible chromatic flicker. Nat. Neurosci. 10, 657–662. https://doi.org/10.1038/nn1879 (2007).
Carstensen, M. S. et al. 40 Hz invisible spectral flicker and its potential use in Alzheimer’s light therapy treatment. In Hamblin, M. R., Carroll, J. D. & Arany, P. (eds.) Mechanisms of Photobiomodulation Therapy XV, vol. 11221, 20, https://doi.org/10.1117/12.2544338 (SPIE, 2020).
Agger, M. P. et al. Novel invisible spectral flicker induces 40 Hz neural entrainment with similar spatial distribution as 40 Hz stroboscopic light. J. Alzheimers Dis. 88, 335–344. https://doi.org/10.3233/JAD-220081 (2022).
Hajós, M. et al. Safety, Tolerability and efficacy of 40Hz sensory stimulation for Alzheimer’s disease. medRxiv 2023.03.23.23287637, https://doi.org/10.1101/2023.03.23.23287637 (2023).
Gramfort, A. et al. MEG and EEG data analysis with MNE-Python. Front. Neurosci. https://doi.org/10.3389/fnins.2013.00267 (2013).
Dominik Welk & Evgenii Kalenkovich. Frequency-tagging: Basic analysis of an SSVEP/vSSR dataset - MNE 1.0.0 documentation (2019).
Bach, M. & Meigen, T. Do’s and don’ts in Fourier analysis of steady-state potentials. Doc. Ophthalmol. 99, 69–82. https://doi.org/10.1023/A:1002648202420 (1999).
Taylor, J. E., Rousselet, G. A., Scheepers, C. & Sereno, S. C. Rating norms should be calculated from cumulative link mixed effects models. Behav. Res. Methods https://doi.org/10.3758/s13428-022-01814-7 (2022).
Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 82, 1–26, https://doi.org/10.18637/JSS.V082.I13 (2017).
Christensen, R. H. B. Regression models for ordinal data (2010).
Agger, M. et al. P28- alzlight pilot: Preliminary report on safety and feasibility from a randomized controlled trial of light-based brain stimulation with 40 HZ invisible spectral flickering light in patients with mild-tomoderate Alzheimer’s disease. J. Prevent. Alzheimer’s Dis. 9, 76–76 (2022).
Montés-Micó, R., Cerviño, A., Martínez-Albert, N., García-Marqués, J. V. & Marie, S. Performance of a new device for the clinical determination of light discomfort. Expert Rev. Med. Devices 17, 1221–1230. https://doi.org/10.1080/17434440.2020.1837623 (2020).
Clear, R. D. Discomfort glare: What do we actually know?. Light. Res. Technol. 45, 141–158. https://doi.org/10.1177/1477153512444527 (2013).
Keys, A. E. et al. Patient-reported medication adherence and tolerability: Results of a prospective observational study. J. Am. Coll. Clin. Pharm. 4, 271–279. https://doi.org/10.1002/jac5.1365 (2021).
Birks, J. Cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharm. Res.https://doi.org/10.1002/14651858.CD005593 (2006).
Perry, R. J. & Hodges, J. R. Attention and executive deficits in Alzheimer’s disease: A critical review. Brain 122, 383–404. https://doi.org/10.1093/BRAIN/122.3.383 (1999).
Gulbinaite, R., Roozendaal, D. & VanRullen, R. Attention effects on steady-state visual evoked potentials in response to 3–80 Hz flicker. J. Vis. 17, 977–977. https://doi.org/10.1167/17.10.977 (2017).
Acknowledgements
This work was partially supported with electroencephalography equipment and electrodes provided by the medical device company, OptoCeutics ApS, in addition to their equipment, the visual stimulation system (VSS) 1.0 to the following academic research project at DTU Fotonik: PhD Project in Photonics Technologies for Treatment & Diagnostics of Alzheimer’s and Dementia, Marcus H. Carstensen. The authors would like to express their gratitude to the department at DTU Compute for their technical support, to Kristoffer Hougaard Madsen, for his advice, to Manja Gersholm Grønberg for her comments concerning our statistical analyses, and to Maibritt Horning, Tjalfe Egholm Rude, Henrik Enggaard Hansen, and Kristoffer Hougaard Madsen for their assistance reviewing the manuscript prior to publication. Finally, the authors extend their thanks to the Innovation Fund Denmark (Case Number: 1044-00177A) for partially funding the PhD project of MAH.
Author information
Authors and Affiliations
Contributions
TA, MHC, LSH, MAH designed the experiments. LSH and MAH collected data. LSH, MAH, MHC analyzed the data. MHC, MTS determined the parameters of the light stimuli. NMN contributed to equipment sponsoring of the light stimuli and EEG. All authors contributed to manuscript preparation and review before submission. All authors discussed the results and implications and commented on the manuscript at all stages.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
The initiators of this project are TSA and PMP. The project has not received any funding. TSA declares that he has no conflict of interest. PMP owns shares in the company OptoCeutics ApS. MHC is CTO and owns shares in the company OptoCeutics ApS. LSH is a full-time employee of OptoCeutics ApS, but initiated the work while at DTU Photonics. MAH is an industrial Ph.D. student enrolled at DTU Compute and partially funded by OptoCeutics ApS and the Innovation Fund Denmark (Case number: 1044-00177A). Both LSH and MAH have stock options in the company OptoCeutics ApS. MTS had a student research position at OptoCeutics ApS, however, he conducted his work while being a Master’s student at DTU. NMN is CEO and owns shares in the company OptoCeutics ApS. JEB owns shares in the company OptoCeutics ApS.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hansen, L.S., Carstensen, M.H., Henney, M.A. et al. Light-based gamma entrainment with novel invisible spectral flicker stimuli. Sci Rep 14, 29747 (2024). https://doi.org/10.1038/s41598-024-75448-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-75448-4