Abstract
Neurogenic lower urinary tract dysfunction (NLUTD) is a frequent consequence of spinal cord injury (SCI), leading to symptoms that significantly impact quality of life. Although many life-saving techniques are available, current treatment strategies for managing NLUTD still exhibit limitations and drawbacks. Here, we introduce a new electrical neuromodulation strategy involving electrical stimulation of the major pelvic ganglion (MPG) to initiate bladder contraction, in conjunction with innovative programmable (IPG) electrical stimulation on the pudendal nerve (PN) to induce external urethral sphincter (EUS) relaxation in freely moving or anesthetized SCI mice. Furthermore, we conducted the void spot assay, and cystometry coupled with EUS electromyography (EMG) recordings to evaluate voiding function, and monitor bladder pressure and EUS activity. Our findings demonstrate that our novel electrical neuromodulation approach effectively triggers coordinated bladder muscle contraction and EUS relaxation, effectively counteracting SCI-induced NLUTD. Additionally, this electrical neuromodulation method enhances voiding efficiency, closely resembling natural reflexive urination in SCI mice. Thus, our study offers a promising electrical neurostimulation approach aimed at restoring physiological coordination and potentially offering personalized treatment for improving voiding efficiency in individuals with SCI-associated NLUTD.
Similar content being viewed by others
Introduction
The normal functions of the lower urinary tract include the storage and periodic elimination of urine. During storage, the bladder muscle relaxes while the urethral sphincter contracts. Conversely, during voiding, the bladder muscle contracts and the urethral sphincter relaxes. The regulation of these functions relies on a spino-bulbospinal reflex mediated by neurons in the pontine micturition center (PMC) of the brainstem1. The PMC sends direct projections to the lumbosacral spinal cord to coordinate autonomic and somatic efferent pathways2. However, spinal cord injury (SCI) disrupts normal bladder function by hindering the transmission of afferent information to the periaqueductal gray (PAG) and PMC, as well as efferent commands to the lower spinal centers that regulate bladder motor neurons and the external urethral sphincter (EUS). Most patients with SCI progressively develop neurogenic lower urinary tract dysfunction (NLUTD), which not only poses a significant risk for renal function deterioration, but also impairs health-related quality of life such as urinary incontinence. Left untreated or unrecognized, NLUTD may exacerbate over time, leading to serious morbidities, such as hydronephrosis, urinary tract infections, vesicoureteral reflux, bladder damage, calculus formation, and renal failure due to elevated residual urine in the bladder and increased intravesical pressures3.
Traditional treatment strategies, such as intermittent catheterization, pharmacological approaches, and surgical interventions, have been employed to manage NLUTD4,5,6,7. Although these treatments achieve storage and evacuation of urine without upper urinary tract damage, they fail to address the fundamental loss of control associated with SCI and are not without drawbacks. Electrical neuromodulation is a reversible and efficient therapeutic method. Over the past few decades, various electrical neurostimulation methods and devices have emerged as promising alternatives to address NLUTD in patients with SCI, aiming to restore control over urination8. Pelvic nerve stimulation targeting the preganglionic parasympathetic fibers innervating the bladder muscle has been found to induce bladder contractions8,9. However, reflexive co-activation of sphincter contraction triggered by pelvic nerve stimulation often impedes proper micturition10. Sacral anterior root stimulation (SARS) combined with posterior rhizotomy has shown positive effects on bladder control and efficient voiding in patients with SCI, despite the drawbacks of a complex surgical procedure and irreversible posterior rhizotomy8,11,12. Subsequent studies have explored the blockage of EUS contractions via high-frequency electrical stimulation of the pudendal nerve (PN), acknowledging that this method is confined to enhancing micturition efficiency by blocking the afferent pudendal nerve fibers and may not be suitable for all patients. Overall, current treatment options for NLUTD are limited3,13, necessitating the development of novel therapeutic interventions to restore urinary voiding and regain control over urination in individuals affected by NLUTD.
In this study, we introduced a novel electrical neuromodulation strategy involving stimulation of the major pelvic ganglion (MPG) in conjunction with PN stimulation for the treatment of NLUTD in SCI mice. We applied this electrical neuromodulation approach in freely moving or anesthetized SCI mice, and conducted a void spot assay, and cystometry combined with EUS electromyography (EMG) recordings to evaluate urination patterns and monitor bladder pressure and EUS activity. Our findings demonstrate that this new electrical neuromodulation strategy effectively induced coordinated bladder muscle contraction and EUS relaxation, thereby overcoming SCI-induced NLUTD and successfully mimicking the natural reflexive urination process. In summary, our study introduces a novel and effective neuromodulation therapy to restore urination control.
Results
Deep analysis of the functional patterns obtained by cystometry and EUS electromyography
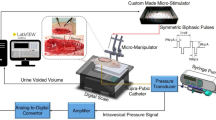
Unlike previous studies investigating treatments for NLUTD, such as complete sacral posterior root rhizotomy and pudendal nerve block14,15,16, we propose a novel intervention that uses dual-site electrical stimulation to mimic the coordinated activity of the bladder muscle and EUS during normal voiding, with the goal of restoring coordination between the bladder and urethral sphincter. To capture the functional patterns of bladder muscle contraction and EUS relaxation during normal reflexive urination, we examined the intravesical pressure (IVP) and EMG data obtained through cystometry combined with EUS-EMG recording in urethane-anesthetized mice (Fig. 1A). Each reflexive voiding event corresponded to an IVP change (bladder pressure increase followed by decrease) accompanied by EUS-EMG bursting activity (Fig. 1A, n = 12 mice). Notably, we observed that the onset of bladder contraction (threshold pressure) preceded the initiation of EUS-EMG bursting activity (latency: 0.8 ± 0.11 s; Supplementary Fig. 1a). The difference (∆P) between threshold pressure and end pressure was approximately 3 cmH2O (∆P: 3.11 ± 0.55 cmH2O; Fig. 1A, Supplementary Fig. 1b).
Characteristics of IVP and EUS-EMG activity during reflexive urination. (A) Representative bladder pressure trace (top); and time-locked EUS-EMG signal (bottom). The latency refers to the time between the sudden rise in bladder pressure (threshold pressure) and the onset of EUS-EMG activity. ∆P represents the pressure difference between the threshold pressure (the first circular black dot) and the end pressure (the second circular black dot). (B) Total duration of representative EMG data including the bursting phase (EUS bursting activity duration, T1) and the tonic phase (tonic activity duration, T2). The bursting (phasic) pattern of activity (T1) produces the pulsatile release of urine. An enlarged representation of the T1 period (bottom), including the bursting EUS contraction period (active period, AP) and the EUS relaxation period (silent period, SP). (C) Quantification of the duration of T1 and T2. n = 12 mice. (D) Quantification of the maximum amplitude of EMG signals. n = 12 mice. (E) Quantification of the duration of AP and SP. Student’s t-test (two-tailed, paired). ***p < 0.001 n = 12 mice. (F) Quantification of the total number of active phases (APs) in each EMG data during the EUS bursting phase. n = 12 mice.
Subsequently, we conducted a comprehensive analysis of the temporal and spectral features of EUS-EMG data (Fig. 1B–F). During a voiding event, the total duration of EUS-EMG activity was 5.55 ± 0.28 s, comprising EUS bursting activity duration (T1, 4.51 ± 0.24 s) and tonic activity duration (T2, 1.04 ± 0.13 s; Fig. 1B,C). EUS-EMG bursting activity consisted of a bursting EUS contraction period (active period, AP) and an EUS relaxation period (silent period, SP) (Fig. 1B). The bursting activity had a maximum spike amplitude of 0.94 ± 0.06 mV, with a total of 21 ± 1 APs observed (Fig. 1D,E). Each AP lasted for 32.64 ± 1.94 ms, while each SP lasted for 184.40 ± 10.97 ms (Fig. 1F). Spectral analysis of the EUS-EMG data revealed frequencies ranging from 0 to 4 kHz during the bursting duration, whereas frequencies were within the 0–1.5 kHz range during the tonic duration (Supplementary Fig. 1c). These findings suggest distinct activity patterns in the bladder and of the EUS during normal reflexive urination.
Novel pudendal nerve stimulation elicits urethral sphincter relaxation
EUS receives dense innervation from sacral motor neurons located in Onuf’s nucleus of the spinal cord, with their axons traversing through PN17. This innervation was confirmed by injecting CTB into the urethral sphincter (Supplementary Fig. 2a,b). To assess the feasibility of generating artificial phasic bursting activity in EUS through electrical stimulation of the PN, we unilaterally stimulated the PN using various electrical stimulation parameters (Tables 1; 2), while simultaneously monitoring changes in bladder pressure via cystometry and EUS-EMG signals in urethane-anesthetized mice (Supplementary Fig. 2c). Stimulation frequencies of 25 Hz, 80 Hz, and 800 Hz, each with a stimulation peak voltage of 1 V for 5.3 s (Tables 1; 2), failed to evoke EUS-EMG discharges characteristic of normal reflexive urination or induce IVP changes during bladder filling (Fig. 2A,B, n = 9 mice). In contrast, our innovative programmable (IPG) electrical stimulation, combining 800 Hz and 80 Hz with a stimulation peak voltage of 1 V for 5.3 s (Table 1), successfully induced EUS-EMG bursting activity, synchronous reduction in IVP, and fluid elimination via the urethra (Fig. 2C,D). The success rate of IPG electrical stimulation in eliciting EMG bursting activity and IVP reduction, resulting in urination, was 100% (Fig. 2E).
Innovative programmable (IPG) electrical stimulation of pudendal nerve induces EUS relaxation. (A) Representative bladder pressure traces (top) and time-locked EUS-EMG signals (bottom) for stimulation frequencies of 800 Hz, 80 Hz, and 25 Hz. (B) Enlarged view of the dashed line boxes in panel (A). (C) Representative bladder pressure traces (top) and time-locked EUS-EMG signals (bottom) recorded during IPG stimulation. (D) Enlarged view of the dashed line boxes in panel (C). (E) Success rate of PN stimulation eliciting EMG bursting activity and IVP reduction under different stimulation frequencies. “Program” refers to the execution of 144 critical parameters in sequence as listed in Table 1. n = 9 mice. Wilcoxon test (two-tailed, paired). ***p < 0.001. (F) Spectral analysis of EUS electromyography induced by IPG electrical stimulation of the PN in panel (D). (G) Frequency-amplitude response curves for normal EUS electromyography and electromyography induced by IPG electrical stimulation.
Moreover, the frequencies of IPG electrical stimulation-induced EMG signals fell within the 0–4 kHz range during the bursting activity duration (Fig. 2F), similar to the physiological EUS-EMG frequencies during normal reflexive urination (Supplementary Fig. 1c). Within this frequency ___domain, the frequency-amplitude response curves of IPG electrical stimulation-induced EMG closely matched those of physiological EUS-EMG (Fig. 2G). In summary, our results demonstrate that IPG electrical stimulation of the PN efficiently induces EUS relaxation.
Coordinated electrical stimulation of MPG and PN drives reflexive urination in intact mice
Previous studies have demonstrated a direct neural connection between the MPG and bladder muscle18 and electrical stimulation of the MPG can induce bladder muscle contraction10,19,20. In this study, this connection was confirmed by injecting CTB into the bladder muscle, which labeled neurons widely distributed across the MPG (Supplementary Fig. 3a,c,d). A stimulation voltage of 1 V was used to evoke bladder muscle contractions (Table 2). To investigate the functional effects of coordinated electrical stimulation of the MPG and PN on bladder activity, EUS function and voiding outcomes, we individually implanted electrodes into the MPG and PN and performed cystometry and EUS-EMG recording in urethane-anesthetized intact mice (Fig. 3A, n = 9 mice). The coordinated stimulation parameters aimed to replicate the co-activation of bladder contraction and EUS relaxation during natural reflexive urination, involving 2 s of MPG stimulation (25 Hz frequency, stimulation voltage of 1 V; Table 2) followed by 5.3 s of our IPG stimulation of the PN (a combination of 800 Hz and 80 Hz, stimulation peak voltage of 1 V; Table 1; Fig. 3B).
Simultaneous electrical stimulation of the MPG and PN triggers reflexive urination. (A) Experimental scheme for electrical stimulation of the MPG and PN while simultaneously monitoring intravesical pressure (IVP) and EUS-EMG. (B) Timeline for experimental procedures. The MPG electrical stimulation lasted for 2 s (25 Hz, 1 V). PN electrical stimulation (IPG electrical stimulation) began 1 s after MPG stimulation and lasted for 5.3 s. (C) Representative IVP traces (top) and time-locked EUS-EMG signals (bottom) for each group. Each voiding event is denoted with an asterisk. (D) Enlarged view of the dashed line boxes in panel (C). (E) Success rate of electrical stimulation at two peripheral nerve sites (TPNS) inducing urination, Stimulation, Stim. n = 9 mice. (F) Quantification of the time interval between the initiation of IVP oscillations and the onset of EUS-EMG bursting activity. n = 9 mice for each group. Student’s t-test (two-tailed, paired). (G) Quantification of the variation in IVP (∆P) during urination in each group. n = 9 mice. Wilcoxon test (two-tailed, paired). (H) Time–frequency curve after performing spectral analysis of the EUS-EMG data from panel (D). (I) Comparison of the root mean square (RMS) of EUS-EMG data obtained from each group. n = 9 mice . Wilcoxon test (two-tailed, paired).
Based on our results shown in Fig. 1A and Supplementary Fig. 1a, MPG stimulation commenced 1 s before PN stimulation (Fig. 3B). We observed that combined stimulation at these two peripheral nerve sites (TPNS) effectively induced urination, with a success rate of 100% (Fig. 3C–E), and the pattern of IVP change and EUS-EMG bursting activity induced by coordinated electrical stimulation closely resembled natural reflexive urination (Fig. 3C–E). No significant differences in latency or bladder pressure changes were noted between the stimulated and control groups (Fig. 3F,G). Furthermore, the spectrum of stimulation-induced EUS-EMG signals in the stimulated group resembled that of the natural EUS-EMG signals during reflexive urination in the control group (Fig. 3H). There were also no significant differences in the root mean square (RMS) of EUS-EMG between the groups (Fig. 3I). Taken together, these findings suggest that our novel method of simultaneous electrical stimulation of MPG and PN can replicate natural urination by artificially coordinating bladder contraction and EUS relaxation.
High-level complete spinal cord injury resulted in NLUTD
To examine the potential of our innovative simultaneous electrical stimulation technique of MPG and PN to artificially synchronize bladder contraction with EUS relaxation in SCI mice, we developed a spinal cord injury mouse model (Supplementary Fig. 4a). Four weeks after SCI, immunostaining revealed a reduction in the density of NeuN+ cells and an increase in the expression of GFAP (a marker of inflammation) at the lesion site in SCI mice (Supplementary Fig. 4b). To assess the effects of high-level complete SCI on voiding behavior and urodynamics, we conducted void spot assays and filling cystometry combined with EUS-EMG recordings in male WT mice that underwent complete transection of the spinal cord at the thoracic (T) 10 level (Supplementary Fig. 4a).
The results of the void spot assay revealed significant differences in voiding behavior between SCI and sham-operated mice (Fig. 4A,B, n = 12 mice). Void spots in sham-operated mice were predominantly observed at the corners of the cage, whereas SCI mice exhibited a different voiding pattern, with spots distributed across the entire floor of the cage, indicating loss of positional preference (Fig. 4B). Additionally, SCI mice demonstrated impaired urination function, as evidenced by a decreased voiding interval (29.8 ± 3.48 min in sham-operated mice versus 0.96 ± 0.11 min in SCI mice; p < 0.001; Fig. 4C), reduced urine volume (average void spot area: 25.3 ± 2.22 cm2 in sham-operated mice versus 1.09 ± 0.15 cm2 in SCI mice; p < 0.001; Fig. 4D), and smaller void spot diameter (average void spot diameter: 5.62 ± 0.25 cm in sham-operated mice versus 1.15 ± 0.08 cm in SCI mice; p < 0.001; Fig. 4E) were observed compared to sham-operated mice.
High-level spinal cord injury caused NLUTD. (A) Schematic of the void spot assay setup. (B) Examples of void spots from sham mice (left) and SCI mice (right) illustrating impaired urination function. (C–E) Quantification of the interval for each void (C), the average area of each void spot (D), and the average diameter of each void spot (E). n = 12 mice for each group. Student’s t-test or Mann-Whitney U test (two-tailed, unpaired). ***p < 0.001. (F) Diagram depicting cystometry combined with external urethral sphincter (EUS) electromyography (EMG) recording using a multi-channel physiological recording instrument. (G) Representative intravesical pressure (IVP) traces (top) and time-locked EUS-EMG signals (bottom) during continuous transvesical infusion cytometry in anesthetized SCI mice or sham mice. Each urination event is denoted with an asterisk. Non-urination events are denoted with an arrowhead. Intravesical pressure data were subjected to Savitzky–Golay filtering in panel (G). (H) Enlarged view of the dashed line boxes in panel (G).
Representative recording traces of conventional filling cystometry combined with EUS-EMG recording in sham-operated mice exhibited a regular increase in bladder pressure (voiding contraction) during the voiding phase, accompanied by EUS bursting (phasic activity) to relax EUS for urine release (Fig. 4F–H). In contrast, SCI mice displayed significant changes in these parameters, characterized by non-voiding contractions and increased tonic EUS activity concurrent with rising bladder pressure (Fig. 4G, right; Fig. 4H, bottom), indicating that bladder contraction occurred simultaneously with EUS activation. These findings suggest that high-level complete SCI can lead to NLUTD.
Coordinated electrical stimulation of the MPG and PN improves voiding efficiency in SCI mice
To evaluate the efficacy of our combined stimulation (two peripheral nerve sites, TPNS) of the MPG and PN in treating NLUTD in SCI mice, we implanted stimulating electrodes into the MPG and PN of SCI mice. We then conducted void spot assays in freely moving mice before and after electrical stimulation, and we also performed cystometry and EUS-EMG recording in urethane-anesthetized mice. The results of the void spot assay revealed a significant increase in the diameter of voiding spots in SCI-TPNS stimulation mice compared to SCI mice without electrical stimulation (SCI, 0.63 ± 0.04 cm; SCI + Stim, 2.37 ± 0.05 cm; p = 6.4006E−09; Fig. 5A,B, n = 9 mice).
Coordinated electrical stimulation of MPG and PN enhances urinary efficiency in SCI mice. (A) Example of voiding spots deposited in sham mice (left). Example of voiding spots deposited in SCI mice (middle). Examples of voiding spots induced by combined electrical stimulation of the PN and the MPG in SCI mice (right, Stimulation group, Stim). (B) Quantification of the voiding spot diameters in each group, two peripheral nerve sites, TPNS. n = 9 mice for each group. Student’s t-test (two-tailed, unpaired). ***p < 0.001. (C,D) Representative IVP trace (top) and time-locked EUS-EMG signal (bottom). Each non-voiding or voiding event is denoted by arrows or asterisks. (E,F) Enlarged view of the dashed line boxes in panel (C,D).
Representative urodynamic recording traces of SCI mice without electrical stimulation displayed non-voiding contractions and overflow incontinence (Fig. 5C,E). Conversely, each combined stimulation event in SCI-TPNS mice corresponded to IVP changes, EUS-EMG bursting activity, as well as voiding (Fig. 5D,F). The change in IVP during voiding contraction was significantly higher in SCI-stimulation mice compared to SCI mice (SCI, 0.88 ± 0.11 cmH2O; SCI + TPNS Stim, 4.54 ± 0.61 cmH2O; p = 0.00016; Supplementary Fig. 5a). Overall, these findings support the notion that by overcoming NLUTD, coordinated electrical stimulation of the MPG and PN improves voiding efficiency in SCI mice.
Discussion
In this study, we developed an innovative electrical neurostimulation method to replicate reflexive urination control in awake or urethane-anesthetized SCI mice based on the characteristic activities of the bladder muscle and sphincter during natural reflexive voiding. This new electrical neurostimulation approach comprised innovative programmable (IPG) electrical stimulation targeting the PN to relax EUS and electrical stimulation of the MPG to control bladder contraction. Our results demonstrate that this novel stimulation induced coordinated bladder contraction and sphincter relaxation, leading to successful reflexive voiding. Our findings present a potent electrical neurostimulation strategy aimed at treating NLUTD, reconstructing bladder function, and restoring urinary control in patients with SCI. Additionally, they serve as a reference for personalized treatment approaches and the application of unidirectional brain-machine interfaces in urinary function recovery.
SCI impairs the transmission of urinary signals from the PMC to the spinal cord, often leading to NLUTD13,21,22. In recent decades, electrical stimulation therapy has become a key treatment for NLUTD due to its reversibility and controllability. It enhances voiding efficiency post-SCI by delivering artificial signals to the nerves. In this study, given the intact anatomical connections between the MPG and bladder muscle and the PN innervating the EUS post-SCI2,23,24, we explored whether artificial simultaneous stimulation of the MPG and PN could control both the bladder muscle contraction and EUS relaxation, thereby achieving artificial control of voiding post-SCI. Previous studies have shown that electrical stimulation of the MPG triggers bladder contractions8,20,25. Thus, the primary challenge of this study was to determine how to artificially control the PN to achieve EUS relaxation.
Our study presents a novel approach to controlling EUS relaxation, diverging from the traditional PN blocking method, which works through efferent blockade16,26. In contrast, our method leverages direct electrical stimulation of PN to mimic physiological control mechanisms. This approach allows us to artificially transmit signals that facilitate controlled relaxation of the EUS, aligning more closely with natural physiological processes. The feasibility of controlling EUS through electrical stimulation was assessed in a mouse model. Although the parameters for achieving artificial control were complex, involving implementing 144 critical parameters in a specified sequence (as detailed in Table 1), the results were highly promising. Notably, serial numbers, total time, and inter-group durations are adjustable, but the number of serial numbers used must be at least half of those listed. Specifically the number of serial numbers can be selected or adjusted between 4 and 20. The application of electrical signals designed to replicate EUS functional patterns led to a notable reduction in bladder pressure in a filled bladder and produced electromyographic (EMG) signals that mirrored normal physiological activity (Fig. 2C,D). These findings suggest that artificial electrical stimulation might achieve EUS relaxation. To further validate the specificity of our simulated electrical stimulation and rule out potential crosstalk during simultaneous electrical recording, we used a single frequency from the simulated information for stimulation. The results showed a continued increase in bladder pressure in the filled bladder, and the simultaneous EUS recordings did not show physiological-like EUS activity (Fig. 2A,B). This underscored the unique effectiveness of our approach in achieving targeted EUS relaxation, distinguishing it from conventional methods.
The potential efficacy of this stimulation pattern may arise from its ability to induce excitatory postsynaptic potentials (EPSPs), leading to a significant recruitment of acetylcholine (ACh) at the synaptic terminals of the PN without causing substantial fatigue in the sphincter21,27. Le Feber and Van Asselt’s study, which showed that the pudendal nerve induces urethral relaxation mediated by acetylcholine, supports this mechanism28. We considered other potential explanations, such as PN stimulation modulating EUS activity through the spinal segmental reflex pathway. Through the activation of pudendal afferents, phasic bursting of EUS increases voiding efficiency. Experiments at another laboratory, which used electrical stimulation of the pudendal motor branch increased voiding efficiency in both rats and cats, showed the possibility of this mechanism29.
The successful induction of EUS relaxation demonstrates the potential for achieving the key medical objective: protecting the upper urinary tract by keeping bladder pressure low. In conclusion, these findings indicate that artificial electrical stimulation of the PN can achieve EUS relaxation. This presents a new possibility for the artificial control of EUS and laying the foundation for future artificial coordinated control of voiding and human–machine interaction. With the advancement of artificial intelligence and brain–machine technologies, human–machine interaction has become possible. Our study lays the groundwork for future human–machine interactions that aime to achieve coordinated control of the bladder muscle and EUS.
Our current findings have been validated in mice under specific conditions, but further investigation is need across different animal models, injury sites, and patterns. Additionally, considering wireless electrical stimulation could enhance mobility in future interventions. In conclusion, our innovative electrical neurostimulation approach mimics reflexive urination control and may offer an alternative therapy for addressing NLUTD, improving voiding efficiency in patients with SCI.
Materials and methods
Animals
All experimental procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the animal use protocol, reviewed and approved by the Institutional Animal Use and Care Committee at the Third Military Medical University (AMUWEC, 20223602). All methods are reported in accordance with ARRIVE guidelines. Wild-type C57BL/6J mice were housed under standard laboratory conditions with free access to food and water provided by the SPF (Beijing) Biotechnology Co., Ltd. Mice of similar age and weight were randomly allocated to either the experimental or the control group. To ensure experimental reproducibility and meet statistical analysis requirements, a total of 105 mice were used in this study. After excluding mice with missing data and those with significant weight loss post-SCI, 66 mice were included in the final analysis and data evaluation. To minimize the number of mice used while meeting experimental design needs, SCI mice underwent void spot assays before proceeding to cystometry and EUS-EMG testing. Specific n values for all experiments are detailed in the corresponding figure legends.
Spinal cord injury
Adult male C57BL/6J mice, 8 weeks old, were anesthetized with isoflurane (3% induction, 0.5% maintenance) and placed in a prone position on a temperature-controlled pad. A 1 cm-long incision was made along the midline of the back, followed by laminectomy to remove the lamina over the eighth thoracic (T8) vertebral landmarks corresponding to T10 spinal cord segments30. Subsequently, the T10 spinal cord segment was severed by using a sterile surgical blade. Notably, prior to complete injury, all the mice underwent body weight monitoring and manual bladder voiding. After the injury, the laminectomy was closed by suturing the muscle with 5-0 prolene, and the skin incision was closed using 7.5-mm Michel’s wound clips (FineScience Tools). Enrofloxacin (5 mg/kg, i.p.) was given for 10 days post-injury to prevent infection. Gentle manual bladder expression was performed twice daily throughout the study period.
Immunohistochemistry
Four weeks after spinal cord injury, mice with hind limb motor function were eliminated from the study. The spinal cords of the remaining mice were subjected to immunofluorescent labeling for NeuN and GFAP (astrocyte marker). Mice were anesthetized with 1% pentobarbital sodium and sequentially perfused first with saline and then with PBS containing 4% PFA. For immunostaining experiments, transverse spinal cord cryostat slices of 70 μm thickness were obtained and processed free-floating. Sections were initially rinsed in PBS and then subjected to a blocking phase consisting of 10% normal donkey serum and 0.1% Triton X-100 in PBS for 2 h at room temperature. Subsequently, primary antibodies were prepared in the same blocking solution and incubated overnight at the following concentrations: chicken anti-NeuN (1:200, Abcam, ab134014), and rabbit anti-GFAP (1:200, Abcam, ab7260). After three rinses in washing buffer (PBS with 0.1% Triton X-100), the slices were treated for 2 h at room temperature with secondary antibodies. Following three additional washes in washing buffer, the slices were stained with DAPI, rinsed in PBS, transferred to Super Frost slides, and mounted with mounting material under glass coverslips. Finally, the slices were imaged using a Zeiss LSM 980 confocal microscope or an Olympus BX53-F2 epifluorescence microscope.
Cholera toxin B subunit tracing
Spinal cord intact C57BL/6J male mice, 8 weeks old, were anesthetized using pentobarbital sodium at a dose of 100 mg/kg and positioned in a supine position on a temperature-controlled board. A 1 cm midline abdominal incision was made to expose the bladder and external urethral sphincter. Cholera toxin B subunit conjugated with Alexa-555 dye (CTB555, Invitrogen, C34776) was injected into the bladder dome (1 μl volume) and the external urethral sphincter (2 μl volume) separately using a microinjection pump (Nanoject III™, Drummond Scientific Corp., USA) at a concentration of 1 μg/μl. The injection rate was set at 100 nl/min. After completing the CTB555 injection, the pump was stopped, and a 3-min waiting period was observed before slowly withdrawing the injection needle. Closure of the abdominal muscle and skin was performed using 5-0 sutures. Mice were then returned to their housing cages, and tissue samples were collected through a cardiac perfusion procedure ten days post-surgery.
Cystometry and EUS-EMG
Cystometry was performed according established procedures19. In the present study, we assessed the changes in the activity of the detrusor and the urethral sphincter following spinal cord injury by performing bladder catheterization and implantation of electrodes into the EUS at the fourth week post-SCI33. Intravesical pressure was measured during cystometry to assess bladder function without considering abdominal pressure. Male C57BL/6J mice were anesthetized with isoflurane (3% induction, 0.5% maintenance) and positioned supine on a temperature-controlled pad. Eye ointment (Bepanthen®, Bayer) was applied to safeguard the eyes. Following shaving of the abdominal fur, a 1 cm incision was made along the midline to expose the bladder and external urethral sphincter. A 7 cm-long PE10 catheter was inserted into the bladder apex using a 20 G (0.9*80TWLB) guiding needle. The PE10 catheter was affixed to the bladder apex with 6-0 non-absorbable suture thread, and its other end was passed through the abdomen and exited through the dorsal neck skin. A silver wire electrode, measuring 1–2 mm in length and made of 36 AWG wire, was implanted into the external urethral sphincter. The opposite end of the electrode was similarly passed through the abdomen using a guiding needle and secured with suture thread alongside the PE10 catheter. The connections were additionally reinforced using Vetbond Tissue Adhesive (3 M, 1469SB). Subsequently, mice received intraperitoneal injections of ketoprofen (5 mg/kg, i.p.) for pain relief and enrofloxacin (5 mg/kg, i.p.) for postoperative care.
For anesthetized simultaneous recordings, the mice were administered with urethane (1 g/kg, i.p.). A multi-channel physiological recording system (RM6240E, Chengdu Instrument Factory, China) was employed, with one channel linked to the external urethral sphincter electrode and four channels connected to the pressure transducer (YPJ01H; Chengdu Instrument Factory, China). The pressure transducer system was attached to the PE10 catheter in the mouse’s neck. The infusion pump (RWD404; RWD Technology Corp., Ltd., China) facilitated infusion rates ranging from 5 to 50 μl/min. EMG recording parameters were set at a sampling rate of 8 kHz, sensitivity of 1 mV, and signal acquisition between 1 Hz and 10 kHz. Pressure recording parameters included a sensitivity of 12.5 cmH2O, no hardware high-pass filtering, and a low-pass filter set at 30 Hz. Post-recording analysis encompassed EMG and pressure data, involving parameters such as amplitude, cycle, and root mean square for EMG, as well as peak pressure and pressure differentials for pressure data. RM6240E analysis tools facilitated data extraction, which was subsequently exported to Excel for analysis using SPSS 23 software (IBM, USA) for statistical interpretation.
Implantation of stimulation electrodes in the PN and MPG
Spinal cord intact or SCI male C57BL/6J mice were anesthetized with isoflurane (3% induction, 0.5% maintenance) and positioned supine on a temperature-controlled pad. A 1-cm incision was made along the midline of the abdomen. Blunt dissection was performed using forceps to separate the tissues surrounding the prostate for implantation of nearby stimulation electrodes. Two 1.5 mm stimulation electrodes, made of 36 AWG wire, were placed on the MPG, typically on the left side, using a 20 G guiding needle. Silicone adhesive (Kwik-Sil, World Precision Instruments, USA) was used to secure the electrodes. Abdominal muscles and skin were sutured separately using a 5-0 suture thread.
For the placement of stimulation electrodes on the pudendal nerve (PN), the mice were placed in a prone position on a temperature-controlled pad. A 1 cm-long incision was made at the skin and corresponding muscle along the iliac bone landmark to facilitate exposure. Blunt dissection was used to expose both the sciatic nerve and the PN Two 1.5 mm stimulation electrodes were positioned on one PN, typically on the left side, using a guiding needle. Silicone adhesive (Kwik-Sil, World Precision Instruments, USA) was used to secure the electrodes. The muscle was sutured with a 5-0 suture thread and the skin was closed with a 7 mm wound clip. Postoperatively, mice were administered intraperitoneal injections of ketoprofen (5 mg/kg, i.p.) and enrofloxacin (5 mg/kg, i.p.). All procedures, including catheter placement, EUS-EMG electrode implantation, and PN and MPG electrode implantations, were completed within 45 min in the same surgery session. PN and MPG electrodes were implanted after the catheter and EUS-EMG electrodes.
Electrical stimulation methods
To generate analog electrical signals, we connected the stimulation electrodes to the output of an NI acquisition card (NI USB-6009, National Instruments, USA) and operated them using a Labview-based program (Labview 2017, National Instruments, USA). To stimulate the MPG, we set the stimulation parameters to a frequency of 25 Hz, with voltage ranging from 0.25 to 2 V, and a duration of 5 s. For PN stimulation, we divided the stimulation protocol into two phases: burst and tonic. The burst phase lasted 4.2 s with a frequency of 800 Hz, mimicking the burst characteristics of EMG discharges in the urethral sphincter muscle. Each burst lasted approximately 32.86 ms, followed by a silent interval averaging 163.33 ms. The stimulation peak voltages ranged from 0.5 to 1 V. The tonic phase featured an 80 Hz frequency, lasting a total of 1.1 s, with stimulation voltages ranging from 0.01 to 0.1 V. It is worth noting that for PN electrical stimulation, the minimum voltage for each stimulation cluster is one-third of the peak voltage. During the combined stimulation, we applied a voltage of 1 V to the MPG with a frequency of 25 Hz and a duration of 2 s. PN stimulation began 1 s after MPG stimulation was initiated, following the parameters described for PN stimulation. More detailed stimulation parameters are listed in Tables 1 and 2.
Statistical analysis
All data were assessed for normality using Shapiro–Wilk tests and histograms in SPSS23 software (IBM, USA) to determine if they followed a normal distribution. All statistical analyses were two tailed. For normally distributed data, we employed both paired and unpaired two-tailed t-tests. Data that did not adhere to a normal distribution were analyzed using the Wilcoxon test or Mann–Whitney U test. All p-values < 0.1 are indicated in the figures. *p < 0.05; **p < 0.01; ***p < 0.001; ns indicates no significant difference. Error bars represent mean ± standard error of the mean (s.e.m.).
Data availability
The data are available from the corresponding author upon reasonable request.
References
Benarroch, E. E. Neural control of the bladder: Recent advances and neurologic implications. Neurology 75, 1839–1846 (2010).
Fowler, C. J., Griffiths, D. & de Groat, W. C. The neural control of micturition. Nat. Rev. Neurosci. 9, 453–466 (2008).
Li, H., Nahm, N., Borchert, A., Wong, P. & Atiemo, H. Contemporary treatment of detrusor sphincter dyssynergia: A systematic review. Curr. Bladder Dysfunct. Rep. 13, 206–214 (2018).
Mangera, A. et al. An updated systematic review and statistical comparison of standardised mean outcomes for the use of botulinum toxin in the management of lower urinary tract disorders. Eur. Urol. 65, 981–990 (2014).
Terpenning, M. S., Allada, R. & Kauffman, C. A. Intermittent urethral catheterization in the elderly. J. Am. Geriatr. Soc. 37, 411–416 (1989).
Warren, J. W., Muncie, H. L., Hebel, J. R. & Hall-Craggs, M. Long-term urethral catheterization increases risk of chronic pyelonephritis and renal inflammation. J. Am. Geriatr. Soc. 42, 1286–1290 (1994).
Reynard, J. M., Vass, J., Sullivan, M. E. & Mamas, M. Sphincterotomy and the treatment of detrusor–sphincter dyssynergia: Current status, future prospects. Spinal Cord. 41, 1–11 (2003).
McGee, M. J., Amundsen, C. L. & Grill, W. M. Electrical stimulation for the treatment of lower urinary tract dysfunction after spinal cord injury. J. Spinal Cord. Med. 38, 135–146 (2015).
Lee, J. W. et al. Emerging neural stimulation technologies for bladder dysfunctions. Int. Neurourol. J. 19, 3–11 (2015).
Gaunt, R. A. & Prochazka, A. Control of urinary bladder function with devices: successes and failures. In Progress in Brain Research vol. 152, 163–194 (Elsevier, 2006).
Van Kerrebroeck, P. E. V., Koldewijn, E. L. & Debruyne, F. M. J. Worldwide experience with the Finetech-Brindley sacral anterior root stimulator. Neurourol. Urodyn. 12, 497–503 (1993).
Krasmik, D., Krebs, J., Van Ophoven, A. & Pannek, J. Urodynamic results, clinical efficacy, and complication rates of sacral intradural deafferentation and sacral anterior root stimulation in patients with neurogenic lower urinary tract dysfunction resulting from complete spinal cord injury. Neurourol. Urodyn. 33, 1202–1206 (2014).
Bacsu, C., Chan, L. & Tse, V. Diagnosing detrusor sphincter dyssynergia in the neurological patient. BJU Int. 109, 31–34 (2012).
Panicker, J. N., Fowler, C. J. & Kessler, T. M. Lower urinary tract dysfunction in the neurological patient: Clinical assessment and management. Lancet Neurol. 14, 720–732 (2015).
Burks, F. N., Bui, D. T. & Peters, K. M. Neuromodulation and the neurogenic bladder. Urol. Clin. N. Am. 37, 559–565 (2010).
Tai, C., Roppolo, J. R. & De Groat, W. C. Block of external urethral sphincter contraction by high frequency electrical stimulation of pudendal nerve. J. Urol. 172, 2069–2072 (2004).
Holstege, G. Micturition and the soul. J. Comp. Neurol. 493, 15–20 (2005).
Bertrand, M. M., Korajkic, N., Osborne, P. B. & Keast, J. R. Functional segregation within the pelvic nerve of male rats: A meso- and microscopic analysis. J. Anat. 237, 757–773 (2020).
Tai, C., Booth, A. M., De Groat, W. C. & Roppolo, J. R. Bladder and urethral sphincter responses evoked by microstimulation of S2 sacral spinal cord in spinal cord intact and chronic spinal cord injured cats. Exp. Neurol. 190, 171–183 (2004).
Lee, S. et al. Mechano-neuromodulation of autonomic pelvic nerve for underactive bladder: A triboelectric neurostimulator integrated with flexible neural clip interface. Nano Energy 60, 449–456 (2019).
De Groat, W. C., Griffiths, D. & Yoshimura, N. Neural control of the lower urinary tract. In Comprehensive Physiology (ed. Terjung, R.) 327–396 (Wiley, 2014).
Castro-Diaz, D. & Taracena Lafuente, J. M. Detrusor-sphincter dyssynergia. Int. J. Clin. Pract. 60, 17–21 (2006).
Rao, Y. et al. Ventrolateral periaqueductal gray neurons are active during urination. Front. Cell. Neurosci. 16, 865186 (2022).
Hou, X. H. et al. Central control circuit for context-dependent micturition. Cell 167, 73–86 (2016).
Holmquist, B. & Olin, T. Electromicturition in male dogs at pelvic nerve stimulation: An urethrocystographic study. Scand. J. Urol. Nephrol. 2, 115–127 (1968).
Tai, C., Roppolo, J. R. & De Groat, W. C. Response of external urethral sphincter to high frequency biphasic electrical stimulation of pudendal nerve. J. Urol. 174, 782–786 (2005).
Jeffery, N. D. Pathophysiology, clinical importance, and management of neurogenic lower urinary tract dysfunction caused by suprasacral spinal cord injury. J. Vet. Intern. Med. 30, 1575–1588 (2016).
Le Feber, J. & Van Asselt, E. Pudendal nerve stimulation induces urethral contraction and relaxation. Am. J. Physiol. Regul. Integr. Compar. Physiol. 277, R1368–R1375 (1999).
Langdale, C. L. & Grill, W. M. Phasic activation of the external urethral sphincter increases voiding efficiency in the rat and the cat. Exp. Neurol. 285, 173–181 (2016).
Harrison, M. Vertebral Landmarks for the Identification of Spinal Cord Segments in the Mouse (2013).
Yao, J. et al. A corticopontine circuit for initiation of urination. Nat. Neurosci. 21, 1541–1550 (2018).
Yao, J. et al. Simultaneous measurement of neuronal activity in the pontine micturition center and cystometry in freely moving mice. Front. Neurosci. 13, 663 (2019).
Saito, T. et al. Time-dependent progression of neurogenic lower urinary tract dysfunction after spinal cord injury in the mouse model. Am. J. Physiol. Renal Physiol. 321, F26–F32 (2021).
Funding
This study was supported by Grants from the National Natural Science Foundation of China (31970946), China Postdoctoral Science Foundation (2021M700602), and Talent Project of Chongqing (4246ZP1252).
Author information
Authors and Affiliations
Contributions
Jiwei Yao, Junan Yan, and Yuangui Chen conceived and designed the study. Jun Li, Guoxian Deng, and Xianping Li performed the experiments and collected the data. Lingxuan Yin, Chunhui Yuan, and Wei Shao performed post-hoc histological and behavioral experiments. Jun Li, Xianping Li, and Lingxuan Yin analyzed the data. Jiwei Yao, Junan Yan, Yuangui Chen and Jun Li wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Deng, G., Li, X. et al. An innovative electrical neurostimulation approach to mimic reflexive urination control in spinal cord injury models. Sci Rep 14, 25305 (2024). https://doi.org/10.1038/s41598-024-76499-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-76499-3
Keywords
This article is cited by
-
SST neurons in the periaqueductal gray regulate urination and bladder function
Communications Biology (2025)