Abstract
Luminal breast cancer exhibits a high incidence of bone recurrence when metastasizing to distant organs. The mechanisms underlying the organotropism of luminal breast cancer cells remain unclear. In this study, we aimed to determine the role of WWP1 (WW ___domain-containing E3 ubiquitin protein ligase 1)-PTEN (phosphatase and tensin homolog deleted on chromosome ten) interaction in bone tropism in luminal breast cancer. We observed that WWP1 was overexpressed in luminal breast cancer tissues and associated with poor prognosis in breast cancer patients. In luminal breast cancer cells, WWP1 was found to mediate PTEN ubiquitination, resulting in the functional loss of PTEN. As a result, we demonstrate that WWP1 contributes to bone tropism in luminal breast cancer cells via the polyubiquitination of PTEN. Consequently, WWP1-mediated PTEN polyubiquitination contributed to the early metastasis of luminal breast cancer cells to the bone. Thus, our study provides a mechanistic insight into the bone tropism of luminal breast cancer cells and proposes a potential therapeutic strategy for mitigating cancer metastasis to the bone.
Similar content being viewed by others
Introduction
The bone is the most common site of metastasis in breast cancer, particularly in the luminal subtype1,2. Over two-thirds of breast cancer patients develop bone metastasis, resulting in severe pain, pathological fractures, and other skeletal-related events, which are associated with poor prognosis for breast cancer patients3. Clinical studies have revealed that luminal breast cancer is more likely to metastasize to the bone than to the non-bone organs4,5. However, the molecular mechanisms that regulate this bone selectivity remain unclear. Intricate molecular interactions within cancer cells, which are highly context-dependent, contribute to the development of bone metastasis6. In particular, phosphatase and tension homolog deleted on chromosome ten (PTEN) is a significant tumor suppressor that cancer cells cannot afford to lose entirely before maturation7. By antagonizing the PI3K (phosphatidylinositol 3-kinase)/AKT (protein kinase B) signaling pathway, PTEN can govern fundamental cellular processes in various cancer cells8. Mice with PTEN overexpression have achieved a tumor-suppressive metabolic state, leading to a lifespan extension7. Therefore, the active and reactive functions of PTEN are considered promising therapeutic opportunities for human health. In terms of molecular mechanisms, the activity, expression, and localization of PTEN are frequently dysregulated through post-translational modifications, such as ubiquitylation9. The ubiquitylation of PTEN can be promoted by several E3 ubiquitin ligases, affecting its protein stability or dimer formation, both of which are highly consequential for tumor initiation, progression, and metastasis10,11,12,13,14. Post-translational modification is a complex process influenced by various molecules within different cells, so ubiquitylation is context-dependent15. Various evidence has demonstrated the critical role of PTEN post-translational modification in bone metastases, as well as lung, breast, and prostate cancer16.
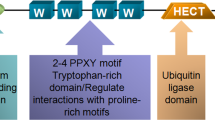
Several E3 ubiquitin ligases have been reported to promote PTEN polyubiquitylation for degradation10,13. However, WW ___domain-containing E3 ubiquitin protein ligase 1 (WWP1) was found to regulate PTEN dimerization and membrane recruitment, rather than degradation, through K27-linked polyubiquitination in prostate cancer cells8. WWP1 belongs to the NEDD4 (neural precursor cell expressed, developmentally down-regulated 4) - like protein family, characterized by an N-terminal C2 ___domain, four WW domains, and a C-terminal catalytic HECT (homologous to the E6-associated protein carboxyl terminus) ___domain17. After binding to PTEN, WWP1 mediates polyubiquitylation of lysine residue 342, 344, or both8. Thus, WWP1 may suppress PTEN dimerization and membrane recruitment, ultimately resulting in neoplastic transformation8. Among various cancers, breast cancer, one of the most common bone tropism cancers, is the most sensitive to PTEN dysfunction18. In addition, WWP1 was reported to be a potential therapy target for breast cancer. Many studies have demonstrated that WWP1 played a potent role in breast cancer tumorigenesis or drug resistance by the ubiquitination of PTEN, MUC1, CXCL12, and other breast cancer-promoting or suppressing proteins19,20,21,22. Though PTEN and WWP1 are reported to influence bone metastasis, reports regarding the role of the WWP1-PTEN interaction in luminal breast cancer are lacking16,22. Therefore, the purpose of our study was to investigate the role of WWP1-PTEN interaction in bone tropism in luminal breast cancer and to explore their mechanisms.
In this study. We demonstrated that WWP1 was upregulated in breast cancer and contributed to the tumorigenesis. We confirmed the interaction between WWP1 and PTEN in luminal breast cancer cells. Moreover, we verified K27-linked polyubiquitination of PTEN by WWP1 in luminal breast cancer cells. In addition, we found that WWP1 could promote luminal breast cancer cell migration in vitro and metastasis in vivo. Generally, our study highlights the critical role of the WWP1-PTEN interaction in luminal breast cancer bone metastasis and suggests a new potential approach for managing breast cancer metastasis.
Results
WWP1 was overexpressed and related with a poor prognosis in breast cancer
To elucidate the potential role of WWP1 in breast cancer, we first analyzed data from TCGA (The Cancer Genome Atlas) and GTEx (Genotype-Tissue Expression) databases. The results revealed a general upregulation of WWP1 mRNA in breast cancer (Fig. 1A). We further validated this finding by confirming strong WWP1 expression in the tumors of most breast cancer patients with spinal metastasis, but minimal expression in their adjacent noncancerous breast tissues (Fig. 1B and C). This observation indicates that WWP1 is barely expressed in normal breast tissues but is frequently overexpressed in breast tumor tissues that metastasize to bone. Moreover, the WWP1 expressions are also analyzed between luminal breast cancer patients with and without spinal metastases. As shown in Fig. 1D and E, the luminal breast cancers that developed spinal metastasis (M) had a relatively higher WWP1 expression than those that did not develop metastasis (T). In addition, Kaplan–Meier analysis demonstrated that breast cancer patients with low WWP1 expression achieved a relatively more prolonged overall survival (Fig. 1F). These results indicated that WWP1 might be a risk factor in breast cancer metastasis.
WWP1 was overexpressed and related with a poor prognosis in breast cancer. (A) Pan-cancer analysis of WWP1 expression between normal and tumor tissues from TCGA and GETx. (B) Western blot analysis of WWP1 expression in paired samples of breast cancer spinal metastasis (M) and adjacent nontumorous tissues (N). (C) Quantification of WWP1 protein levels relative to GAPDH in (B). (D) Western blot analysis of WWP1 expression in samples of luminal breast cancer with spinal metastasis (M) and without spinal metastasis (T). (E) Quantification of WWP1 protein levels relative to GAPDH in (D). (F) Overall survival analysis for breast cancer patients based on WWP1 expression, categorized into high (red) and low (blue) expression. P-values were calculated using the log-rank test. *P < 0.05, **P < 0.01, ***P < 0.001, results are representative of at least three independent experiments and shown as mean ± SD.
WWP1 interacts with PTEN in luminal breast cancer cell lines
To determine the underlying mechanisms of WWP1 in breast cancer metastasis, we first assessed the protein levels of WWP1 in various breast cancer cell lines. As shown in Fig. 2A, WWP1 exhibited an upregulation in luminal cell lines compared to other cell lines. The highest expression of WWP1 in luminal breast cancer cell lines, especially in MCF7 cells, was further verified by immunoblot analysis (Fig. 2B and C). These results indicated the critical role of WWP1 in luminal breast cancer metastasis.
WWP1 interacts with PTEN in luminal breast cancer cell lines. (A) Western blot analysis of WWP1 expression levels in different breast cancer cell lines. (B,C) Quantifications of WWP1 levels relative to GAPDH among different breast cancer cell lines are shown. (D) BT474, MCF7, and T47D cells transfected with Flag-WWP1 were subjected to immunoprecipitation using anti-Flag antibodies. Both cell lysates and immunoprecipitates were then blotted. (E–G) Interaction between WWP1 and PTEN in MCF7 cells, T47D cells, and BT474 cells was confirmed through Co-IP assays. Cell lysates were immunoprecipitated using control IgG, anti-WWP1, and anti-PTEN antibodies. The immunoprecipitates were then analyzed.
PTEN is well known to be dysregulated, such as through posttranslational modification, during the breast cancer progression. And WWP1 was reported to mediate PTEN posttranslational modification in prostate cancer. Therefore, we explored whether WWP1 could regulate PTEN in luminal breast cancer cells, as posttranslational modification is context-dependent. Binding assays revealed a strong interaction between WWP1 and PTEN in luminal breast cancer. As shown in Fig. 2D, WWP1 exhibited strong binding capability to PTEN in luminal breast cancer lines (MCF7, BT474, and T-47D), suggesting a promising role for WWP1 in regulating PTEN in luminal breast cancer cells. Co-immunoprecipitation experiments confirmed the binding between endogenous WWP1 and PTEN proteins in the three cell lines (Fig. 2E–G). These observations verified the interaction between WWP1 and PTEN in luminal breast cancer cells and suggested that WWP1 should be a regulator of PTEN in the luminal breast cancer metastasis.
WWP1 polyubiquitylates PTEN
As WWP1 is an E3 ubiquitin ligase, we went on to determine the possibility that WWP1 mediates polyubiquitination of PTEN, which had not been studied in luminal breast cancer cells. According to Fig. 2A–C, WWP1 had high expression in MCF7 cells, so we silenced endogenous WWP1 in MCF7 cells using shRNAs (Fig. 3A), and immunoblot analysis confirmed the successful knockdown of WWP1 in MCF7 cells (Fig. 3B). Moreover, from Fig. 3A, we can see that the knockdown of WWP1 did not influence the PTEN protein levels, and the immunoblot analysis confirmed this conclusion (Fig. 3B). Consequently, the downregulation of WWP1 by shRNA reduced PTEN polyubiquitination in MCF-7 cells (Fig. 3C). Immunoblot analysis exhibited a significant decrease in PTEN ubiquitination level when WWP1 was knocked down (Fig. 3D). Conversely, ectopic overexpression of wild-type WWP1 (WWP WT) in MCF7 cells, treated with WWP1 shRNA, recused the PTEN polyubiquitination levels. In contrast, the ectopic expression of WWP1 C890A (WWP1 CA), a catalytically inactive mutation, abolished this effect (Fig. 3E,F). As ubiquitination is a context-dependent and intricate process, these results suggested an indispensable role of WWP1 in triggering PTEN polyubiquitination in luminal breast cancer cells, and the WWP1-mediated PTEN polyubiquitination is dependent on the catalytic activity of WWP1.
WWP1 polyubiquitylates PTEN. (A) WWP1 was knocked down in MCF7 cells using shRNA, and knockdown efficiency was verified through western blotting. (B) Quantification of WWP1 (left) and PTEN (right) protein levels normalized to GAPDH in (A) is shown. (C) Effects of WWP1 knockdown on WWP1-mediated PTEN polyubiquitination. WWP1-silenced MCF7 cells were transfected with the indicated constructs, and PTEN polyubiquitination was analyzed through western blotting. (D) Quantification of the ubiquitination levels of PTEN in (C) is shown. (E) Analysis of PTEN polyubiquitination level in WWP1-knockdown MCF7 cells with ectopic overexpression of either wide-type WWP1 (WT) or C890A WWP1 (CA, a catalytically inactive mutant of WWP1). (F) Quantification of the ubiquitination levels of PTEN in (F) is shown. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, results are representative of at least three independent experiments and shown as mean ± SD.
WWP1 did not affect PTEN stability
Previous studies have shown that the stabilization of PTEN protein suppresses breast cancer tumorigenesis23. However, according to Fig. 3A,C,E, WWP1 did not affect the PTEN protein levels. Indicating polyubiquitination by WWP1 did not lead to PTEN degradation. To further examine the effects of WWP1 on PTEN stability, we subsequently assessed the correlation between the protein turnover rate and WWP1-mediated polyubiquitination of PTEN in luminal breast cancer cells. The cycloheximide (CHX) treatment experiment in MCF7 cells showed the turnover rate of PTEN was not affected by overexpression of WWP1 (Fig. 4A,B). Moreover, the knock-down of WWP1, using shRNA, had no influence on the half-life of PTEN (Fig. 4C,D). Together, these results showed that WWP1 did not mediate the stability of PTEN in MCF7 cells.
WWP1 did not affect PTEN stability. (A) MCF7 cells transfected with WWP1 were treated with cycloheximide (10 µg/ml), and analyzed at the indicated times for western blot. (B) Quantification of PTEN levels relative to GAPDH in (A) is shown. (C,D) MCF7 cells stably expressing WWP1 shRNA were treated with cycloheximide (10 µg/ml), and the half-life of PTEN was analyzed at the indicated times through western blot and relative quantification analysis. (E) Western blot analysis of PTEN expression in samples of luminal breast cancer with spinal metastasis (M) and without spinal metastasis (T). (F) Quantification of PTEN protein levels relative to GAPDH in (C). (G) Effect of ubiquitin K27R (Lys to Arg) or K-only (K27) ubiquitin on WWP1-regulated PTEN polyubiquitination in MCF7 cells. WWP1-mediated PTEN K27-linked ubiquitination was confirmed in MCF7 cells. *P < 0.05, **P < 0.01, and ***P < 0.001. Data are representative of at least three independent experiments and are presented as mean ± SD.
In addition, we compared the PTEN expressions between luminal breast cancers with and without spinal metastasis. Hardly any noticeable difference could be detected from the western blot imaging and analysis (Fig. 4E,F). Given that WWP1 had a higher expression in luminal breast cancers that developed spinal metastasis against those without spinal metastasis (Fig. 1D,E), these findings demonstrated that WWP1-regulated polyubiquitination does not lead to PTEN degradation. Indeed, recently, WWP1 was found to affect the dimerization and membrane recruitment of PTEN mediated by the K27 ubiquitination, which defunctionalized PTEN8. Subsequently, we verified the K27 ubiquitination of PTEN mediated by WWP1 in MCF7 cells (Fig. 4G). Together, these results emphasize the crucial role of WWP1 in regulating PTEN function in luminal breast cancer cells rather than PTEN stabilization.
WWP1 promotes luminal breast cancer cells migration via PTEN polyubiquitination
The above data lead us to propose that WWP1 might function as a promotor for luminal breast cancer metastasis through the regulation of PTEN. To verify this hypothesis, we generated WWP1-knockdown derivatives of MCF7 cells using a lentiviral shRNA vector in MCF7 cells, and then stable expressing WT or C890A (CA) WWP1 in the WWP knock-down MCF7 cells (Fig. 5A). WWP1 knockdown downregulated Akt phosphorylation without affecting the PTEN and Akt protein levels (Fig. 5A). Overexpressing WWP1 WT instead of CA rescued the Akt phosphorylation (Fig. 5A). Subsequently, we carried out transwell assays to evaluate the migration ability of WWP1-knockdown MCF7 cells. As shown in Fig. 5B, WWP1 knockdown substantially suppressed the migration ability of MCF7 cells in vitro. However, only WWP1 WT, but not WWP1 CA, was able to reverse the migration phenotype (Fig. 5B,C), suggesting the essential role of WWP1 catalytic activity, which functioned in PTEN ubiquitination, in mediating MCF7 migration. Taken together, these results demonstrated that WWP1 could promote the migration ability of luminal breast cancer cells through the polyubiquitination of PTEN, as evidenced by its requirement for catalytic activity.
WWP1 promotes migration of luminal breast cancer cells in vitro and early bone metastasis in vivo. (A) WWP1, PTEN, and Akt signaling protein levels were analyzed in MCF7 cells with WWP1 knock-down, and stably transfected with WWP1 WT or CA in this WWP1 knock-down MCF7 cells. (B) Effects of WWP1 on MCF7 cells migration were accessed using Transwell assays. (C) Quantification of MCF7 cell numbers in different groups in (B). (D) BLI analyses of early bone metastasis in nude mice 2 weeks after IIA injection of MCF7 control cells, WWP1 knockdown, and WT overexpression in WWP1-knockdown cells (n = 5 mice in each group). (E) BLI signals of all nude mice in each group of (D) at 2 weeks. (F) WWP1, PTEN, and Akt signaling protein levels in tumors were analyzed. (G) Percentage of metastasis-free nude mice per group. *P < 0.05, **P < 0.01, and ***P < 0.001. Data are representative of at least three independent experiments and are presented as mean ± SD.
WWP1 contributes to early-stage bone metastasis of luminal breast cancer in vivo
Encouraged by the in vitro results, we further explored the function role of WWP1 in breast cancer metastasis via an intra-iliac artery (IIA) injection model in nude mice. This model enables monitoring of early-stage bone colonization24, which is particularly relevant for luminal breast cancer due to its strong bone tropism. As shown in Fig. 5D, WWP1 knockdown markedly reduced the bone metastasis burden following the IIA injection of cancer cells in mice. Bioluminescent imaging (BLI) quantification substantiated the decrease in metastatic signal in the hind limbs within the first two weeks after cancer cell inoculation (Fig. 5E). Meanwhile, the stable expression of WWP1 in WWP1 knock-down MCF7 cells promoted the cancer cell metastasis ability in vivo (Fig. 5D,E). WWP1, PTEN, and proteins relevant to Akt in the tumors were analyzed. As shown in Fig. 5F, in line with the in vitro analysis, the knockdown of WWP1 downregulated the Akt phosphorylation in the MCF7 cells injected into mice that metastasized to bone. Besides, survival analyses confirmed that mice injected with WWP1 overexpression clones of MCF7 cells had significantly shorter survival periods (Fig. 5G). Akt signaling is essential for PTEN-mediated tumor metastasis. Therefore, these results underscore the pivotal role of WWP1 in bone metastasis of luminal cancers.
Discussion
Numerous studies have focused on bone metastasis in breast cancer. Breast cancer is categorized into different subtypes based on its molecular types25. Identifying molecules responsible for tumor progression, metastasis, and chemotherapy resistance can shed light on breast cancer treatment26. Several tumor-derived molecules, such as IL-6, MMP1, and Jagged127, have been identified in studies of ER− cell lines. However, while ER is reported to have a significant clinical association with the bone tropism of breast cancer, only a few studies have provided partial explanations for the bone preference in ER+ luminal breast cancer2,28. Our study identified the E3 ligase WWP1 as a critical regulator of bone preference in ER+ luminal breast cancer cells. WWP1 was upregulated in most breast cancer and luminal breast cancer cell lines, and this upregulation was linked to poor prognosis in breast cancer patients29. The interaction between WWP1 and the tumor suppressor PTEN, which regulates early bone metastasis, was detected in ER+ luminal breast cancer cells. Mechanistically, the increased abundance of WWP1 promotes its binding with PTEN in luminal breast cancer cells, thus increasing the K27-linked polyubiquitination of PTEN8. This polyubiquitination of PTEN, regulated by WWP1, directly inhibits the formation of dimers and membrane recruitment8, ultimately promoting bone metastasis in MCF7 cells.
Increasing evidence has shown a significant relationship between HECT E3 ubiquitin ligases and the progression of various human cancers30,31. WWP1 belongs to the NEDD4 family, characterized by C2, HECT, and four WW domains17. According to the data from TCGA and GETx, WWP1 is frequently upregulated and amplified in breast and prostate cancers. These cancers have a higher propensity to metastasize to bone2,32,33. Aberrant WWP1 expression is significantly associated with the progression of human cancers17. WWP1 could meditate chemotherapy resistance of MCF7 cells and metastasis of MDA-MB-231 cells21,22. However, no study has previously focused on its function in luminal breast cancer metastasis. Accordingly, our study highlights the promising role of WWP1 in promoting luminal breast cancer metastasis to the bone, providing a new therapeutic target for bone metastasis in breast cancer.
In luminal breast cancer cells, WWP1 targets PTEN for polyubiquitination, promoting metastasis to the bone. Polyubiquitination mediated by WWP1 regulates PTEN dimer formation and membrane recruitment rather than PTEN stability8. The function of PTEN is strictly controlled through its subcellular localization, post-translational modifications, or both8. Maintaining the balance of PTEN plays a vital role in the proliferation and metastasis of various human cancers23. Breast, lung, and prostate cancer that tend to metastasize to bone are subjected to PTEN dysfunction16. Moreover, PTEN also suppresses primary and metastatic bone cancer16,34. HER2-positive breast cancer progression and metastasis were reported to be suppressed by PTEN16. However, whether PTEN dimer formation and membrane recruitment can prevent luminal breast cancer cells from metastasizing to specific organs remains to be elucidated. To investigate the potent bone tropism of luminal breast cancer cells, we silenced WWP1 using shRNA. Consequently, the polyubiquitination of PTEN decreased, and the migration ability of MCF7 cells was reduced, as assessed using the transwell assay. Subsequent overexpression of WWP1 restored PTEN polyubiquitination and, thus, the migration ability of MCF7 cells. These results were further verified in the animal experiments. In summary, our study revealed that WWP1 could promote the early bone metastasis of luminal breast cancer cells by regulating PTEN function through polyubiquitination.
However, more details need to be further studied before we can target WWP1-PTEN for the treatment of ER+ breast cancer metastasis to bone. Firstly, more breast cancer samples need to be collected according to their subtype to analyze the WWP1 expression and the correlation between WWP1 and PTEN. Besides, the binding site between WWP1 and PTEN needs to be well-studied for designing targeted drugs. Ultimately, future studies are needed to demonstrate the precise mechanisms between PTEN ubiquitination and luminal breast cancer bone tropism. Fortunately, the identification of WWP1 as a critical regulator for luminal breast cancer bone metastasis may serve as a valuable treatment strategy.
In conclusion, this study is the first to establish the nonproteolytic ubiquitination of PTEN mediated by WWP1 in luminal breast cancer cells. It underscores the critical role of WWP1 in mediating bone colonization of luminal breast cancer cells by preventing PTEN dimer formation and membrane recruitment (Fig. 6). This non-proteolytic ubiquitination of PTEN mediated by WWP1 in luminal breast cancer cells could represent a general mechanism underlying their preference for bone metastasis, offering a novel insight into the bone preference of luminal breast cancer metastasis. Therefore, targeting the WWP1-PTEN interaction holds promise for ER+ breast cancer patients at high risk of bone metastasis.
Methods
Clinical analysis
Expression analysis of breast cancer patients was downloaded from GEPIA (Gene Expression Profiling Interactive Analysis) 2.0 (http://gepia2.cancer-pku.cn/). Frozen samples of breast cancer bone metastasis, adjacent tissues, and breast cancer tissues were acquired from the Department of Orthopedic Oncology of Changzheng Hospital (Navy Medical University), and the basic information of these samples was listed in Table SI. We obtained written informed consent and received approval from the Ethics Committee of Changzheng Hospital. Informed consent was provided by the patients.
Cell culture
All the cell lines used in this study were purchased from ATCC (American Type Culture Collection), and were authenticated by STR (Short Tandem Repeat) profiling. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS and 1% penicillin–streptomycin. The cells were grown at 37 ℃ in a 5% CO2 incubator and were free from mycoplasma contamination.
Plasmids and antibodies
Plasmids: pSPAX2, pMD2.G, His-Ub, His-Ub K27R, His-Ub K27, Flag-PTEN, HA-PTEN, Flag-WWP1, Flag-WWP1-C890A (Flag-WWP1 CA) were stored in our laboratory. Antibodies: anti-WWP1 (Abcam, ab227213 and ab104440), anti-PTEN (Abcam, ab32199 and ab267787), anti-GAPDH (Sigma-Aldrich, G9545), anti-Flag (Abcam, ab205606), anti-His (Abcam, ab200537), anti-HA (Abcam, ab314237), normal IgG (Santa Cruz, sc-2025). Anti-pThr308-Akt (CST, 9275), anti-Akt (CST, 9272), and anti-pSer473-Akt (CST, 4060, D9E).
Immunoprecipitation and western blotting
Immunoprecipitation was performed as previously described8, using different antibodies and reagents. Western blotting was conducted as previously described35.
Lentivirus infection
We cotransfected 293T cells with plasmids pSPAX2, pMD2.G, and the lentivirus-based constructs as indicated. The supernatant was collected to harvest recombinant lentivirus. Lentiviruses carrying short hairpin RNA (shRNA) targeting human WWP1 (TRCN0000003395: ATTGCTTATGAACGCGGCTTT and TRCN0000003396: ACAACACACCTTCATCTCCGT)19 were used to infect MCF7 cells with Polybrene (10 µg/ml). MCF7 cells stable expressing WWP1 WT or WWP1 C890A were also generated through lentivirus transduction. The infected MCF7 cells were selected with puromycin (2 µg/ml) to generate stable clones.
Ubiquitination assay
For the ubiquitination analysis of PTEN, MCF7 cells were transfected with the indicated constructs, along with His-ubiquitin. Cells were lysed as previously described8. Briefly, cells were lysed in buffer A [6 M guanidine HCl, 10 mM imidazole, and Na2HPO4/NaH2PO4 (pH 8.0)]. The lysates were incubated with Ni-NTA agarose for 3 h at 37 ℃, followed by western blot analysis. WWP1 WT and WWP1 C890A were cloned into pFlag-CMV-2; PTEN was cloned into pCMV-HA; ubiquitin was cloned into pcDNA3.1/His-N. All the plasmids were stored in our lab.
Animal experiments
Female nude mice (6 weeks old), housed under SPF (Specific Pathogen Free) facilities, were used in animal experiments. These experiments were approved by the Committee for Animal Welfare of the Naval Medical University, and all the experiments in this article were in accordance with ARRIVE guidelines. Intra-iliac artery (IIA) injection was performed according to previous reports24,36 after anesthetized by inhalation of 2.5% isoflurane. In summary, 1 × 105 luciferase-labeled cells were injected into the intra-iliac artery for early bone metastasis analysis. Mice were euthanized by intraperitoneal injection of pentobarbital sodium (200 mg/kg).
Experimental statement
The experiments and methods used in this article were all in accordance with relevant guidelines and regulations.
Statistical analyses
All data are representative of at least three independent experiments and are presented as the mean ± SD, analyzed by GraphPad Prism 8.0. Statistical differences between the two groups were assessed using Student’s t-test. Statistical analysis was performed using 1-way ANOVA and 2-way ANOVA test followed by Tukey’s or Fisher’s least significant difference multiple comparisons for multigroup data sets. P < 0.05 means significant (*P < 0.05, **P < 0.01, and ***P < 0.001).
Data availability
The data presented in this study are available on request from the corresponding author.
References
Clézardin, P. et al. Bone metastasis: mechanisms, therapies, and biomarkers. Physiol. Rev. 101(3), 797–855 (2021).
Nolan, E., Lindeman, G. J. & Visvader, J. E. Deciphering breast cancer: from biology to the clinic. Cell 186(8), 1708–1728 (2023).
van Uden, D. J. P. et al. Metastatic behavior and overall survival according to breast cancer subtypes in stage IV inflammatory breast cancer. Breast Cancer Res. 21(1), 113 (2019).
Falato, C., Schettini, F., Pascual, T., Brasó-Maristany, F. & Prat, A. Clinical implications of the intrinsic molecular subtypes in hormone receptor-positive and HER2-negative metastatic breast cancer. Cancer Treat. Rev. 112, 102496 (2023).
Kim, S. et al. Genomic profile of metastatic breast cancer patient-derived xenografts established using percutaneous biopsy. J. Transl. Med. 19(1), 7 (2021).
Waning, D. L., Guise, T. A. & Mohammad, K. S. A connexin responsible for the fatal attraction of Cancer to bone. Cell Metab. 29(1), 6–8 (2019).
Liu, A., Zhu, Y., Chen, W., Merlino, G. & Yu, Y. PTEN dual lipid- and protein-phosphatase function in tumor progression. Cancers (Basel). 14(15), 3666 (2022).
Lee, Y. R. et al. Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway. Science. 364(6441), eaau0159 (2019).
Álvarez-Garcia, V., Tawil, Y., Wise, H. M. & Leslie, N. R. Mechanisms of PTEN loss in cancer: it’s all about diversity. Semin. Cancer Biol. 59, 66–79 (2019).
Dai, C. et al. Aagab acts as a novel regulator of NEDD4-1-mediated Pten nuclear translocation to promote neurological recovery following hypoxic-ischemic brain damage. Cell Death Differ. 28(8), 2367–2384 (2021).
Guo, Y. et al. Linear ubiquitination of PTEN impairs its function to promote prostate cancer progression. Oncogene 41(44), 4877–4892 (2022).
Iwase, R. et al. Semisynthetic approach to the analysis of tumor suppressor PTEN ubiquitination. J. Am. Chem. Soc. 145(11), 6039–6044 (2023).
Lee, Y. R. et al. WWP1 gain-of-function inactivation of PTEN in cancer predisposition. N. Engl. J. Med. 382(22), 2103–2116 (2020).
Van Themsche, C., Leblanc, V., Parent, S. & Asselin, E. X-linked inhibitor of apoptosis protein (XIAP) regulates PTEN ubiquitination, content, and compartmentalization. J. Biol. Chem. 284(31), 20462–20466 (2009).
Cockram, P. E. et al. Ubiquitination in the regulation of inflammatory cell death and cancer. Cell. Death Differ. 28(2), 591–605 (2021).
Xi, Y. & Chen, Y. Oncogenic and therapeutic targeting of PTEN loss in bone malignancies. J. Cell. Biochem. 116(9), 1837–1847 (2015).
Hu, X. et al. The emerging role of WWP1 in cancer development and progression. Cell. Death Discov. 7(1), 163 (2021).
Gao, X. et al. PTENP1/miR-20a/PTEN axis contributes to breast cancer progression by regulating PTEN via PI3K/AKT pathway. J. Exp. Clin. Cancer Res. 38(1), 256 (2019).
Kishikawa, T. et al. WWP1 inactivation enhances efficacy of PI3K inhibitors while suppressing their toxicities in breast cancer models. J. Clin. Investig. 131(24), e140436 (2021).
Liao, C. et al. WWP1 targeting MUC1 for ubiquitin-mediated lysosomal degradation to suppress carcinogenesis. Signal Transduct. Target. Therapy 6(1), 297 (2021).
Subik, K. et al. The ubiquitin E3 ligase WWP1 decreases CXCL12-mediated MDA231 breast cancer cell migration and bone metastasis. Bone 50(4), 813–823 (2012).
Zhou, Z., Liu, R. & Chen, C. The WWP1 ubiquitin E3 ligase increases TRAIL resistance in breast cancer. Int. J. Cancer 130(7), 1504–1510 (2012).
Zhang, P. et al. Ubiquitin ligase CHIP regulates OTUD3 stability and suppresses tumour metastasis in lung cancer. Cell. Death Differ. 27(11), 3177–3195 (2020).
Bado, I. L. et al. The bone microenvironment increases phenotypic plasticity of ER(+) breast cancer cells. Dev. Cell 56(8), 1100–17e9 (2021).
Waks, A. G. & Winer, E. P. Breast cancer treatment: a review. JAMA 321(3), 288–300 (2019).
Hofbauer, L. C. et al. Novel approaches to target the microenvironment of bone metastasis. Nat. Rev. Clin. Oncol. 18(8), 488–505 (2021).
Satcher, R. L. & Zhang, X. H. Evolving cancer-niche interactions and therapeutic targets during bone metastasis. Nat. Rev. Cancer. 22(2), 85–101 (2022).
Coleman, R. E. et al. Bone metastases. Nat. Rev. Dis. Primers 6(1), 83 (2020).
Behera, A. & Reddy, A. B. M. WWP1 E3 ligase at the crossroads of health and disease. Cell. Death Dis. 14(12), 853 (2023).
Bernassola, F., Chillemi, G. & Melino, G. HECT-type E3 ubiquitin ligases in cancer. Trends Biochem. Sci. 44(12), 1057–1075 (2019).
Cruz Walma, D. A., Chen, Z., Bullock, A. N. & Yamada, K. M. Ubiquitin ligases: guardians of mammalian development. Nat. Rev. Mol. Cell. Biol. 23(5), 350–367 (2022).
Liang, Y., Zhang, H., Song, X. & Yang, Q. Metastatic heterogeneity of breast cancer: molecular mechanism and potential therapeutic targets. Semin. Cancer Biol. 60, 14–27 (2020).
Kfoury, Y. et al. Human prostate cancer bone metastases have an actionable immunosuppressive microenvironment. Cancer Cell 39(11), 1464–78e8 (2021).
Zheng, C. et al. PTEN in osteosarcoma: recent advances and the therapeutic potential. Biochim. Biophys. Acta Rev. Cancer. 1874(2), 188405 (2020).
Zhang, Z. et al. OTUB2 promotes cancer metastasis via Hippo-independent activation of YAP and TAZ. Mol. Cell 73(1), 7–21e7 (2019).
Zhang, W. et al. Bone metastasis initiation is coupled with bone remodeling through osteogenic differentiation of NG2+ cells. Cancer Discov. 13(2), 474–495 (2023).
Author information
Authors and Affiliations
Contributions
Conceptualization, Hao Jiang and Jianru Xiao; Methodology, Hao Jiang and Jianru Xiao; Software, Hao Jiang; Validation, Jianru Xiao; Formal Analysis, Hao jiang, Zhenxi Li, and Wei Xu; Investigation, Hao jiang, Zhenxi Li, and Wei Xu; Resources, Jianru Xiao; Data Curation, Hao Jiang; Writing – Original Draft Preparation, Hao jiang, Zhenxi Li, and Wei Xu; Writing – Review & Editing, Jianru Xiao; Visualization, Hao jiang; Supervision, Jianru Xiao; Project Administration, Jianru Xiao; Funding Acquisition, Jianru Xiao.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jiang, H., Li, Z., Xu, W. et al. WWP1 targeting PTEN for polyubiquitination to promote bone metastasis of luminal breast cancer. Sci Rep 14, 29950 (2024). https://doi.org/10.1038/s41598-024-81541-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-81541-5