Abstract
Acute kidney injury (AKI) is a common clinical problem, and patients who survive AKI have a high risk of chronic kidney disease (CKD). The acute protective effects of Cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors in AKI have been examined, there is still relatively little known regarding the impact of acute CDK4/6 inhibition on the chronic sequence of AKI. Therefore, we utilized the CDK4/6 inhibitor Palbociclib to examine the long-term effects of CDK4/6 inhibition in a rodent model of ischemic AKI. Palbociclib (Palb) was administered during the acute stage or post the acute stage of AKI and mice were sacrificed 21 days post injury. We found that Palb could cause renal senescence and renal fibrosis. Furthermore, dasatinib (D) plus quercetin (Q) were used to eliminate senescent cells in ischemic AKI murine model and Palb was administered post the treatment of D + Q. We found that Palb could reverse the senolytic and antifibrotic effects induced by D + Q, which indicates that the profibrotic effect of Palb could be ascribed to its pro-senescent effects. Our results demonstrate that CDK4/6 inhibitors treatment might be deleterious on the chronic sequence of AKI.
Similar content being viewed by others
Introduction
Despite extensive research into its pathophysiology and a variety of proposed therapeutic interventions, acute kidney injury (AKI) persists as a formidable clinical challenge1. Current consensus emphasizes the primacy of preventative strategies and supportive care in managing AKI, given the absence of definitive treatment modalities2. Although recovery is possible for many patients, the potential for AKI to develop into chronic kidney disease (CKD), is becoming increasingly acknowledged3. This underscores the need for renewed research efforts to understand the mechanism of AKI developing into CKD and discover effective treatments to mitigate its long-term consequences4.
The intricate and multifaceted pathophysiological processes underlying nephrotoxic and ischemic AKI involve a complex interplay between various elements. These include endothelial damage, apoptotic and necrotic tubular cells, inflammatory cytokines, and inflammatory cells infiltration, all contributing to the manifestation and intensity of the AKI phenotype5. Traditionally, research has examined these processes in isolation, leading to therapeutic attempts with mixed success rates in animal models. Additionally, these interventions have mainly concentrated on acute outcomes and histological changes over a short period following the initial injury. The lasting impact of these acute phase treatments on long-term renal health is a crucial question that has not yet been fully answered6.
During AKI, residual quiescent tubular epithelial cells demonstrate a capacity for rapid proliferation. Inhibition of cell cycle progression may safeguard these cells and facilitate repair processes. Multiple studies have corroborated the protective role of cell cycle inhibitors in the context of AKI7,8,9. These investigations reveal that the timely administration of cell cycle inhibitors can mitigate damage to renal tubular epithelial cells and enhance the restoration of renal function. Notably, after the resolution of acute stressors, it is crucial to alleviate cell cycle arrest to prevent subsequent fibrotic changes. Prior research has linked sustained G2-M arrest following AKI with an increased risk of renal fibrosis10. In contrast, the implications of prolonged G1-S arrest on fibrogenesis in the post-AKI setting remain under-explored. The advancement from G1 to S phase is driven by Cyclin-Dependent Kinases 4 and 6 (CDK4/6), which, upon forming complexes with cyclin D, propels cell cycle progression. Several CDK4/6 inhibitors, such as Palbociclib (Palb) and Ribociclib, have been devised to interrupt this phase transition11. Nonetheless, the impact of CDK4/6 inhibitors on the progression of renal fibrosis post-AKI warrants further investigation.
The purpose of this study was to examine the effect of prolonged administration of Palb on the progression of renal fibrosis after AKI. We investigated the mechanism of Palb-induced renal fibrosis and found a correlation between renal senescence and renal fibrosis. Furthermore, by using dasatinib (D) and quercetin (Q) to eliminate senescent cells and subsequently administering Palb, we confirmed the role of senescence induction by Palb in the progression of renal fibrosis post-AKI.
Results
Palbociclib promotes renal senescence and inhibit proliferation in the renal tissue
To evaluate the impact of Palb on normal kidney tissue, we administered Palb orally for a duration of seven days (Fig. 1A). Our findings indicate a significant upregulation of both mRNA and protein expressions of P16 and P21 in the kidney tissue, suggesting that Palb induces cellular senescence (Fig. 1B-F). This observation was further substantiated by an increase in the number of SA-β-Gal positive senescent cells in normal renal tissues (Fig. 1G). Furthermore, a decrease in the expression of LaminB1, a marker associated with alterations in the nuclear envelope, was observed (Fig. 1H). To gain a deeper comprehension of the impact of Palb on cellular proliferation, we investigated the expression of Ki-67, a marker associated with proliferation, within the renal cells of mice with normal physiological conditions. Following the administration of Palb, we observed a notable reduction in the expression of Ki-67 in comparison to the control group, signifying an impediment in cellular proliferation (Fig. 1G). These discoveries imply that Palbociclib prompts cell cycle arrest and hampers cellular proliferation within the renal tissue of normal mice, consequently augmenting the population of senescent cells.
Palbociclib promotes renal senescence and inhibit proliferation in the renal tissue. Palbociclib was administered to C57BL/6 mice via gavage for 7 days (A). Relative mRNA expression levels of P16 and P21 are shown (B, C). Representative and quantitative immunoblot analyses of P16 and P21 are depicted (D, E, F). Representative immunostaining of SA-β-Gal activity (scale bar, 100 μm) and Ki67 in renal tissue (scale bar, 50 μm) is presented (G). Representative immunofluorescence staining of LaminB1 in renal tissue (scale bar, 20 μm) is shown (H). The qPCR and immunoblot results are shown as the fold change compared with the Veh group. Data are expressed as mean ± SD. n = 4 for the Veh group and n = 6 for all other groups. *p < 0.05, **p < 0.01.
Palbociclib treatment in the acute stage of AKI promotes renal senescence and fibrosis
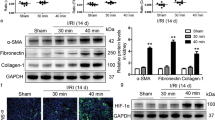
To ascertain the effects of sustained Palb administration during the acute phase of AKI on fibrotic progression, mice were treated with Palb for seven days commencing 24 h post-ischaemia/reperfusion (I/R) injury, with a subsequent observation period of 14 days. Renal samples were harvested on day 21 following I/R injury (Fig. 2A). Transcriptomic analysis demonstrated elevated mRNA levels of senescence-associated markers P16 and P21 in the renal tissue of the unilateral I/R injury (uIRI) plus Palb group (Fig. 2B, C), coupled with an increase in SA-β-galactosidase staining, in contrast to the uIRI group (Fig. 2D). These data suggest that Palb treatment in the immediate aftermath of renal injury promotes the expression of senescent phenotypes within the kidney over time. Extending the analysis to include fibrotic indicators post-Palb administration revealed a pronounced elevation in the mRNA expression of fibrosis biomarkers, including Collagen I and Fibronectin, when compared with the uIRI group (Fig. 2E, F). Immunohistochemical analyses corroborated the increased deposition of Collagen I and Fibronectin within the renal sections of the uIRI plus Palb group (Fig. 2G, I, J), and macrophage infiltration was found to be upregulated in the uIRI plus Palb group relative to the uIRI control (Fig. 2H, K). Collectively, these observations substantiate the hypothesis that prolonged Palbociclib intervention during the acute stage of AKI may exacerbate the severity of subsequent renal fibrosis.
Palbociclib administration in the acute stage of AKI induces renal senescence and fibrosis. Palbociclib was administered to C57BL/6 mice via gavage from day 1 to day 7, and the mice were euthanized on day 21 post-renal ischemia (A). Relative mRNA expression levels of P16 and P21 are shown (B, C). Representative immunostaining of SA-β-Gal activity (scale bar, 100 μm) is presented (D). Relative mRNA expression levels of Collagen I and Fibronectin are depicted (E, F). Representative immunohistochemical staining of Collagen I and Fibronectin in renal tissue (scale bar, 50 μm) is shown (G), along with semi-quantitative analysis (I, J). Representative immunohistochemical staining of F4/80 (scale bar, 50 μm) is presented (H), and semi-quantitative analysis of macrophage infiltration in renal tissue is provided (K). The qPCR results are shown as the fold change compared with the Veh group. Data are expressed as mean ± SD. n = 4 mice. *p < 0.05, **p < 0.01, ***p < 0.001.
Palbociclib treatment post-acute stage of AKI promotes renal senescence and fibrosis
To determine the prolonged effects of Palb administration on fibrosis progression subsequent to the acute phase of AKI, we initiated Palb treatment on day 8 post-I/R injury, continuing for a span of seven days. Renal specimens were procured on day 21 after the insult (Fig. 3A). Notably, Palb treatment was associated with elevated P16 and P21 mRNA levels in the renal tissue, implicating an augmented senescent phenotype in the uIRI plus Palb group (Fig. 3B, C). Further appraisal of fibrotic marker expression revealed that Palb treatment in the later phase can potentiate the transcriptional upregulation of Collagen I and Fibronectin in the uIRI plus Palb condition (Fig. 3D, E). Moreover, immunohistochemical analysis disclosed an intensified deposit of α-SMA, Collagen I, and Fibronectin in the renal sections from the uIRI plus Palb group (Fig. 3F, H, I, J), allied with an enhanced macrophage infiltration compared to the uIRI alone (Fig. 3G, K). These results suggest that continued Palbociclib intervention post-acute phase of AKI may accelerate the progression of renal fibrosis.
Palbociclib administration post-acute stage of AKI promotes renal senescence and fibrosis. Palbociclib was administered to C57BL/6 mice via gavage from day 8 to day 14, and the mice were euthanized on day 21 post-renal ischemia (A). Relative mRNA expression levels of P16 and P21 are shown (B, C). Relative mRNA expression levels of Collagen I and Fibronectin are depicted (D, E). Representative immunohistochemical staining of α-SMA, Collagen I, and Fibronectin in renal tissue (scale bar, 50 μm) is presented (F), along with semi-quantitative analysis of α-SMA, Collagen I, and Fibronectin positive areas in renal tissue (H, I, J). Representative immunohistochemical staining of F4/80 (scale bar, 50 μm) is shown (G), and semi-quantitative analysis of macrophage infiltration in renal tissue is provided (K). The qPCR results are shown as the fold change compared with the Veh group. Data are expressed as mean ± SD. n = 4 mice. *p < 0.05, **p < 0.01, ***p < 0.001.
Palbociclib reverses the senescence reducing and anti-fibrosis effects of D + Q in uIRI murine model
Building on previous evidence that the D + Q cocktail alleviates renal fibrosis in an uIRI mouse model by ablating senescent cells [12], we aimed to investigate the effects of Palb on renal senescence and fibrosis in this paradigm, after D + Q administration (Fig. 4A). Our results reveal that while D + Q diminished the uIRI-induced augmentation of P16 and P21 mRNA levels, subsequent Palb administration could partly reverse this attenuation (Fig. 4B, C). Consistant with transcript patterns, protein expressions of P16, P21, and P53 manifested a similar trend (Fig. 4D-H). Furthermore, Palb was found to amplify SA-β-galactosidase staining in renal tissues, a senescence marker repressed by D + Q in the uIRI context (Fig. 4I. These data imply that Palb may negate D + Q-mediated senescent cell clearance. Beyond senescence markers, we delineated that Palb can not only offset the reduction in renal senescence but also potentiate fibrosis, an effect abrogated by D + Q treatment. Specifically, Palb treatment led to an upregulation in the protein abundance of αSMA and Collagen I, which had previously been downregulated by D + Q in the uIRI model (Fig. 5A-C). In parallel, immunohistochemical staining for Sirius Red, αSMA, Collagen I, and Fibronectin, which were ameliorated by D + Q, were intensified following Palb treatment (Fig. 5D-H). Altogether, these outcomes robustly argue for a role of Palbociclib in counteracting the attenuation of renal fibrosis, by fostering renal senescent cell accumulation in the uIRI mouse model.
Palbociclib increases the renal senescence reduced by D + Q in uIRI murine model. D + Q was administered weekly for a total of three doses, followed by a 7-day treatment course with Palbociclib. Mice were sacrificed on day 22 following renal ischemia (A). Relative mRNA expression levels of P16 and P21 in renal tissue are shown (B, C). Representative and quantitative immunoblot analysis of P16, P21, and P53 in renal tissue is depicted (D-H). Representative renal tissue sections stained for SA-β-Gal activity (scale bar, 200 μm) are presented (I). The qPCR and immunoblot results are shown as the fold change compared with the Sham group. Data are expressed as mean ± SD. n = 4 for the Sham group and n = 6 for all other groups. *p < 0.05, **p < 0.01, ***p < 0.001.
Palbociclib increases the renal fibrosis reduced by D + Q in uIRI murine model. Immunoblot analyses illustrating representative blots and quantitative data for α-SMA and Collagen I in renal tissue are shown (A-C). Representative blots are presented (A), with quantification of α-SMA and Collagen I level shown in (B) and (C), respectively. Representative immunohistochemical staining of Sirius Red, α-SMA, Collagen I, and Fibronectin in renal tissue (scale bar, 50 μm) is depicted (D). Semi-quantitative analysis of stained renal tissue sections, indicating the positive areas for Sirius Red (E), α-SMA (F), Collagen I (G), and Fibronectin (H), is provided. The qPCR and immunoblot results are shown as the fold change compared with the Sham group. Data are expressed as mean ± SD. n = 4 for the Sham group and n = 6 for all other groups. *p < 0.05, **p < 0.01, ***p < 0.001.
D + Q mitigates the pro-senescence and pro-fibrosis effects of palbociclib in uIRI murine model
Building upon findings that Palb can negate the senescence-reducing and anti-fibrosis effects of the D + Q cocktail, we further explored D + Q’s impact on renal senescence and fibrosis following Palb treatment in a uIRI murine model (Fig. 6A). Our study demonstrates that D + Q effectively reduced the elevated mRNA levels of P21 and P53 that were induced by Palb (Fig. 6B, C). This suggests that D + Q could counteract the senescence signals enhanced by Palb treatment. Beyond these markers of senescence, we observed a notable decrease in renal fibrosis, which was initially induced by Palb. Specifically, D + Q treatment led to a decrease in the protein abundance of αSMA and Collagen I, which had previously been upregulated by Palb in the uIRI model (Fig. 6D-F). Immunohistochemical staining showed reductions in Sirius Red, Collagen I, and Fibronectin – all of which were increased by Palb treatment (Fig. 6G-J). These findings collectively suggest that the removal of senescent cells, driven by D + Q, can significantly attenuate renal senescence and fibrosis, thus countering the adverse effects observed with Palb administration in the uIRI mouse model.
D + Q decreases the renal senescence and renal fibrosis induced by palbociclib in uIRI murine model. Palbociclib was administered to C57BL/6 mice via gavage from day 1 to day 7, then treated with D + Q weekly for a total of two doses. Mice were sacrificed on day 21 following renal ischemia (A). Relative mRNA expression levels of P21 and P53 in renal tissue are shown (B, C). Representative and quantitative immunoblot analysis of Collagen I and αSMA in renal tissue is depicted (D-F). Representative immunohistochemical staining of Sirius Red, Collagen I, and Fibronectin in renal tissue (scale bare, 50 μm) is depicted (G). Semi-quantitative analysis of stained renal tissue sections, indicating the positive areas for Sirius Red (H), Collagen I (I), and Fibronectin (J) is provided. The qPCR and immunoblot results are shown as the fold change compared with the Sham group. Data are expressed as mean ± SD. n = 4 mice. **p < 0.05, ***p < 0.01, ****p < 0.001.
Discussion
In this study, we observed that prolonged administration of Palb during the early stage of AKI or thereafter may potentially accelerate the development of renal fibrosis. Furthermore, Palb was observed to trigger senescence in the renal tissue, concomitant with the progression of renal fibrosis. Additionally, our findings indicate that Palb’s promotion of renal fibrosis post-AKI is reliant on its senescence-inducing effects.
Palbociclib, a suppressor of CDK4/6, functions similarly to the P16 protein, which plays a key role in inhibiting the activity of CDK4 and CDK6. In a unilateral ureteral obstruction (UUO) model, the absence of P16 led to increased tubular and interstitial cell proliferation, ultimately contributing to the development of fibrosis following renal trauma12. Notably, P16 is considered a regulatory switch that hinders proliferation and matrix formation, thereby mitigating fibrosis. Additionally, in an ischemia-reperfusion injury (IRI) model, P16ablation promoted tubular cell proliferation and reduced apoptosis in the initial stage, while also increasing interstitial capillary density during the recovery phase13. Consequently, divergent perspectives on the role of P16 have emerged in the context of acute injury. The cell cycle arrest induced by P16 can have dual effects: it can be beneficial for DNA damage repair but detrimental for fibrosis progression. These effects manifest differently in various stages following acute injury. The use of P16 knockout mice may complicate the understanding of its specific role in each stage. Therefore, more precise methods, such as the use of CDK4/6 inhibitors, are needed to investigate the role of CDK4/6 in acute injury and repair stages.
Previous studies have investigated the effects of CDK4/6 inhibitors on renal tubular epithelial cells in the acute phase of AKI in mouse models. De Rocco et al. found that PD0332991 could prevent early cell death and support the regeneration of damaged tubules in an ischemia-reperfusion injury (IRI) mouse model. Their findings suggested that inducing cell cycle arrest can facilitate DNA repair and prevent abnormal cell divisions7. Additionally, Kim et al. demonstrated that ribociclib reduces damage to renal tubular epithelial cells in a cisplatin-induced AKI mouse model, with the protein Rb1 mediating ribociclib’s protective effects9. These studies focused on the short-term effects of CDK4/6 inhibitors during the acute phase of AKI. However, the translation of these acute protective effects into favorable long-term outcomes remains unclear. Our study initially investigated the effects of prolonged Palb administration during the acute phase of AKI on the subsequent development of renal fibrosis. We found that administering Palb daily for seven days during the acute phase of AKI exacerbated fibrosis on day 21 post-IRI. We also evaluated the impact of sustained Palb treatment post the acute phase of AKI on the progression of renal fibrosis. Our results indicated that a similar seven-day regimen of Palb post-acute AKI phase also increased fibrosis by day 21 post-IRI. Consistent with our observations, another study found that Palb therapy post-IRI (administered either four or ten days after the ischemic event) did not have protective effects at 28 days post-injury14. Considering these findings, prolonged Palb therapy may contribute to the progression of fibrosis, warranting further investigation into the underlying mechanisms responsible for fibrosis augmentation induced by prolonged Palb treatment.
In addition to their cell cycle blocking effects, CDK4/6 inhibitors also exhibit non-canonical effects, such as inducing senescence15. Our administration of tubular epithelial cells with Palb revealed the induction of senescence in these cells. Furthermore, prolonged administration of Palb in vivo confirmed its pro-senescent effects. Several studies have verified the pro-senescent effects of CDK4/6 inhibitors16,17. Unlike classical senescence, senescence induced by CDK4/6 inhibitors can be reversible, as demonstrated by the reacquisition of proliferative capability by cells in the senescent state after drug removal. The mechanisms by which CDK4/6 inhibitors induce senescence are still not fully understood. Additionally, we observed that prolonged administration of Palb could modulate renal senescence in a uIRI murine model, which correlated with the progression of fibrosis. Previous studies have investigated the relationship between renal senescence and renal fibrosis, and renal senescence has been confirmed as a crucial mechanism in the progression of renal fibrosis following acute injury18,19. Based on these findings, we speculate that senescence induced by Palb is vital for renal fibrosis progression following AKI.
CDK4/6 inhibitors, in addition to inducing senescence, may have various effects such as metabolic changes and immunomodulatory actions20,21. The metabolic and immunomodulatory actions can also impact on fibrosis22. Therefore, it is crucial to understand whether the impact of Palb on the development of renal fibrosis following AKI is dependent on its pro-senescent properties. To address this question, a modified set of criteria like “Koch’s postulates” has been suggested23. The combination of D + Q is known as a method for eliminating senescent cells in various disease models. In previous research, D + Q was used to eliminate senescence in a murine model of AKI-CKD progression induced by IRI, resulting in a reduction in both senescence and fibrosis18. This confirmed a connection between the removal of senescent cells and the reduction of fibrotic progression. In this study, we investigated the effects of Palb treatment following D + Q intervention in an IRI-induced AKI-CKD murine model. Our finding reveal that Palb reinstated both renal senescence and fibrosis, which had been alleviated by D + Q. Further investigation into the sequence of D + Q intervention post-Palb treatment in uIRI murine model shed light on the mechanism involved, showing that D + Q effectively reduced both renal fibrosis and senescence induced by Palb. Therefore, it can be inferred that the pro-fibrotic effects of Palb could be attributed to its pro-senescent effects.
It is necessary to recognize the limitations of our study. A critical next step is to determine whether our findings are reproducible in different forms of AKI-CKD murine models. Additionally, evaluating the effects of CDK4/6 inhibitors on cancer patients with AKI remains a pivotal area of exploration. Further research is essential to comprehensively address these aspects, enhancing the applicability and relevance of our findings in broader clinical contexts.
In conclusion, our research demonstrates that the administration of CDK4/6 inhibitor may contribute to the advancement of renal fibrosis following AKI. This phenomenon can be attributed to the pro-senescent properties of CDK4/6 inhibitor. Therefore, it is imperative to acknowledge the potential pro-fibrotic effects when utilizing CDK4/6 inhibitors in clinical settings.
Method
Animal models
Male C57BL/6 mice, aged 6–8 weeks and weighing 25–30 g, were obtained from the Guangdong Medical Laboratory Animal Center in Guangdong, China. Ethical review was conducted, and all animal experiments were approved by the Institutional Animal Care and Use Committee of Southern Medical University. The study was conducted in accordance with the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals and the ARRIVE guidelines. Anesthesia was induced via intraperitoneal injection of 1.5% pentobarbital. The left kidney was exposed by a midline incision in the abdomen, and the renal pedicle was clamped with mouse artery clips for 35 min to induce ischemia.
The CDK4/6 inhibitor, Palbociclib, was formulated by dissolution in a 50 mM sodium lactate solution. The study aimed to assess the impact of Palb on renal fibrosis after AKI. Mice received an oral gavage of Palb at a dosage of 150 mg/kg, either 24 h post UIRI over a duration of 7 days or commencing 8 days following uIRI for an equivalent period. A control group was treated with a matching volume and concentration of sodium lactate solution through oral gavage. All mice were humanely euthanized 21 days post-uIRI for subsequent analysis. To evaluate the potential role of Palb-induced senescence in the development of renal fibrosis, we administered a combination of dasatinib (5 mg/Kg) and quercetin (50 mg/Kg) orally once a week for three doses, commencing 24 h after uIRI. Following this, the mice were subjected to Palb treatment for a duration of 7 days, and ultimately sacrificed 21 days after uIRI. We also administered Palb continually for 7 days, commencing 24 h after uIRI. Then we treated a combination of dasatinib and quercetin orally once a week for two doses and sacrificed 21 days after uIRI.
SA-β-Gal assay
SA-β-Gal staining was performed using a staining kit (Cell Signaling Technology). The fresh mice renal tissue was embedded in OCT solution, 6-µm cryosections were fixed in 1% formaldehyde at room temperature. After the fixative was removed, cryosections were incubated overnight at 37℃with staining solution (>12 h). The images were obtained under Olympus microscope, and the percentage of positive cells stained by SA- β-Gal was quantified.
Immunofluorescence assay
Following deparaffinization and dehydration, the 4 μm paraffin kidney tissue sections underwent antigen retrieval and were sealed with a 2.5% BSA buffer solution. Subsequently, the sections were incubated overnight at 4℃ with LaminB1 antibody (1:200, Proteintech, 12987-1-AP), washed with PBS, and then exposed to a secondary antibody (1:200, CoraLite488-Goat Anti-Rabbit IgG, Proteintech, SA00013-2) at room temperature for 1 h. The tissue was counterstained with DAPI and imaged using a confocal microscope (Olympus, Shanghai, China), followed by analysis using Image J software.
Immunohistochemical assay
Fresh kidney tissue was fixed in 4% paraformaldehyde and embedded in paraffin, and 4 μm paraffin sections were mounted on glass slides. After deparaffinization and dehydration, the sections were subjected to antigen retrieval and blocked with hydrogen peroxide solution before incubation in primary antibodies against ki67 (1:200,Abconal, USA), Collagen I (1:500,BOSTER, China), FN (1:500,Sigma-Aldrich ) and α-SMA (1:200,Sigma-Aldrich), overnight at 4℃ in the cassette (> 18 h), and incubated for 1 h at room temperature. DAB kit (ZSGB-BIO, Beijing, China) was used for staining, then hematoxylin re-staining was performed, and tissue staining was observed under Olympus microscope.
Sirius red staining assay
The 4 μm paraffin sections of kidney tissue was deparaffined and dehydrated, about 20ul of Sirius red dye was added, and left for 1 h at room temperature. After cleaning with PBS, it was added into the concentration gradient ethanol solution for dehydration. After sealing, images were acquired under Olympus microscope.
PCR assay
Total RNA from kidney tissues was extracted using RNAIso Plus (Takara, Kusatsu, Japan), and 2 µg of total RNA was used for reverse transcription using the PrimeScriptRT reagent kit (Takara). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using a SYBR Green Supermix kit (Novizan Biotechnology Co., LTD., China). Relative expression was normalized to the expression levels of GAPDH or actin. The primers are listed in Table 1.
Statistical analysis
Data are expressed as means ± SD of at least three independent experiments. All statistical analysis was performed using SPSS 25.0 software. Differences between two groups were performed by the Student’s t test and multiple groups were evaluated by one-way ANOVA, Comparison between pair-to-pair sample means was performed using LSD for homogeneous variance and Dunnett ‘T3. P values < 0.05 were considered statistically significant.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Change history
14 January 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-84533-7
References
Mehta, R. L. et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 385 (9987), 2616–2643 (2015).
Kellum, J. A. et al. Acute kidney injury. Nat. Rev. Dis. Primers. 7 (1), 52 (2021).
See, E. J. et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 95 (1), 160–172 (2019).
James, M. T. et al. Long-term outcomes of acute kidney injury and strategies for improved care. Nat. Rev. Nephrol. 16 (4), 193–205 (2020).
Zuk, A. & Bonventre, J. V. Acute kidney Injury. Annu. Rev. Med. 67, 293–307 (2016).
Dagher, P. C. et al. The p53 inhibitor pifithrin- alpha can stimulate fibrosis in a rat model of ischemic acute kidney injury. Am. J. Physiol. Ren. Physiol. 302, F284–F291 (2012).
DiRocco, D. P. et al. CDK4/6 inhibition induces epithelial cell cycle arrest and ameliorates acute kidney injury. Am. J. Physiol. Ren. Physiol. 306 (4), F379–F388 (2014).
Pabla, N. et al. Mitigation of acute kidney injury by cell-cycle inhibitors that suppress both CDK4/6 and OCT2 functions. Proc. Natl. Acad. Sci. U S A. 112 (16), 5231–5236 (2015).
Kim, J. Y. et al. Ribociclib mitigates cisplatin-associated kidney injury through retinoblastoma-1 dependent mechanisms. Biochem. Pharmacol. 177 (March), 113939 (2020).
Yang, L. et al. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 16 (5), 535–543 (2010).
Goel, S., Bergholz, J. S. & Zhao, J. J. Targeting CDK4 and CDK6 in cancer. Nat. Rev. Cancer. 22 (6), 356–372 (2022).
Wolstein, J. M. et al. INK4a knockout mice exhibit increased fibrosis under normal conditions and in response to unilateral ureteral obstruction. Am. J. Physiol. Physiol. 299 (6), F1486–F1495 (2010).
Lee, D. H. et al. INK4a deletion results in improved kidney regeneration and decreased capillary rarefaction after ischemia-reperfusion injury. Am. J. Physiol. Physiol. 302 (1), F183–F191 (2011).
Osaki, Y. et al. Blocking cell cycle progression through CDK4/6 protects against chronic kidney disease. JCI Insight 7(12). (2022).
Wagner, V. & Gil, J. Senescence as a therapeutically relevant response to CDK4/6 inhibitors. Oncogene. 39 (29), 5165–5176 (2020).
Wang, B. et al. Pharmacological CDK4/6 inhibition reveals a p53‐dependent senescent state with restricted toxicity. EMBO J. 41 (6), 1–16 (2022).
Crozier, L. et al. CDK4/6 inhibitors induce replication stress to cause long-term cell cycle withdrawal. EMBO J. 41 (6), 1–20 (2022).
Li, C. et al. Senolytic therapy ameliorates renal fibrosis postacute kidney injury by alleviating renal senescence. FASEB J. 35 (1), 1–16 (2021).
Li, S. et al. Tubular cell senescence promotes maladaptive kidney repair and chronic kidney disease after cisplatin nephrotoxicity. JCI Insight 8(8). (2023).
Sager, R. A. et al. Therapeutic potential of CDK4/6 inhibitors in renal cell carcinoma. Nat. Rev. Urol. 19 (5), 305–320 (2022).
Zhang, S. et al. Immunomodulatory effects of CDK4/6 inhibitors. Biochim. Biophys. Acta Rev. Cancer. 1878 (4), 188912 (2023).
Henderson, N. C., Rieder, F. & Wynn, T. A. Fibrosis: from mechanisms to medicines. Nature. 587 (7835), 555–566 (2020).
Kirkland, J. L. & Tchkonia, T. Cellular Senescence: a translational perspective. EBioMedicine. 21, 21–28 (2017).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81270826, 81470972, 82070708 to J.W.), Natural Science Foundation of the Xinjiang Uygur Autonomous Region of China (2021D01C012 to J.G.), Natural Science Foundation of the Xinjiang Uygur Autonomous Region of China (2022D01C847 to S.Z.), The First People’s Hospital of Kashi “Pearl River Scholars Tianshan Talents” Cooperation Expert Studio (KDYY202205 to S.H.). State Key Laboratory of Dampness Syndrome of Chinese Medicine &The First People’s Hospital of Kashi Fund (SZGZZ20240040 to J.G.).
Author information
Authors and Affiliations
Contributions
Conceptualizations: J.W., J.G.Methodology: S.Z., R.P., Y.S, L.H.Investigation: S.Z., R.P., Y.S, L.H., S.H.Supervision: J.W., J.G.Writing: S.Z., R.P., J.W.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: In the original version of this Article the author Jie Guo was incorrectly affiliated. Full information regarding this can be found in the correction published with this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, S., Peng, R., Shen, Y. et al. The deleterious effects of CDK4/6 inhibition on renal recovery post-acute kidney injury. Sci Rep 14, 28143 (2024). https://doi.org/10.1038/s41598-024-79663-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-79663-x