Abstract
Although serum uric acid (SUA) is a risk factor for cardiometabolic outcome, but it remains unclear which patients with coronary artery disease (CAD) benefit the most from SUA lowering therapy (ULT). The association of SUA level, systemic inflammation and cardiometabolic risk is still unclear. The current study is aimed to examine whether SUA-associated cardiometabolic risk is modulated by systemic inflammation in CAD patients. A total of 16,598 CAD patients with baseline high-sensitivity C-Reactive Protein (hsCRP) and SUA available were included. Baseline and follow-up data were collected. The primary endpoint was major adverse cardiovascular and cerebrovascular events (MACCE), including death, myocardial infarction and stroke. In patients with hsCRP ≥ 2 mg/L, increasing quintiles of SUA were significantly associated with increased rates of 2-year MACCE (adjusted p < 0.001 for trend, p = 0.037 for interaction). Each unit increase in SUA levels was associated with a 11.3% increased risk of MACCE (adjusted p < 0.001, p = 0.002 for interaction). However, in patients with hsCRP < 2 mg/L, increasing quintiles of SUA were not associated with increased MACCE (adjusted p = 0.120). Elevated SUA levels are related to MACCE when hsCRP levels are 2 mg/L or more but not less than 2 mg/L. This finding suggests a potential benefit of combined ULT and anti-inflammation therapy in patients with hyperuricemia and greater systemic inflammation.
Similar content being viewed by others
Introduction
Coronary artery disease (CAD) still expands threats to global health and remains one of the leading cause of morbidity and mortality worldwide1. Despite standard secondary prevention and guideline recommended therapy, CAD patients are still confronted with risk of recurrent adverse cardiovascular and cerebrovascular events. Although intensive management of traditional risk factors such as LDL-C, hypertension, diabetes mellitus and smoking have made progress on reducing ischemic events, novel risk factors remains to be recognized to further improve clinical prognosis.
As the prevalence of hyperuricemia has been increasing worldwide2,3, several studies have reported that hyperuricemia was associated with increased CAD risk and adverse cardiometabolic outcomes in CAD patients4,5,6,7,8. Serum uric acid (SUA) level is used to diagnose hyperuricemia and to monitor and guide urate-lowering therapy (ULT). The pathogenesis of increased SUA level and atherosclerosis was not fully interpretated. High-sensitivity C-reactive protein (hsCRP) is a widely used biomarker for systematic inflammation and a novel anti-inflammation therapy target for atherosclerotic disease9,10. Interestingly, previous study reported that inflammation played important roles in various signal pathways for SUA activated atherosclerosis11 and uric acid induced hs-CRP express in vascular cell proliferation12. However, whether hs-CRP could modulate SUA-associated cardiometabolic risk was still unclear. Accordingly, we tested the hypothesis that SUA-associated adverse cardiovascular and cerebrovascular outcome would be significantly modulated by systemic inflammation in a large, multicenter, contemporary cohort of CAD patients.
Method
Study population
We conducted a post-hoc analysis of data from the PRospective Observational Multicenter cohort for Ischemic and hEmorrhage risk in coronary artery disease patients (the PROMISE cohort). Briefly, the PROMISE cohort was a multicenter prospective cohort, enrolled 18,701 patients with coronary artery disease at 9 centers in China from January, 2015 to March, 2019. Inclusion criteria included patients of at least 18 years old, diagnosis of CAD, indication for at least one antiplatelet drug. Exclusion criteria were a life expectancy of fewer than 6 months and current participation in another interventional clinical trial. The selection of a treatment strategy was dependent on clinician and patient preference in accordance with contemporaneous guidelines13. Coronary angiography and intervention were performed by experienced coronary interventional cardiologists. The decision for percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) was made by a heart team composed of experienced cardiologists and surgeons. The definition of chronic kidney disease is based on persistent reduction of renal functional component(a reduction of estimated glomerular filtration rate, the cutoff value is eGFR < = 60 mL/min/1.73m2) for at least 3 months. Patients with acute kidney injury weren’t included in this population.
Biomarker assessment
Blood samples were taken by direct vein puncture with 24 h after admission. The samples were collected into general vacuum tube without anticoagulation. SUA was measured by Uricase Colorimetric Assay and the upper reference limit (URL) was 1.33 mg/dl (to convert millimoles per liter to milligram per deciliter, divided by 59.5). And hs-CPR was measured by immunoturbidimetry and the URL was 3 mg/L. According to several important clinical trials such as CANTOS, PROVE-IT trial and et al.9,10, the definition used for high residual inflammatory risk was hs-CRP > = 2 mg/dl, which stood for increased adverse clinical outcomes. In this study, we also chose hs-CRP > = 2 mg/dl as the cut-off value for high residual inflammatory risk.
Clinical end points
All patients were evaluated by clinic visit or by phone at 1, 6, 12 and 24 months. The ischemic and bleeding endpoints were recorded at follow up. The primary endpoint was major adverse cardiovascular and cerebrovascular events (MACCE), a composite of death, myocardial infarction (MI) and stroke. The secondary endpoint were death, MI and stroke. MI was diagnosed in accordance with the contemporaneous Universal Definition of Myocardial Infarction14,15. Strokes included ischemic and hemorrhagic strokes according to the World Health Organization classification of diseases16. Investigator training, blinded questionnaire filling, and telephone recording were performed to obtain high-quality data. All endpoints were adjudicated centrally by 2 independent cardiologists, and disagreement was resolved by consensus.
Statistical analysis
Continuous variables are reported as mean and SD values for normally distributed variables or median and interquartile range values for non-normally distributed variables. Categorical variables are reported as frequency and percentage. Multivariable Cox stepwise hazards regression modeling was used to assess the relationship between MACCE and SUA, including interactions, while adjusting for important covariates. Hazard ratios and 95% CIs are reported. Tests of trend were performed across the SUA quintiles. Kaplan-Meier curves illustrate the incidence of MACCE during follow up. The number at risk at each 200 days increment is given under each plot. Missing values were imputed using the median for continuous variables or the mode for categorical variables. All P values were from 2-tailed tests and results were deemed statistically significant at P < 0.05. Analysis was performed using SPSS, version 24.0 (IBM Corp., Armonk, NY, USA). Figures were created using GraphPad Prism, version9.0.0(86) (GraphPad Software, LCC).
Results
Patients and baseline characteristics
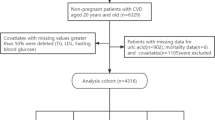
The present study included patients with both SUA and hsCRP available within 24 h after admission. As such, 16,598 of the enrolled 18,701 patients in PROMISED cohort met the criteria and were included in the present analysis (Fig. 1).
Table 1 describes the baseline clinical and biomarker characteristics of the current population stratified according to achieved hsCRP levels (< 2 mg/L vs. ≥2 mg/L). The average age of the present population was 60 ± 10 years old and most patients were male (12410[67.5%]), 5389(32.5%) patients had diabetes mellitus, 10,857(65.4%) and 12,675(76.4%) had hypertension and hyperlipidemia respectively. 15,903(95.8%) patients received aspirin, 15,203(91.6%) received P2Y12 receptor antagonist, 15,763(95.0%) received statins. The average levels of SUA was 5.79 ± 1.65 mg/dl and hsCRP was 3.87 ± 5.61 mg/L. Figures S1 and S2 illustrate the distribution of hsCRP and SUA levels in patients using histograms. Table S1 shows the baseline characteristics of the population according to SUA quintiles. Figure S3 depicts the correlation between these two variables with a scatter plot (Correlation Coefficient = 0.052, Spearman p<0.001). As shown in Table S2 and S3, when baseline characteristics were stratified by hsCRP levels, patients with hsCRP ≥ 2 mg/L had higher body mass index, low-density lipoprotein cholesterol, Lipoprotein(a) and baseline Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX) score. Patients with hsCRP ≥ 2 mg/L had more comorbidity of hypertension, chronic kidney disease, prior stroke, smoking history, heart failure of reduced ejection fraction (HFrEF) and acute myocardial infarction.
Clinical outcomes
A total of 16,164(97.4%) patients completed 2-year follow up. During 2-year follow up, 459(2.8%) patients had death, 325(2.0%) had cardiac death, 235(1.4%) had myocardial infarction, 382(2.3%) had stroke and 1795(10.8%) had MACCE. Table 2 shows the 2-year cardiovascular and cerebrovascular events according to sCRP levels. Patients with hsCRP levels ≥ 2 mg/L experienced higher risk of 2-year death (4.3% vs. 1.4%, p < 0.001), cardiac death (3.1% vs. 0.9%, p < 0.001), myocardial infarction (1.9% vs. 1.0%, p < 0.001), stroke (2.7% vs. 2.0%, p = 0.004), and MACCE (7.5% vs. 3.9%, p < 0.001) than patients with hsCRP < 2 mg/L.
Figure 2 shows the incidence of first MACCE stratified according to SUA quintiles in the setting of hsCRP < 2 mg/L (Fig. 2a) or ≥ 2 mg/L (Fig. 2b) over 2-year follow up. Only in those with hsCRP ≥ 2 mg/L, increasing quintiles of SUA levels were associated with a higher incidence of 2 years MACCE (P < 0.001 for trend). However, in those with hsCRP levels < 2 mg/L, increasing quintiles of SUA levels were not associated with a higher incidence of MACCE (P = 0.403 for trend). The incidence of MACCE according to SUA quintiles in the whole population and different hsCRP levels are shown in Figure S4 and S5.
Table 3 shows univariable and multivariable adjusted relationships between MACCE stratified according to hsCRP and SUA levels. In the overall population, compared to hsCRP level < 2 mg/L, higher hsCRP level (≥ 2 mg/L) was significantly associated with higher risk of MACCE (HR = 1.45, 95%CI: 1.25–1.68, p < 0.001). And the continuous hsCRP levels were also associated with MACCE (HR = 1.02, 95%CI: 1.01–1.02, p < 0.001). In the overall population, SUA levels were associated with MACCE (HR = 1.06, 95%CI: 1.04–1.06 p < 0.001). However, after being stratified by hsCRP of 2 mg/L, SUA levels were significantly associated with MACCE only when hsCRP level ≥ 2 mg/L and each unit increase of SUA levels was associated with 11.3% increased risk of MACCE (HR = 1.13, 95%CI: 1.08–1.18, p < 0.001). In patient with hsCRP level < 2 mg/L, SUA levels were not significantly associated with higher MACCE (HR = 1.04, 95%CI: 0.99–1.08, p = 0.120). There was a significant interaction for MACCE between hsCRP dichotomy and SUA levels (P = 0.002 for interaction).
Figure 3 shows the restricted cubic splines to flexibly model and visualize the adjusted relation of predicted SUA level with MACCE. In the whole population, the risk of MACCE was linearly increased with SUA levels and after reaching SUA level of 5.63 mg/dl, MACCE risk was significantly higher with increased SUA levels (p < 0.001) (Fig. 3A). In patients with hsCRP level < 2 mg/L, the curve was relatively flat when SUA levels were below 5.58 mg/dl and there was no significant association between MACCE risk and SUA levels (p = 0.12) (Fig. 3B). However, in population with hsCRP level ≥ 2 mg/L, the plot showed a sharp increase in the risk of MACCE, especially within the higher range of predicted SUA level (above 5.68 mg/dl). The increased MACCE risk became significantly related to predicted SUA levels. (p < 0.001) (Fig. 3C).
Association of adjusted SUA levels with MACCE in the whole population (3A), hsCRP < 2 mg/dl (3B) and hsCRP ≥ 2 mg/dl (3C). We used continuous SUA levels to analysis the association with MACCE risk in adjusted models. The multivariable model adjusted for sex, age, acute myocardial infarction, previous myocardial infarction, hypertension, heart failure with reduced ejection fraction, smoking, chronic kidney disease, percutaneous coronary intervention. The lines represent adjusted hazard ratios (solid lines) and 95% confidence intervals (shaded areas). The dashed vertical lines in 3 A, 3B and 3 C were separately stood for SUA levels of 5.63 mg/dl, 5.58md/dl and 5.68 mg/dl. SUA, serum uric acid; hsCRP, high-sensitivity C-reactive protein; MACCE, major adverse cardiovascular and cerebrovascular disease.
Table 4 describes the associations between hsCRP and SUA quintiles. After fully adjusted multivariable analysis, hsCRP ≥ 2 mg/L subgroup, SUA quintiles were associated with higher MACCE(p for trend < 0.001), and high SUA quintiles(Q3 [6.05-6.98 mg/dl]and Q4[6.99-61.65 mg/dl]) were significantly associated with a greater risk of MACCE. However, when hsCRP < 2 mg/L, high SUA quintiles were not associated with higher risk of MACCE (p for trend = 0.278). There was a significant interaction for MACCE between SUA quintiles and hsCRP dichotomy (P = 0.037 for interaction).
Subgroup analysis
Further Subgroup analysis were conducted between groups of age, sex, whether PCI was performed, heart failure with reduced ejection fraction, acute myocardial infarction, diabetes mellitus, chronic kidney disease and baseline LDL-C levels. After fully adjusted multivariable analysis, except for HFrEF subgroup with hsCRP ≥ 2 mg/L, the association of SUA quintiles and 2 years MACCE were consistent across subgroups (p for interaction > 0.05). In the setting of hsCRP ≥ 2 mg/L, higher SUA levels were similarly associated with higher risk of MACCE in patients underwent PCI, with acute myocardial infarction, diabetes mellitus and baseline LDL-C less than 1.4mmol/L (Fig. 4).
Subgroup analysis of major adverse cardiovascular and cerebrovascular events according to high-sensitivity C-reactive protein (hsCRP) and serum uric acid (SUA) levels in a Fully Adjusted Multivariable Model. *Multivariable model adjusted for sex, age, acute myocardial infarction, previous myocardial infarction, hypertension, heart failure with reduced ejection fraction, smoking, chronic kidney disease, percutaneous coronary intervention.
Discussion
To our knowledge, we demonstrated for the first time that SUA-associated cardiometabolic risk might be mediated by hsCRP levels, a marker for systemic inflammation, in a large multicenter cohort of CAD patients. The major findings of the current study are as follows: (1) After fully adjusted multivariable analysis, positive correlation of SUA levels and increased MACCE risk was only found in patients with greater degrees of systemic inflammation (hsCRP ≥ 2 mg/L); (2) Each unit increase of SUA levels was associated with 11.3% increased risk of MACCE only when hsCRP level ≥ 2 mg/L; (3) The association of SUA levels and 2 years MACCE were consistent across subgroups including age, sex, whether PCI was performed, acute myocardial infarction, diabetes mellitus, chronic kidney disease and baseline LDL-C levels less than 1.4mmol/L or not. These findings might elucidate the interdependence effect of the two known mediators on ASCVD risk: SUA and systemic inflammation.
Uric acid is the end-product of purine metabolism, which is mainly regulated by xanthine oxidoreductase (XOR), converting hypoxanthine to xanthine and finally to uric acid17. Hyperuricemia is closely associated with gout, chronic kidney disease and hypertension2,18, which are closely related to atherosclerosis. Recently, accumulating evidence demonstrated that uric acid may play significant roles in pathogenesis of cardiovascular disease and cardiovascular death4,5. The underline pathogenesis of uric acid related cardiovascular risk is complicated, however oxidative stress and inflammation induced by uric acid are the main mechanism11. It is known that uric acid itself is chemically an important antioxidant in the extracelluar space. However, in the intracellular space, uric acid acts as a pro-oxidant agent, which induces endothelial dysfunction by intracellular oxidative stress and leads inflammation in vascular endothelial and smooth muscle cells19,20.
Inflammation has already been known to play an important role in the pathophysiology of atherogenesis and ischemic events21. Sufficient experimental and clinical evidence implicates that inflammation provide a series of pathways that link traditional risk factors, such as lipid and hypertension, to atherosclerosis22,23. The biomarker hsCRP, which stands for systematic inflammation, is validated usefully for predicting both atherosclerotic and ischemic risk24,25. Recent clinical trials have showed that therapy targeted on inflammation can reduce cardiovascular events in patients already received guideline recommended standard therapy. The “Canakinumab Anti-inflammatory Thrombosis Outcomes Study” (CANTOS) enrolled 10,061 patients with previous myocardial infarction and a hsCRP level of 2 mg/L or more despite of standard medical therapy. The participants were allocated randomly to receive three doses of canakinumab, an antibody that neutralizes the proinflammatory cytokine IL-1β. In patients who received 300-mg canakinumab every 3 months, the median level of hsCRP was reduced 41% greater than that with placebo, and the rate of recurrent cardiovascular events were significantly lowered beyond the effect of lipid lowering9. The results of the CANTOS trial affirmed that residual inflammation risk assessed by hsCRP levels was associated with future recurrent cardiovascular risk.
Previous study of in vitro human vascular cells had demonstrated that through stimulating expression of CRP, UA altered proliferation/migration of smooth muscle cell and nitric oxide release of endothelial cells19 in the pathogenesis of UA-associated atherosclerosis. In this study, we reported in patients with hsCRP level ≥ 2 mg/L, each unit increase of SUA levels was associated with 11.3% increased risk of MACCE. This finding provided clinical evidence that greater degree of systemic inflammation was associated with adverse SUA-induced cardiovascular risk. However, we also found a large portion of patients with high SUA levels but low systemic inflammation, in whom SUA levels were not associated with adverse prognosis. This finding implied that although SUA mediated CRP expression of vascular cells, more complex underlying mechanism of systemic inflammation in vivo might play important role in SUA-induced cardiovascular risk.
In general, SUA levels are higher in male than female for estrogen protection26. However, whether SUA-associated cardiovascular risk is different between female and male is still controversial. Sun et al.27 reported SUA levels were significantly associated with coronary atherosclerosis evaluated by 256-detector-row coronary computed tomographic angiography in female but not male. But Ando et al.28 found there was no difference in SUA-associated lipid-rich plaques by intravascular ultrasound between genders. In the current study, our subgroup analysis supported the consistency of SUA-associated cardiovascular risk in female and male. This finding suggested that when it comes to SUA related cardiovascular risk, systemic inflammation but not gender was the important factor that we should pay more attention to.
The improvement of cardiovascular outcomes of urate-lowering therapy (ULT) is still controversial. Allopurinol and febuxostat are both clinical commonly used ULT drugs reducing UA by inhibition of xanthine oxidase. Singh et al.29 reported that longer allopurinol use duration (at least 181days) was associated with reduced myocardial infarction in 29,298 elderly patients. Although with a relatively large sample size, the observational design was its major limitation. Subsequent small sample size randomized trials demonstrated benefit of ULT on surrogate endpoints such as blood pressure, endothelial function and carotid intima-media30,31,32. A recent systematic review including 6,458 participants from 28 trials with 506 major adverse cardiovascular events reported that ULT did not produce benefits on clinical outcomes including major adverse cardiovascular events33. Thus, lowering SUA alone to improve cardiovascular outcomes may not be effective. Our study reveals that SUA-associated cardiovascular risk appears to be significantly mediated by systemic inflammation, suggesting a potential benefit of combined ULT and anti-inflammation therapy in patients with greater systemic inflammation.
Limitation
There are a number of limitations in the current study. First, the current analysis of the study was based on data from a large multicenter cohort. The observational design may preclude any definitive conclusion. Second, only baseline SUA and hsCRP levels were recorded in the current study and the conclusion focused on in-hospital SUA levels and inflammation status. With no follow-up results on SUA and hsCRP levels available, the effect of systemic inflammation and SUA trajectory related cardiovascular risk were still unclear. Thirdly, the data of proportion of urate-lowering therapy were not collected in our cohort, which might have effect on the follow-up SUA levels and cardiovascular risk. Fourthly, two years follow-up duration was comparatively short for evaluating long-term outcomes. Finally, our study categorized participants into SUA quartiles and did not produce a certain cut-off value, which could hamper its clinical applicability.
Conclusion
The present study demonstrates in a large multicenter prospective cohort that SUA-associated MACCE risk appears to be mediated by concomitant levels of systemic inflammation. This finding suggests a potential benefit of combined ULT and anti-inflammation therapy in patients with hyperuricemia and greater systemic inflammation.
Data availability
The datasets generated or analysed during the current study are not publicly available due to the participants did not agree for their data to be shared publicly but are available from the corresponding author on reasonable request.
References
G.B.D.C.o.D. Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the global burden of Disease Study 2017. Lancet 392(10159), 1736–1788 (2018).
Singh, G., Lingala, B. & Mithal, A. Gout and hyperuricaemia in the USA: Prevalence and trends. Rheumatology 58(12), 2177–2180 (2019).
Chen-Xu, M. et al. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: The National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 71(6), 991–999 (2019).
Bos, M. J. et al. Uric acid is a risk factor for myocardial infarction and stroke: The Rotterdam study. Stroke 37(6), 1503–1507 (2006).
Krishnan, E. et al. Hyperuricemia and the risk for subclinical coronary atherosclerosis–data from a prospective observational cohort study. Arthritis Res. Ther. 13(2), R66 (2011).
Tscharre, M. et al. Uric acid is associated with long-term adverse cardiovascular outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Atherosclerosis 270, 173–179 (2018).
Morikawa, N. et al. Serum urate trajectory in young adulthood and incident cardiovascular disease events by middle age: CARDIA study. Hypertension 78(5), 1211–1218 (2021).
Virdis, A. et al. Identification of the uric acid thresholds predicting an increased total and cardiovascular mortality over 20 years. Hypertension 75(2), 302–308 (2020).
Ridker, P. M. et al. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl. J. Med. 377(12), 1119–1131 (2017).
Hoogeveen, R. C. & Ballantyne, C. M. Residual cardiovascular risk at low LDL: Remnants, Lipoprotein(a), and inflammation. Clin. Chem. 67(1), 143–153 (2021).
Kimura, Y., Tsukui, D. & Kono, H. Uric acid in inflammation and the pathogenesis of atherosclerosis. Int. J. Mol. Sci. 22(22), 12394 (2021).
Kang, D. H. Uric acid-induced C-reactive protein expression: Implication on cell proliferation and nitric oxide production of human vascular cells. J. Am. Soc. Nephrol. 16(12), 3553–3562 (2005).
Smith, S. C. Jr. et al. ACC/AHA guidelines of percutaneous coronary interventions (revision of the 1993 PTCA guidelines)—Executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee to revise the 1993 guidelines for percutaneous transluminal coronary angioplasty). J. Am. Coll. Cardiol. 37(8), 2215–2239 (2001).
Thygesen, K. et al. Third universal definition of myocardial infarction. Eur. Heart J. 33(20), 2551–2567 (2012).
Thygesen, K. et al. Fourth universal definition of myocardial infarction (2018). J. Am. Coll. Cardiol. 72(18), 2231–2264 (2018).
Sartorius, N. et al. Progress toward achieving a common language in psychiatry. Results from the field trial of the clinical guidelines accompanying the WHO classification of mental and behavioral disorders in ICD-10. Arch. Gen. Psychiatry 50(2), 115–124 (1993).
Saito, Y. et al. Uric acid and cardiovascular disease: A clinical review. J. Cardiol. 78(1), 51–57 (2021).
Ohno, I. Relationship between hyperuricemia and chronic kidney disease. Nucleosides Nucleotides Nucleic Acids 30(12), 1039–1044 (2011).
Kang, D. H. et al. Uric acid-induced C-reactive protein expression: Implication on cell proliferation and nitric oxide production of human vascular cells. J. Am. Soc. Nephrol. 16(12), 3553–3562 (2005).
Sharaf, E., Din, U. A. A., Salem, M. M. & Abdulazim, D. O. Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: A review. J. Adv. Res. 8(5), 537–548 (2017).
Libby, P. & Hansson, G. K. From focal lipid storage to systemic inflammation: JACC Review topic of the Week. J. Am. Coll. Cardiol. 74(12), 1594–1607 (2019).
Xiao, L. & Harrison, D. G. Inflammation in hypertension. Can. J. Cardiol. 36(5), 635–647 (2020).
Puri, R. et al. Effect of C-Reactive protein on lipoprotein(a)-associated cardiovascular risk in optimally treated patients with high-risk vascular disease: A prespecified secondary analysis of the ACCELERATE trial. JAMA Cardiol. 5(10), 1136–1143 (2020).
Ridker, P. M. A test in context: High-sensitivity C-Reactive protein. J. Am. Coll. Cardiol. 67(6), 712–723 (2016).
Liu, Y. et al. Relationship between high-sensitivity C-Reactive protein and long-term outcomes in Elderly patients with 3-Vessel disease. Angiology 73(1), 60–67 (2022).
Adamopoulos, D. et al. The relationship of sex steroids to uric acid levels in plasma and urine. Acta Endocrinol. (Copenh) 85(1), 198–208 (1977).
Sun, Y. et al. A cross-sectional analysis of the relationship between uric acid and coronary atherosclerosis in patients with suspected coronary artery disease in China. BMC Cardiovasc. Disord 14, 101 (2014).
Ando, K. et al. Impact of serum uric acid levels on coronary plaque stability evaluated using integrated backscatter intravascular ultrasound in patients with coronary artery disease. J. Atheroscler Thromb. 23(8), 932–939 (2016).
Singh, J. A. & Yu, S. Allopurinol reduces the risk of myocardial infarction (MI) in the elderly: A study of Medicare claims. Arthritis Res. Ther. 18(1), 209 (2016).
Soletsky, B. & Feig, D. I. Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension 60(5), 1148–1156 (2012).
Higgins, P. et al. Allopurinol reduces brachial and central blood pressure, and carotid intima-media thickness progression after ischaemic stroke and transient ischaemic attack: A randomised controlled trial. Heart 100(14), 1085–1092 (2014).
George, J. et al. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation 114(23), 2508–2516 (2006).
Chen, Q. et al. Effect of urate-lowering therapy on cardiovascular and kidney outcomes: A systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 15(11), 1576–1586 (2020).
Acknowledgements
We thank all staff members for data collection, data entry, and monitoring as part of this study.
Funding
This work was supported by the CAMS Innovation Fund for Medical Sciences (2020-I2M-C&T-B-049); CAMS Innovation Fund for Medical Sciences (2023-I2M-C&T-B-061); the National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, Chinese Academy of Medical Sciences (NCRC2022003); the National High Level Hospital Clinical Research Funding (2024-GSP-TJ-8).
Author information
Authors and Affiliations
Contributions
Jinqing Yuan, Yaling Han, Xueyan Zhao, Jue Chen, Runlin Gao, Lei Song Participated in the study design. Ying Song, Weiting Cai, Lin Jiang, Jingjing Xu, Yi Yao, Na Xu, Xiaozeng Wang, Zhenyu Liu, Zheng Zhang, Yongzhen Zhang, Xiaogang Guo, Zhifang Wang, Yingqing Feng, Qingsheng Wang, Jianxin Li Participated in data analysis. Ying Song Wrote the manuscript. All authors participated in data interpretation and critical revision of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The Institutional Review Board of Fuwai Hospital approved the study protocol, and the IRB number of this study is IRB2012-BG-006. The entire research was conducted in accordance with relevant regulations, and informed consent was obtained from all participants. The research was performed in accordance with the Declaration of Helsinki.
Consent for publication
Written informed consent for publication was obtained from all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Song, Y., Cai, W., Jiang, L. et al. Effect of high sensitivity C-reactive protein on uric acid-related cardiometabolic risk in patients with coronary artery disease—a large multicenter prospective study. Sci Rep 14, 29350 (2024). https://doi.org/10.1038/s41598-024-75508-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-75508-9