Abstract
Chronic high-altitude hypoxia (CHH) induces irreversible abnormalities in various organisms. Emerging evidence indicates that CHH markedly suppresses bone mass and bone strength. Targeting senescent cells and the consequent senescence-associated secretory phenotype (SASP) with senolytics is a recently developed novel therapy for multiple age-related diseases. The combination of dasatinib and quercetin (DQ) has been proven to selectively target senescent cells and attenuate SASP in multiple tissues. In this study, experimental mice were subjected to an environment simulating 5,000 m above sea level for 8 weeks to induce CHH conditions. Our results indicated that DQ supplementation was well-tolerated with negligible toxicity. In vivo, DQ prevented reductions in BMD and BMC and improved bone microarchitecture against CHH-induced changes. Biomechanical testing demonstrated that DQ significantly improved the mechanical properties of femoral bones in CHH-exposed mice. Furthermore, DQ mitigated senescence in LepR + BMSCs and decreased the population of senescent cells, as evidenced by reduced senescence markers and SA-β-Gal staining. An analysis of serum and bone marrow aspirates showed that DQ treatment preserved angiogenic and osteogenic coupling in the bone marrow microenvironment by maintaining type H vessels and angiogenic growth factors. The results suggest that DQ has significant anti-senescence effects on BMSCs and a positive impact on the bone marrow microenvironment, supporting its clinical investigation as a therapeutic agent for CHH-related osteoporosis.
Similar content being viewed by others
Introduction
Sudden exposure to high altitudes is well-documented to induce acute mountain sickness, primarily driven by the decreased atmospheric partial pressure of oxygen and resulting in tissue hypoxia1,2,3. Moreover, chronic exposure to such hypoxia contributes to irreversible abnormalities across several systems like respiratory, cardiovascular, haematological, and neurological4,5. Among these, the impact on bone health, specifically bone deterioration due to high-altitude exposure, has increasingly commanded attention6. Evidence from recent studies suggests a notable decrease in bone mass and mechanical strength in rodents subjected to both acute and chronic high-altitude conditions, alongside aberrations in skeletal metabolism marked by escalated bone resorption and diminished bone formation activities7,8,9,10,11. Similarly, clinical studies have corroborated these findings in humans, revealing significant reductions in bone mineral density and bone-forming activity at high to extreme altitudes, with a challenging full recovery post-return to sea level6,12,13. Despite growing interest, the quest for effective and safe interventions against high-altitude-induced bone deterioration remains largely unfulfilled, underscoring an urgent need for novel therapeutic paradigms.
The phenomenon of cellular senescence denotes an inevitable fate for aging cells, characterized by an irreversible halt in proliferation, potent mitogenic signals, telomere shortening, DNA damage, and increased reactive oxygen species (ROS) production14,15. Senescent cells exhibit a senescence-associated secretory phenotype (SASP), inherently pro-inflammatory and contributing to various diseases14. Notably, the role of cellular senescence in osteoporosis16, previously underrecognized, has emerged as significant, with senescence hindering osteoblast progenitor cell activity, inhibiting bone formation, and enhancing osteoclastic bone resorption17,18,19,20. The targeted elimination of senescent cells through senolytic drugs or genetic interventions has shown promise in mitigating age-related osteoporosis21,22, suggesting a potential therapeutic intervention for osteoporosis treatment.
Under hypoxic conditions typical of high altitudes, the exacerbated oxidative stress and resultant ROS surge impact bone remodeling23. Awareness of hypoxia’s role in osteoporosis has steered drug development against oxidative stress as a strategic maneuver for treating high-altitude-induced osteoporosis24. Emerging studies link chronic hypoxia-hypobaria (CHH) with amplified cellular senescence, spotlighting a potential contributory mechanism to CHH-related osteoporosis23,25,26.
Dasatinib and quercetin (DQ), heralded as a pioneering senolytic treatment combo, has demonstrated efficacy in selectively targeting senescent cells (SnCs) and mitigating SASP across various tissues22,27,28,29. Confirmatory studies have underscored its effectiveness in combating a range of diseases, including diabetes, Alzheimer’s disease30, and osteoporosis22, as well as in enhancing bone regeneration31. Ongoing clinical feasibility trials and advancements in the development of other senolytic agents are contributing to a burgeoning field of research32,33. Evidence supporting DQ’s therapeutic benefits spans multiple age-related disorders, from type II diabetes34 to intervertebral disc degeneration28, atherosclerosis35, diabetic kidney disease36, and idiopathic pulmonary fibrosis37. Despite this, investigations into DQ’s efficacy in combating osteoporosis, specifically in cases of CHH are notably limited.
This study investigates the impact of DQ on CHH-related osteoporosis using a CHH animal model exposed to a commercial hypobaric hypoxia chamber. Our comprehensive analyses, including radiographic, histological, and molecular dimensions reveal that DQ has a significant influence in alleviating osteoporosis by targeting SnCs. This underscores DQ as a promising therapeutic option for CHH-related conditions.
Materials and methods
Animal model establishment and experimental design
Forty male C57BL/6 mice aged 20 weeks were acquired from the Jackson Laboratory (Beijing, China). The mice were housed at 23 ± 2 °C in a normal 12:12 light-dark cycle, in groups of four to five per cage. All animal experiments complied with the ARRIVE guidelines and were approved by the Pizhou Hospital’s Institutional Animal Care and Use Committee (ethics number: 2023-01-012). All animal studies were performed following the National Institutes of Health (NIH) guide for the Care and Use of Laboratory Animals.
The mice were divided into four groups: a normoxic control + Vehicle (Con + Veh, n = 10); a normoxic control + DQ supplementation (Con + DQ, n = 10); a hypobaric hypoxia group + Vehicle (CHH + Veh, n = 10); a hypobaric hypoxia group + DQ supplementation (CHH + DQ, n = 10). The rationale for the number of animals used in each treatment group is based on a power calculation derived from previous experiments. The Con group was maintained at the height of 30 m above sea level, replicating Pizhou city’s altitude. For the CHH-Veh and CHH + DQ groups, a commercial hypobaric chamber (#LATSY01, Liante Medical Equipment Co., Ltd, Suzhou, China) simulated 5,000 m above sea level for 22 h daily over eight weeks. The Con and CHH group mice were fed a standard AIN-93G Diet (Xietong Organism, Jiangsu, China). The CHH + DQ group received 5 mg/kg dasatinib and 50 mg/kg quercetin for the same duration38. DMSO (1%) was used as vehicle. At the end of the experiment, all animals were euthanized by a lethal dose of sodium pentobarbital (80 mg/kg BW), and euthanized by cervical dislocation.
DXA analysis
Post-euthanasia, mice bones were cleared of tissues, fixed in 4% formalin, and assessed ex-vivo for BMD and BMC using dual-energy X-ray absorptiometry (DXA, Lunar Corp., Madison, WI, USA), as described in previous literature39.
Serum biochemical analysis
Blood was sampled by cardiac puncture and centrifuged at 3,000 rpm at 4 °C for 15 min. Serum ALT, AST, BUN, Cr, EPO, ALP, P1NP, TRAcP-5b, CTX-1, IL-6, TNF-α, VEGF, and PDGF-BB were quantitated using ELISA kits according to the manufacturer’s (Jiancheng Biotech Co., Nanjing, China) protocol39.
Micro-computed tomography (µCT) scanning
The right femurs were preserved in 95% ethanol and scanned using the Skyscan 1276 system (Bruker BioSpin, Germany). Utilizing the 3D Creator software bundled with the SkyScan instrument, two-dimensional images were transformed into three-dimensional renderings. The software automatically computed several 3D indices, specifically: the ratio of bone volume to tissue volume (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and the structure model index (SMI), all within the secondary spongiosa region. Additionally, it calculated cortical volume (Ct.V) and cortical thickness (Ct.Th) in the mid-diaphysis.
Bone histomorphometry
Double labeling with Alizarin and Calcein 7 and 14 days before tissue harvest, respectively. Dynamic histomorphometry measurements, including mineral apposition rate and bone formation rates, were conducted on bones following micro-CT analysis. For static histomorphometry, tibias were subjected to HE, Von Kossa, ALP, TRAP, and toluidine blue staining, with quantification of osteoblasts and osteoclasts per bone surface39,40,41,42.
Immunohistochemistry
Immunohistochemical staining was carried out as described previously for osterix, osteocalcin (OCN), collagen-1(Col-1), RANKL, OPG, Leptin receptor (LepR), P16INK4a, IL-6, CD31, and Emcn39,40,41,42. All the antibodies were obtained from Abcam (Cambridge, MA, USA).
Three-point bending biomechanical assays
Left femurs were biomechanically tested using ElectroForce 3200 Series III (Bose, USA)39. Simultaneously, load–displacement curves were plotted and the associated data were automatically collected. These curves were used to obtain parameters such as maximum load (N), yield load (N), ultimate displacement (µm), yield displacement (µm), stiffness (N/mm), and energy absorption (N×mm).
Western blot analysis
Proteins extracted from left tibias were separated by SDS-PAGE followed by electroblotting onto PVDF membranes. The membranes were blotted with primary antibodies against Sclerostin (SOST), ALP, Collagen-1 (Col-1), Osteocalcin (OCN), osterix, Runx2, TRAP, NFATc1, calcitonin receptor (CTR), Cathepsin K, p16, p21, p53, Sirt1, BMI1, γH2A.X, and CD31. β-actin was used as a loading control. All the antibodies were obtained from Abcam (Cambridge, MA, USA). Immunoblotting was carried out as described previously39.
RNA extraction and real-time quantitative PCR (RT-qPCR) assay
Total RNA isolation and real-time PCR were conducted as described in our previous study39,40,41,42. Total RNA was isolated from right tibias was prepared using TRIzol Reagent (Thermo Fisher Scientific) followed by the RNeasy Mini Kit (Qiagen). The RNA was reversed transcribed into cDNA (Applied Biosystems) and real-time PCR analyses were performed using the StepOnePlus Real-Time PCR System (Thermo Fisher Scientific). The mRNA levels were calculated using the 2−ΔΔCt method (relative fold change). β-actin was amplified as an internal control.
Statistical analysis
All data were analyzed with GraphPad Prism version 8 and presented as means ± SD. The normal distribution analyses were adopted from the Kolmosprov–Smirnov test. Two-way ANOVA was used for statistical comparison and to make two-group comparison.
Results
DQ Supplementation shows no pharmacological toxicity
We investigated the effects of DQ supplementation on skeletal alterations under CHH, simulating a high-altitude environment at approximately 5,000 m for an 8-week duration. Using a previously established protocol shown to promote osteogenesis38, we aimed to prevent CHH-induced skeletal damage with DQ supplementation (Fig. 1A). Safety and toxicity of DQ were evaluated through a detailed examination of major organ systems in DQ-administered mice. Compared to the control group, no significant signs of organ toxicity or cellular damage were observed in DQ-treated mice (Fig. 1B). Hepatic and renal functions were assessed by measuring serum levels of liver enzymes (ALT, AST) and renal markers (BUN, creatinine), which remained within normal ranges in the DQ group (Fig. 1C-F). Both serum EPO levels and hematocrit (HCT) levels were significantly increased in the CHH + Veh group. DQ supplementation had no effect on either of these levels (Fig. 1G-H). Additionally, DQ supplementation had no impact on lung weight (Fig. 1I). These results support DQ’s safety and suggest minimal toxicity, reinforcing its potential as a therapeutic for CHH-related osteoporosis.
DQ supplementation demonstrates no pharmacological toxicity. (A) Schematic representation of the experimental workflow, detailing the CHH exposure and subsequent DQ treatment regimen. Mice were exposed to a simulated altitude of 5,000 m (22 h/day) in a hypobaric chamber and received DQ treatment for eight weeks. (B) Histological sections of major organs (liver, lung, cerebrum, kidney, heart, thymus, pancreas, and spleen) stained with hematoxylin and eosin in mice. (C-I) Biochemical analysis of serum levels of ALT, AST, BUN, creatinine, EPO, HCT, and lung weight in DQ-administered mice compared to controls. Data are presented as mean ± standard deviation (SD), with n = 10 per group. Asterisks indicate statistical significance relative to the reference groups, with p-values denoted as *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant.
DQ Supplementation mitigates reduction of BMD and BMC in CHH conditions
DQ’s impact on bone mass in CHH-exposed mice was quantified through DXA, which indicated significant reductions in tibial, femoral, spinal, and total body BMD and BMC following CHH exposure. DQ treatment effectively mitigated these reductions, suggesting its utility in combating CHH-induced osteoporosis (Fig. 2A).
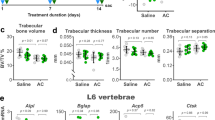
DQ supplementation ameliorates bone loss in CHH-exposed mice. (A) Quantification of BMD and BMC in the tibia, femur, spine, and total body using DXA. (B) Representative µCT images of the right femurs from different groups, accompanied by quantitative µCT analyses of bone parameters, including BV/TV, Tb.N, Tb.Th, Conn.D, Tb.Sp, SMI, Ct.Th, Ct.V, and Ct.Ar. (C) Evaluation of the mechanical properties of left femurs, including maximum load, yield load, ultimate displacement, yield displacement, energy absorption, and stiffness. Data are presented as mean ± SD, with n = 10 per group. Asterisks indicate statistical significance relative to the reference groups, with p-values denoted as *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Effect of DQ on bone microarchitecture and mechanical properties
Micro-computed tomography (µCT) of right femurs revealed trabecular and cortical bone deterioration after CHH exposure, including significant morphological alterations (Fig. 2B). DQ treatment resulted in significantly improved cancellous bone structure and increased cortical thickness, indicating a positive effect on bone architecture.
Biomechanical assessment using three-point bending tests on femora demonstrated a marked deterioration in bone mechanical properties post-CHH exposure. DQ supplementation significantly enhanced these mechanical properties, providing biomechanical evidence of its protective role against CHH-induced bone deterioration (Fig. 2C).
DQ enhances osteoblastic activity impaired by CHH
Histological analyses with toluidine blue indicated a notable decline in osteoblast numbers due to CHH (Fig. 3A). Furthermore, the staining with ALP and von Kossa highlighted a reduction in osteoid synthesis (Fig. 3B, C). Markers of osteoblastic differentiation and matrix production, including osterix (Osx), osteocalcin (Ocn), and collagen 1 (Col-1), showed decreased expression following CHH, which was confirmed by calcein/alizarin double labeling revealing reduced bone formation rates (Fig. 3D-G). CHH also led to a decrease in the serum ALP and P1NP levels compared with the Con + Veh group (Fig. 3H). Gene/protein expression analyses pointed to a significant downregulation of osteogenesis markers (ALP, Col1, Osx, OCN, and Runx2) post-CHH, while DQ supplementation nearly restored these markers (Fig. 3I-J). We also observed an increase in Sost expression in the chronic high altitude group, as depicted in Fig. 3J. Increased Sost expression has been shown to negatively regulate bone formation by inhibiting osteoblast differentiation and activity. This suggests that DQ promotes osteoblastic bone formation in CHH conditions.
DQ enhances osteoblastic activity impaired by CHH. Detailed imaging and quantitative analyses illustrate the effects of DQ on osteogenic differentiation and bone matrix production: (A) Toluidine blue staining, (B) alkaline phosphatase (ALP) activity, (C) Von Kossa staining, (D) Osterix (OSX) expression, (E) osteocalcin (OCN) levels, (F) collagen type 1 (Col-1) expression, and (G) dynamic bone labeling with calcein and alizarin on the trabecular bone surface of mouse tibiae. (H) Serum concentrations of ALP and P1NP measured by ELISA. (I) Relative mRNA levels of osteogenic markers (ALP, Col1a1, Osterix, OCN, and Runx2) in mid-diaphyseal cortical bone of the tibia, evaluated by quantitative RT-PCR. (J) Western blot analysis of sclerostin (SOST), ALP, Col-1, OCN, OSX, and RUNX2 protein expression in the tibia. Data are presented as mean ± SD, with n = 10 per group. Asterisks indicate statistical significance relative to the reference groups, with p-values denoted as *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DQ attenuates CHH-induced osteoclastogenesis
Tartrate-resistant acid phosphatase (TRAP) staining showed an increased osteoclast number in femur and skull following CHH. This increase was mitigated by DQ supplementation (Fig. 4A, B). IHC staining of OPG was markedly lower, while RANKL was significantly higher in the CHH-exposed mice than in the Control mice (Fig. 4C, D). Gene and protein expression analysis of osteoclast markers (NFATc1, calcitonin receptor, cathepsin K, and Trap) suggested elevated osteoclastogenesis under CHH, which was suppressed by DQ administration (Fig. 4E, F). Moreover, serum markers of bone resorption (TRAcP5b, CTX-I) increased with CHH exposure but were significantly reduced by DQ, indicating its inhibitory effect on bone resorption processes (Fig. 4G). Overall, our findings substantiate that DQ supplementation abrogates the detrimental skeletal effects of CHH, advocating its potential in treating osteoporosis provoked by chronic hypoxia.
DQ attenuates CHH-induced osteoclastogenesis. (A B) TRAP staining to detect and quantify osteoclasts on tibia and cranial bone. (C D) Immunohistochemical assessment of RANKL and OPG protein expression in tibial osteocytes. (E) mRNA expression levels of osteoclastogenesis-related genes determined by real-time RT-PCR. (F) Western blot quantification of osteoclastogenesis-related proteins, including TRAP, NFATc1, calcitonin, and cathepsin K in the tibia. (G) Serum concentrations of TRAP-5b and CTX-1 measured by ELISA. Data are presented as mean ± SD, with n = 10 per group. Asterisks indicate statistical significance relative to the reference groups, with p-values denoted as *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DQ inhibits cellular senescence induced by CHH
Previous studies have linked an increased presence of senescent cells (SnCs) in bone tissue with various forms of osteoporosis39,40. However, the role of SnCs in CHH-related osteoporosis remained uncertain. To investigate SnCs’ contribution to this condition, we analyzed SA-β-gal staining across different experimental groups. Notably, there was a significant rise in SA-β-gal + cell numbers at the long bone metaphysis in the femurs of CHH-Veh mice compared with controls (Fig. 5A, B). Conversely, immunostaining showed a marked reduction in SA-β-gal + cells in the DQ-treated mice, suggesting DQ’s prominent anti-senescence activity (Fig. 5A, B). Mesenchymal stromal cells (MSCs) signify a major source of colony-forming unit fibroblasts (CFU-F) and differentiate into bone, cartilage, and adipocytes upon transplantation43. The status of Leptin Receptor-positive (LepR+) BMSCs during CHH was less understood. Our double-labeling analysis for LepR and the senescence marker p16 revealed a significant increase in p16 + expressing LepR + cells in CHH-Veh group mice, indicating cellular senescence. DQ treatment effectively reduced this senescent cell population (Fig. 5A, B). Additionally, western blot analysis underscored that DQ significantly suppressed the expression of senescence markers p16, p21, p53, and γH2A.X, contrasting with their elevated levels in the CHH-Veh group (Fig. 5C). These findings underscore the pivotal role of DQ in mitigating senescence in LepR + MSCs, suggesting DQ’s senolytic capability in addressing CHH-related osteoporosis by clearing SnCs.
DQ inhibits cellular senescence induced by CHH. (A) SA-β-gal staining to identify senescent cells on the tibial trabecular surface. (B) Immunofluorescent staining for leptin receptor (LepR, red) and p16 (green) in the tibial bone matrix. (C) Western blot analysis of senescence-related markers (p16, p21, p53, Sirt1, BMI1, and γ-H2AX), with β-actin as a loading control. Data are presented as mean ± SD, with n = 10 per group. Asterisks indicate statistical significance relative to the reference groups, with p-values denoted as *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DQ counteracts SASP induced by CHH
SASP marker gene expression, including IL-1α, IL-1β, IL-6, IL-8, Mcp1, Mmp13, Activin A, TNFα, TGF-β1, NFκβ, CEBPβ, GATA4, and Pai-1, showed significant upregulation in CHH-Veh mice (Fig. 6A). Similarly, serum levels of pro-inflammatory cytokines IL-6 and TNF-α were significantly elevated in these mice (Fig. 6B). Immunochemistry for IL-6 revealed a marked increase in the CHH-Veh group compared to controls (Fig. 6C). DQ supplementation normalized these markers, demonstrating its efficacy in inhibiting cellular senescence and SASP in CHH conditions.
DQ counteracts SASP induced by CHH. (A) Quantitative RT-PCR to estimate mRNA levels of SASP-related genes. (B) Serum levels of inflammatory cytokines (IL-6, TNF-α) determined by ELISA. (C) Immunohistochemical staining of interleukin-6 (IL-6) in the tibia. Data are presented as mean ± SD, with n = 10 per group. Asterisks indicate statistical significance relative to the reference groups, with p-values denoted as *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DQ preserves angiogenesis-osteogenesis coupling in bone marrow microenvironment
Angiogenesis closely ties with skeletal development, particularly through type H vessels (CD31hiEmcnhi), which are integral to osteogenesis44. Our analysis showed a significant reduction in CD31 + Emcn + type H vessels in the CHH-Veh group compared with controls, a trend reversed by DQ treatment (Fig. 7A). Given the role of Endomucin in vascular and stem cells, we also conducted a western blot for CD31 in bone marrow aspirates, confirming a significant reduction in type H vessels under CHH, reversed by DQ (Fig. 7B). Analysis of angiogenic growth factors, including VEGF and PDGF-BB, in serum and bone marrow (BM), revealed a deficiency in CHH-Veh mice, which was mitigated by DQ treatment (Fig. 7C). Thus, DQ administration over 8 weeks notably curbed CHH-induced vascular and osteogenic disruption by preserving angiogenic growth factors and type H vasculature.
DQ alleviates the suppression of type H vessel formation in CHH-exposed mice. (A) Immunofluorescence staining for Endomucin (Emcn, green) and CD31 (red). Quantitative analysis of the type H vessel (CD31hiEmcnhi, yellow) area proximal to the femoral growth plate across the examined groups. (B) Quantification of vascular endothelial growth factor (VEGF) and platelet-derived growth factor BB (PDGF-BB) in serum and bone marrow by ELISA. (C) Western blot analysis of CD31 in the tibia, with β-actin as a loading control. Data are presented as mean ± SD, with n = 10 per group. Asterisks indicate statistical significance relative to the reference groups, with p-values denoted as *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Discussion
The link between high-altitude exposure and decreased BMD and bone formation rates is well-established in humans6,12,13. Yet, effective strategies to mitigate such skeletal degeneration remain under-researched. In this study, we report that DQ supplementation effectively ameliorates cancellous and cortical bone deterioration in mice exposed to CHH at a simulated altitude of 5,000 m. Our data illustrate that this protective effect results from a dual mechanism: the inhibition of osteoclastic activity and enhancement of osteoblastic bone formation. This study also explored the therapeutic potential of DQ supplementation in mitigating the effects of CHH-induced osteoporosis, with a focus on its senolytic action and modulation of the bone marrow microenvironment. Our findings revealed that DQ not only enhances BMD and architecture but also exerts substantial anti-senescence effects, particularly in LepR + BMSCs, while simultaneously maintaining angiogenesis-osteogenesis coupling. These outcomes suggest a multifaceted role of DQ in preserving bone health under hypoxic conditions.
The observed reinforcement of bone density and structural integrity by DQ is particularly noteworthy. Previous studies have established a strong link between hypoxia and the deterioration of bone quality, primarily through the suppression of osteoblastic activity and the promotion of osteoclastogenesis7,8,9,10,11. In contrast, our research demonstrates DQ’s capacity to counteract these adverse effects, which is in line with recent findings highlighting the protective role of senolytics in bone health22. Unlike traditional approaches that primarily focus on enhancing osteoblast activity or suppressing osteoclast-driven resorption, DQ appears to offer a dual protective mechanism. This is likely due to its ability to mitigate cellular senescence and promote angiogenesis, as suggested by recent findings on senolytic therapies in bone health31,45,46,47. More crucial, our investigation unveils the capacity of DQ to not only reverse this osseous diminution but also restore whole-bone mechanical properties, providing robust evidence for the therapeutic efficacy of senolytics in combating CHH-induced bone loss.
A pivotal aspect of our research centers on the role of cellular senescence in skeletal aging and bone remodeling regulation. Therapeutic elimination and SASP suppression are emerging as promising strategies for managing both physiological bone aging and pathological conditions like osteoporosis. Genetic tactics, such as the removal of p16Ink4a, have been shown to benefically impact longevity and age-related osteoporotic changes22,48. Similarly, pharmacological clearance via senolytics has restored bone architecture in aging22 and radiation-exposure models47. Our observations of decreased SA-β-Gal + cells and the downregulation of senescence markers (p16, p21, p53, γH2A.X) in DQ-treated mice afford compelling evidence of the drug’s senolytic potential. This conclusion is bolstered by the observed reduction in SASP components, which are critical mediators of the deleterious impacts of SnCs49.
The implications of mesenchymal stem cell senescence, particularly in leptin receptor-positive (LepR+) cells, are significant for bone homeostasis. LepR + MSCs are essential for bone and adipocyte formation; therefore, their senescence poses a substantial risk to the skeletal system50,51,52,53,54,55,56,57,58. This research has observed an accumulation of senescent LepR + MSCs in the bone marrow of CHH mice, an effect that was significantly impeded by DQ supplementation. The present study marks a foundational step in delineating the role senescent BMSCs and SnCs play in high-altitude osteoporosis. We have demonstrated for the first time that DQ treatment effectively reduces the senescent phenotype in MSCs and SnCs within the bone microenvironment of CHH mice, thereby attenuating bone loss.
Furthermore, our investigation into DQ’s role in sustaining angiogenesis-osteogenesis coupling adds a critical dimension to understanding bone health in hypoxic conditions. The maintenance of type H vessels, closely associated with osteogenesis, is vital for bone formation and repair59. We found that CHH exposure led to a reduction in these structures, thus impacting bone formation. Importantly, our study revealed that DQ treatment effectively restored the proportion of H-type vessels in the femoral metaphysis of CHH mice, underlining its role in improving impaired angiogenesis and consequently bone health44,60,61.
Throughout our investigations, DQ has displayed a notable safety and biocompatibility profile, which is essential for translating research from bench to bedside. This promising safety profile, coupled with the observed therapeutic effects, positions DQ as a notable candidate for osteoporosis treatment.
In conclusion, our work provides the first evidence that DQ supplementation acts as a preventive measure against bone loss induced by chronic high-altitude exposure, with mechanisms possibly attributed to the alleviation of SnC accumulation, rescue from MSC senescence, reduction of SASP factors, and subsequent promotion of bone-forming activities. This study not only furthers our knowledge of bone physiology under the unique stress of high altitudes but also proposes a compelling therapeutic role for DQ in addressing CHH-induced skeletal deterioration, potentially offering a new horizon for osteoporosis treatments.
Data availability
The data during the current study are available from the corresponding author, Q.G., upon reasonable request.
References
Luks, A. M., Swenson, E. R. & Bartsch, P. Acute high-altitude sickness. Eur. Respiratory Rev. 26(143) (2017).
Paralikar, S. J. & Paralikar, J. H. High-altitude medicine. Indian J. Occup. Environ. Med. 14(1), 6–12 (2010).
Imray, C. et al. Acute mountain sickness: Pathophysiology, prevention, and treatment. Prog. Cardiovasc. Dis. 52(6), 467–484 (2010).
Zepeda, A. B. et al. Cellular and molecular mechanisms in the hypoxic tissue: Role of HIF-1 and ROS. Cell Biochem. Funct. 31(6), 451–459 (2013).
Farias, J. G. et al. Acclimatization to chronic intermittent hypoxia in mine workers: A challenge to mountain medicine in Chile. Biol. Res. 46(1), 59–67 (2013).
Basu, M. et al. Alterations in different indices of skeletal health after prolonged residency at high altitude. High. Alt. Med. Biol. 15(2), 170–175 (2014).
Wang, X. M. et al. Rosamultin attenuates acute hypobaric hypoxia-induced bone injuries by regulation of sclerostin and its downstream signals. High Altitude Medicine & Biology 21(3), 273–286 (2020)..
Brent, M. B. et al. Hypobaric hypoxia deteriorates bone mass and strength in mice. Bone 154, 116203 (2022).
Wang, W. et al. The hypobaric hypoxia environment impairs bone strength and quality in rats. Int. J. Clin. Exp. Med. 10(6), 9397–9406 (2017).
Bozzini, C. et al. Structural and material mechanical quality of femoral shafts in rats exposed to simulated high altitude from infancy to adulthood. High. Alt. Med. Biol. 17(1), 50–53 (2016).
Bozzini, C. et al. Static biomechanics in bone from growing rats exposed chronically to simulated high altitudes. High. Alt. Med. Biol. 14(4), 367–374 (2013).
O’Brien, K. A. et al. Human physiological and metabolic responses to an attempted winter crossing of Antarctica: The effects of prolonged hypobaric hypoxia. Physiological Rep. 6(5) (2018).
Basu, M. et al. Determination of bone mass using multisite quantitative ultrasound and biochemical markers of bone turnover during residency at extreme altitude: A longitudinal study. High Altitude Medicine & Biology 14(2), 150–154 (2013).
Di Micco, R. et al. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell. Biol. 22(2), 75–95 (2021).
Tchkonia, T. et al. Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. J. Clin. Invest. 123(3), 966–972 (2013).
Battmann, A., Schulz, A. & Stahl, U. Cellular senescence: A mechanism of the development of osteoporosis?. Orthopade 30(7), 405–411 (2001).
Kassem, M. & Marie, P. J. Senescence-associated intrinsic mechanisms of osteoblast dysfunctions. Aging Cell. 10(2), 191–197 (2011).
Marie, P. J. Bone cell senescence: Mechanisms and perspectives. J. Bone Min. Res. 29(6), 1311–1321 (2014).
Chen, Q. et al. DNA damage drives accelerated bone aging via an NF-κB-dependent mechanism. J. Bone Min. Res. 28(5), 1214–1228 (2013).
Krishnamurthy, J. et al. Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 114(9), 1299–1307 (2004).
Farr, J. N. et al. Identification of senescent cells in the bone microenvironment. J. Bone Min. Res. 31(11), 1920–1929 (2016).
Farr, J. N. et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 23(9), 1072–1079 (2017).
Dosek, A. et al. High altitude and oxidative stress. Respir Physiol. Neurobiol. 158(2–3), 128–131 (2007).
Yan, C. et al. Resveratrol ameliorates high Altitude Hypoxia-Induced osteoporosis by suppressing the ROS/HIF signaling pathway. Molecules 27(17) (2022).
Srivastava, S. et al. Insight into the role of myokines and myogenic regulatory factors under hypobaric hypoxia induced skeletal muscle loss. Biomarkers 27(8), 753–763 (2022).
Pasha, Q. et al. The Telomere-Telomerase system is detrimental to health at high-altitude. Int. J. Environ. Res. Public. Health 20(3) (2023).
Palmer, A. K. et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell. 18(3), e12950 (2019).
Novais, E. J. et al. Long-term treatment with senolytic drugs Dasatinib and Quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat. Commun. 12(1), 5213 (2021).
Zhu, Y. et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell. 14(4), 644–658 (2015).
Zhang, P. et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat. Neurosci. 22(5), 719–728 (2019).
Honda, Y. et al. Augmentation of bone regeneration by depletion of stress-Induced senescent cells using catechin and senolytics. Int. J. Mol. Sci. 21(12) (2020).
Kirkland, J. L. & Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 288(5), 518–536 (2020).
Partridge, L., Fuentealba, M. & Kennedy, B. K. The quest to slow ageing through drug discovery. Nat. Rev. Drug Discov 19(8), 513–532 (2020).
Wang, L. et al. Targeting p21(Cip1) highly expressing cells in adipose tissue alleviates insulin resistance in obesity. Cell. Metab. 34(1), 186 (2022).
Roos, C. M. et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 15(5), 973–977 (2016).
Hickson, L. J. et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 47, 446–456 (2019).
Justice, J. N. et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 40, 554–563 (2019).
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24(8), 1246–1256 (2018).
Geng, Q. et al. Astaxanthin Attenuates irradiation-induced Osteoporosis in mice by Inhibiting Oxidative Stress, Osteocyte Senescence, and SASP(Food Funct, 2022).
Geng, Q. et al. Pyrroloquinoline Quinone prevents estrogen deficiency-induced osteoporosis by inhibiting oxidative stress and Osteocyte Senescence. Int. J. Biol. Sci. 15(1), 58–68 (2019).
Geng, Q. et al. A soluble bone morphogenetic protein type 1A receptor fusion protein treatment prevents glucocorticoid-Induced bone loss in mice. Am. J. Transl Res. 11(7), 4232–4247 (2019).
Wang, S. et al. Treatment with soluble bone morphogenetic protein type 1A receptor fusion protein alleviates irradiation-induced bone loss in mice through increased bone formation and reduced bone resorption. Am. J. Transl Res. 12(3), 743–757 (2020).
Zhou, B. O. et al. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell. Stem Cell. 15(2), 154–168 (2014).
Xie, H. et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat. Med. 20(11), 1270–1278 (2014).
Wang, X. et al. Enhancement of bone-forming ability on Beta-tricalcium phosphate by modulating Cellular Senescence mechanisms using senolytics. Int. J. Mol. Sci. 22(22) (2021).
Liu, J. et al. Age-associated callus senescent cells produce TGF-β1 that inhibits fracture healing in aged mice. J. Clin. Invest. 132(8) (2022).
Chandra, A. et al. Targeted reduction of senescent cell Burden alleviates focal Radiotherapy-related bone loss. J. Bone Min. Res. 35(6), 1119–1131 (2020).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479(7372), 232–236 (2011).
Coppé, J. P. et al. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
Kfoury, Y. & Scadden, D. T. Mesenchymal cell contributions to the stem cell niche. Cell. Stem Cell. 16(3), 239–253 (2015).
Bonyadi, M. et al. Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice. Proc. Natl. Acad. Sci. U S A 100(10), 5840–5845 (2003).
Li, H. et al. FOXP1 controls mesenchymal stem cell commitment and senescence during skeletal aging. J. Clin. Invest. 127(4), 1241–1253 (2017).
Rosen, C. J. et al. Marrow fat and the bone microenvironment: Developmental, functional, and pathological implications. Crit. Rev. Eukaryot. Gene Expr 19(2), 109–124 (2009).
Chen, Q. et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell. Death Differ. 23(7), 1128–1139 (2016).
Su, J. et al. Cellular senescence mediates the detrimental effect of prenatal dexamethasone exposure on postnatal long bone growth in mouse offspring. Stem Cell. Res. Ther. 11(1), 270 (2020).
Tencerova, M. et al. Obesity-associated hypermetabolism and accelerated senescence of bone marrow stromal stem cells suggest a potential mechanism for bone fragility. Cell. Rep. 27(7), 2050–2062.e6 (2019).
Gao, B. et al. Local delivery of tetramethylpyrazine eliminates the senescent phenotype of bone marrow mesenchymal stromal cells and creates an anti-inflammatory and angiogenic environment in aging mice. Aging Cell. 17(3), e12741 (2018).
Wang, T. et al. Targeting cellular senescence prevents glucocorticoid-induced bone loss through modulation of the DPP4-GLP-1 axis. Signal. Transduct. Target. Ther. 6(1), 143 (2021).
Short, S. et al. Senolytics and senostatics as adjuvant tumour therapy. EBioMedicine 41, 683–692 (2019).
Kusumbe, A. P., Ramasamy, S. K. & Adams, R. H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507(7492), 323–328 (2014).
Ramasamy, S. K. et al. Endothelial notch activity promotes angiogenesis and osteogenesis in bone. Nature 507(7492), 376–380 (2014).
Acknowledgements
This study was supported by the Postdoctoral Research Foundation of China (Grant No. 2020M681739), the Research Funding Project of the Jiangsu Commission of Health (Grant No. Z2021046, LKM2022048), the Research Funding Project of Xuzhou Medical University (Grant No. XZSYSKF2020004), the Xuzhou Institute of Technology (Grant No. KC22273), Health Commission of Changzhou City (Grant No. ZD202340), and Research project of Qinghai Provincial Health Commission (Grant No.2023-wjzdx-106).
Author information
Authors and Affiliations
Contributions
Q.G. and Y.G. wrote the manuscript; Y.G. revised the manuscript; Y.G. conceived and designed the study; Q.G., S.W., J.Z., K.H., L.S., X.S., C.D., H.Z., J.L., F.T., J.H., G.W., Y.G., R.G., Q.L., X.N., K.X., Q.W., W.H., H.Z., Y.Y., J.L., D.Y., and Y.L performed the study and collected the data; Q.G. and Y.G. analyzed the data. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, S., Zhai, J., Heng, K. et al. Senolytic cocktail dasatinib and quercetin attenuates chronic high altitude hypoxia associated bone loss in mice. Sci Rep 14, 30417 (2024). https://doi.org/10.1038/s41598-024-82262-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-82262-5