Abstract
Undescended testis and testicular torsion represent two frequent andrological diseases that affect the pediatric age. Despite these testicular disorders having different causes, they both negatively influence fertility in adulthood mainly due to the accumulation of reactive oxygen species (ROS), which represents the primary molecular damage underlying their long-term effects. The gold standard of treatment for both pathologies is surgery; however, it cannot guarantee an optimal fertility outcome in all clinical cases, underscoring the need to identify effective adjuvant therapies that may target the augmented ROS levels. For this aim, we investigated the pro-proliferative and anti-oxidant effects of PRP (platelet-rich plasma), a hemoderivative product used in regenerative medicine. We confirmed the increased oxidative status in testicular tissue by directly analyzing patients’ biopsies with mass spectrometry and highlighting that three antioxidant proteins are significantly overexpressed compared to healthy testicles. Afterward, we in vitro treated cells derived from patients with cryptorchidism or testicular torsion with PRP, showing that it consistently decreases ROS levels and slightly induces cell proliferation. This study supports the potential use of PRP in patients with testis torsion or cryptorchidism, encouraging its future clinical application as adjuvant therapy to preserve the functionality of this organ by decreasing its ROS levels.
Similar content being viewed by others
Introduction
Many andrological diseases that affect fertility in adulthood originate during childhood, and prompt evaluation and treatment are essential to maximize the testicular functionality and preserve the fertility potential of patients. Two of these andrological diseases of the pediatric age are noteworthy: testicular torsion and cryptorchidism.
Testicular torsion is a frequent pediatric urologic emergency, with an estimated incidence of 3.8 cases per 100,000 males under 18 years and a mean onset age of 10.6 ± 5.8 years1, with a bimodal distribution presenting a peak at neonatal age and a peak at pubertal age2. The causes of testis torsion are different and may be generally associated with trauma, testicular neoplasms3,4, physical activity, and sexual activity. As the testis rotates on itself, venous blood flow stops, leading to stasis and congestion. The testis becomes erythematous and edematous; further progression of torsion results in reduced arterial blood supply, leading to ischemia and subsequent necrosis of the affected tissue. The principal model explaining testicular damage during torsion is the ischemia-reperfusion model: during the first phase of torsion, testicular cells receive an ischemic-type insult, given by the reduction in arterial perfusion, while in the reperfusion phase the damage would be mediated by the accumulation of reactive oxygen species (ROS) and inflammatory mediators. ROS are mainly generated due to the oxygen supply that occurs with the reperfusion phase that follows testis detorsion. In experimental mouse models, it has been shown how alterations in mitochondrial energy production with reperfusion can increase ROS production5, which can damage DNA, endothelium, and germ cells6, giving rise to reduced fertility7,8. The only effective treatment for testicular torsion is represented by surgery, and no adjuvant therapeutic approaches are envisaged. However, we previously showed that by using an in vitro cellular model of primary cells derived from biopsies of patients affected by testis torsion, the adjuvant therapy with human chorionic gonadotropin (hCG) supports cell proliferation, suggesting the potential use of this hormone to improve testicular function also for this pathological condition9.
Cryptorchidism is manifested by the absence of one or both testes in the scrotal sac at birth. Several retrospective studies have identified a prevalence of cryptorchidism in term-born infants between 1.6% and 9%, with an incidence among preterm births as high as 45%10. The descent of the testis occurs in two stages11, with the gubernaculum testis as a key actor. The surgical correction should preferably occur before 12 months or, at the latest, within 18 months since histological examinations of testes from patients of this age have found a decline in germ cell and Leydig cell number12. The effects of cryptorchidism on fertility potential, including a loss of germ cells13, have been reported in numerous articles. To limit damages to the retained testicle, a prompt orchidopexy is crucial. The retainment of the testicle has been shown to increase oxidative stress, which results in increased germ cell apoptosis and hypospermatogenesis14. The main mechanism proposed to explain this event is the increased temperature to which patients’ testes are subjected as long as they remain retained15. At the same time, increased temperature has been associated with reduced function of two antioxidant enzymes, superoxide dismutase (SOD) and catalase15. In addition, Chaki et al. also demonstrated a correlation between oxidative stress in testicular cells of patients with cryptorchidism and reduced testosterone levels16.
PRP, which stands for platelet-rich plasma, is a hemoderived biological product composed of plasma with a high concentration of platelets. Starting from the patient’s blood samples, separating plasma components by density gradient through centrifugation is possible, thus obtaining erythrocytes, PRP, and PPP (platelet-poor plasma). An increase in platelet concentration, growth factors, chemokines, and cytokines is appreciable within PRP. The release of biologically active molecules causes amplification of inflammatory processes, and the fusion of granule vesicles with the cytoplasmic membrane increases the surface area of platelets, improving their effectiveness. Interaction with other immune cells determines the action properties of platelets, and, in particular, interaction with neutrophils regulates the release of ROS and myeloperoxidase and the formation of NETs (neutrophil extracellular traps)17. These findings have justified PRP use as an agent that promotes tissue regeneration and as an immunomodulatory agent18, expanding its utilization from hematology to regenerative medicine and proving popular in orthopedic, dental, dermatological, and aesthetic medicine19.
Due to the safety of using autologous PRP and its broad-spectrum functions, this paper aimed to investigate in vitro the effect of PRP treatment on primary cells derived from pediatric patients affected by testis torsion or cryptorchidism, two pathologies that are characterized by high ROS content, setting the bases for its future application after surgery also in this field.
Results
Testicular torsion
Proteomics reveals an increase in antioxidant protein expression in testicular biopsies of the necrotic rotated testicles
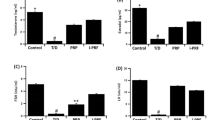
Clinical resolution of testicular torsion requires the surgical derotation of the spermatic cord, which leads to a sudden blood supply to the ischemic testis. Different papers have demonstrated that this ischemic-reperfusion process enhances ROS levels20,21, which could damage cell functionality. As a further confirmation of the induced oxidative stress in testicular cells after the torsion-derotation procedure, we have analyzed the proteome of testicular tissues derived from four individuals: two affected by testicular torsion, both presenting a necrotic testicle (#12 and #13), and two who underwent surgery due to suspected torsion but whose testes were correctly positioned (#61 and #68). It is noteworthy that testicular biopsies of patients #12 and #13 were taken half an hour after surgical de-rotation, suggesting that the ischemic/reperfusion process has taken place. From the output dataset obtained from the proteomic analysis, we focused our attention on the expression levels of antioxidant proteins, and we selected those proteins that followed the statistical and inclusion criteria, as reported in materials and methods. After this analysis, three identified antioxidant proteins were highly expressed in the testicular tissue of patients with torsion compared to control ones, which aligns with the hypothesized ROS enhancement. In Fig. 1A, we reported the expression levels of catalase (CAT), a crucial enzyme that converts hydrogen peroxide into water and oxygen22, and of the two subunits of glutamate-cysteine ligase (the regulatory, M, and the catalytic, C) that is the first enzyme involved in the synthesis of glutathione (GSH) one of the major antioxidant molecule within the cells23. The respective protein levels analyzed through proteomics are 3.95, 12.26, and 100-fold higher in rotated testicles than controls. According to the literature, the strong expression of these proteins supports the involvement of ROS in oxidative damage after surgery, highlighting the necessity to consider this event as detrimental to the well-being and correct function of the testicle.
In vitro model for the study of testis torsion. (A) Fold change of three antioxidant protein levels identified by proteomic analysis of testicular tissues from patients with testis torsion versus testicular tissue from healthy individuals. Catalase (CAT); Glutamate-cysteine ligase regulatory subunit (GCLM); Glutamate-cysteine ligase catalytic subunit (GCLC). (B) Representative bright-field images of gubernacular cells from patient #4 and testicular cells from patient #NR1 during in vitro cell culture. Zoom 10x; scale bar: 100 μm. (C) Fold change of ROS levels is the average of the values analyzed in two patient-derived cells (#4 and #NR1) grown in hypoxia (1% O2) for 6 h and then left in normoxia (atmospheric O2) for 24 h, compared to the same cell types cultured for 24 h in standard condition. Values are the means (± SE) of at least three independent biological replicates. Statistical analysis: * p < 0.05; *** p < 0.001.
Hypoxic/normoxic cell cultures mimic the ischemic-reperfusion phenomenon in vitro
Based on the strong evidence of the involvement of ROS in testis damaged by torsion, we aimed to study cells directly in vitro. For this aim, we included two more patients (#4 and #NR1, both affected by testicular torsion) since they presented the inclusion criteria. In detail, during surgery #NR1 presented a necrotic testicle due to excessive torsion of the spermatic cord and a no-accessible gubernaculum, while on the other hand, in patient #4 the gubernaculum testis was easily accessible; for these reasons, we chose to take a testicular biopsy, instead of gubernaculum, for patient #NR1. Since we previously demonstrated that gubernaculum testis shares many properties with testicular tissue9, we considered both tissues as similar. In both cases, primary cells derived from the respective biopsies proliferated in vitro, giving rise to a mixed population of cells (Fig. 1B). To mimic the in vitro ischemic condition, corresponding to a reduced blood supply and oxygen, we cultured cells in hypoxia (i.e., 37 °C, 5% CO2, and 1% O2) for 6 h; then, to mimic the reperfusion condition, we placed cells back in normoxia (i.e., 37 °C, 5% CO2, and atmospheric O2 concentration) for 24 h (this procedure has been called “hypoxia/normoxia switch”). Our lab has optimized this protocol by testing different hypoxia conditions that, at the same time, maintain good cell viability. To confirm that this procedure mimics the ROS induction manifested in the patient’s testis during the ischemic/reperfusion process, we analyzed ROS levels in living cells derived from the two patients by comparing standard culture conditions, which correspond to atmospheric O2 concentration, with the hypoxia/normoxia switch. Our data show that hypoxia/normoxia switch induces a significant ROS level increase of about 1.4-fold compared to atmospheric oxygen standard concentration (Fig. 1C), thus supporting the consistency of our procedure to mimic in vitro the ischemic/reperfusion event on a ROS level side.
PRP has a diverse effect on cell proliferation and ROS content of the two patients’ derived cells
To study in vitro PRP’s effect, we analyzed cell proliferation in standard and in hypoxia/normoxia conditions, as follows: cells were treated for 24 h with two concentrations of PRP, i.e., 500 and 1000 platelets per µl. It is noteworthy that we also tested higher dosages of PRP, but technical issues occurred, such as the aggregations of its corpuscular components followed by jellification of the medium with the consequence of incorrect gas exchange and cell death. In addition, other authors have tested the in vitro effects of the same PRP treatment duration24,25. Globally, our data underline that cells from the two patients with testis torsion responded differently to the same treatments. Indeed, patient #4 presented a significant induction of cell proliferation with both PRP doses only in hypoxia/normoxia switch, whereas patient #NR1 showed a cell proliferation increase only when treated with 500 platelets/µl in standard conditions (Fig. 2A,B). On the other side, the analysis of ROS content performed with the DCF probe showed that only patient #NR1 presented significantly reduced ROS levels, about 0.5-fold lower, and only when treated with 1000 platelets/µl both in standard and hypoxia/normoxia switch (Fig. 3A,B).
Effect of PRP on the proliferation of cells derived from patients with testicular torsion. Analysis of the effect of PRP on the proliferation of cells derived from two patients with testis torsion (#4 and #NR1) in (A) standard condition (24 h treatment in atmospheric O2) and (B) hypoxic/normoxic condition (6 h treatment with 1% O2 followed by 24 h treatment in atmospheric O2). Histogram legend: white, untreated cells (control); light grey, cells treated with 500 platelets/µl; dark grey, cells treated with 1000 platelets/µl. Values are the means (± SE) of at least three independent biological replicates. Statistical significance is reported as * p < 0.05. “plt” stands for platelets.
Antioxidant effect of PRP on cells derived from patients with testicular torsion. Analysis of the effect of PRP on the analysis of ROS content in cells derived from two patients with testis torsion (#4 and #NR1) in (A) standard condition (24 h treatment in atmospheric O2) and (B) hypoxic/normoxic condition (6 h treatment with 1% O2 followed by 24 h treatment in atmospheric O2). Histogram legend: white, untreated cells (control); light grey, cells treated with 500 platelets/µl; dark grey, cells treated with 1000 platelets/µl. Values are the means (± SE) of at least three independent biological replicates. Statistical significance is reported as * p < 0.05, and ** p < 0.01. “plt” stands for platelets.
Cryptorchidism
Effects of PRP on the proliferation of cells derived from cryptorchid patients
In vitro analyses of PRP effects on cryptorchid patients have been conducted on gubernaculum testis primary cell culture. We chose this experimental model over testicular tissue because, in those patients, retained testicles are generally hypotrophyc due to their permanence in the abdomen or inguinal canal, thus leaving them surgically untouched is the best way to preserve their functionality, if any.
Thus, we cultured in vitro gubernacular biopsies obtained from 4 cryptorchid patients, i.e. patients #2, #20, #94, and #99. Cells from all these patients grew and expanded in vitro (Fig. 4), presenting the typical heterogeneous morphology of this tissue composed of fibroblastic and myofibroblastic cells9. To analyze the effect of PRP on these patients’ derived tissues, we only cultured them in normoxic conditions because the ischemic-reperfusion process is not involved in cryptorchidism pathophysiology. In contrast, the increased ROS levels in gonadal tissues have been suggested to originate from the higher abdomen temperature compared to the physiologic scrotum temperature at 32 °C15. Regarding the effect of PRP on the stimulation of cell proliferation, we show that only one patient (#94) presented a statistically significant induction at 1000 platelets/µl, whereas it is ineffective for the others (Fig. 5A). Regarding ROS levels, two patients (#2 and #20) presented a significant decrease in total intracellular ROS content with both PRP concentrations, with patient #2 presenting less than half of these levels (Fig. 5B), suggesting a healthier state of the cells. Interestingly, patient #94 responded to the PRP anti-oxidant property only at the highest concentration, with a slight but significant decrease in ROS amount. Ultimately, PRP treatment does not affect ROS levels in patient #99 (Fig. 5B), opening the way to further investigations about the reason for this diverse cellular response.
Effects of PRP on proliferation and oxidative status of cells derived from patients with cryptorchidism. Analysis of the effect of PRP on the (A) proliferation rate and (B) ROS content of cells derived from 4 patients with cryptorchidism. Histogram legend: white, untreated cells (control); light grey, cells treated with 500 platelets/µl; dark grey, cells treated with 1000 platelets/µl. Values are the means (± SE) of at least three independent biological replicates. Statistical significance is reported as * p < 0.05, and ** p < 0.01. “plt” stands for platelets.
Discussion
Testicular torsion and cryptorchidism are two conditions that can majorly impact the fertility potential of patients. In the therapeutic management of both pathologies, the gold standard remains surgery. In many cases, however, surgery alone does not prevent the functional deficit of gonadal cells, with effects on fertility only manifested during adulthood26. PRP is a hemoderivative product with antioxidant and immunomodulatory properties. It has long been used in regenerative medicine, a branch that replaces and regenerates damaged cells, tissues, and organs to restore their physiological function27. Although the literature is replete with studies on the antioxidant effects of PRP, the molecular characterization of these mechanisms has not been explored in depth, with only a few papers advancing an explanation regarding its role in oxidative stress. According to our proteomics data, it has been reported that factors released from platelet granules can activate the transcription factor NRF2, which is implicated in the antioxidant response through increased expression of specific ROS detoxifying genes, including those involved in glutathione oxidation28. Hence, in our study, the analysis of testicular tissue derived from patients with necrotic testis torsion showed elevated levels of expression of a protein that regulates glutathione synthesis, glutamate-cysteine ligase (GCL), on both the regulatory and the catalytic subunits, making reduced glutathione more available as a substrate for PRP-stimulated antioxidant actions. Altogether, these observations show the key antioxidant role of PRP, opening the way to its use for different pathologies characterized by oxidative stress.
The purpose of our study was to investigate whether PRP treatment in vitro could limit the exposure of testicular cells to ROS, decreasing oxidative stress caused by (i) the ischemic-reperfusion process in patients with testicular torsion and by (ii) the high temperature of the retained testicle in patients with cryptorchidism, correlating with gonadal cell dysfunction29. Confirming in vitro the antioxidant potential of PRP justifies its future use as a potential adjuvant treatment in clinical practice. For this scope, we initially aimed to study testicular torsion in vitro by mimicking a state of ischemia/reperfusion through the exposure of the cells to a low oxygen level for 6 h, followed by culturing cells in standard conditions for 24 h. Indeed, through this protocol, we detected a significant increase in basal intracellular ROS levels, supporting the consistency of our experimental model. Afterward, we studied the effect of PRP treatment on cell proliferation and ROS content in cells derived from patients with testicular torsion, demonstrating that the effect may be patient-dependent. Indeed, in in vitro standard conditions, which correlate with a state of low damage/torsion due to constant atmospheric oxygen concentration, PRP presents a limited effect on cell proliferation at both dosages, whereas its antioxidant properties are evident only in cells from pt #NR1 at the highest concentration.
Conversely, the ischemic/reperfusion culture model represented a condition that better emphasizes the PRP effects since the proliferation of cells from pt #4 is enhanced at both PRP concentrations. However, the ROS levels of this patient’s cells are not altered, probably due to a less detrimental state of his cells than those of pt #NR1. Indeed, patient #4 had a clinical presentation of moderate severity of testis torsion, with a viable testis after derotation. In this sense, his cells lack the antioxidative effect of PRP, probably reflecting the modest damage the testicular cells suffered. Concerning pt #NR1, his cell proliferation is not incremented by PRP, probably due to the incubation time of 24 h, which could not be enough to demonstrate changes in cell proliferation. Conversely, PRP at the highest concentration has a significant antioxidant effect on his cells, suggesting that an eventual treatment of this patient’s cells would need a high concentration of PRP to receive beneficial effects, in correlation with the more severe clinical presentation of this patient presenting a clear necrotic testicle that has been removed by orchiectomy. Altogether, these data show the different effects of PRP on diverse patients with testis torsion, underlying the importance of identifying the best PRP dosage by in vitro analysis before the patient is administered. Furthermore, these results mirror the clinic presentation of testicle functionality and well-being. Notably, our proteomic results also corroborate a clinical translation of our research, opening the door to performing proteomics analysis in a real-time clinical setting to aid the physician in choosing the most effective treatment for the pediatric patient with andrologic disease. In the case of testicular torsion, quickly becoming aware of the expression of proteins that are implicated in apoptosis or in response to oxidative damage could give information about the patient’s cellular and tissue health status, helping the surgeon decide whether to perform orchidopexy or orchiectomy, whether to administer adjuvant treatments, such as PRP or a combination of PRP/hCG.
Regarding cells derived from cryptorchid patients, we considered one culture condition, i.e., standard condition with atmospheric oxygen concentration, since for this pathology, the alterations are not due to acute damage but rather to prolonged persistence of the testicle in a ___location that is not physiological, with a consequent increase in ROS content, as reported by other authors15. Our data show that only patient #94 presented a significantly induced proliferation after PRP treatment at both concentrations. This suggests that the 24-hour treatment may be too short to test the effect of PRP on this cell event, which generally requires a few days. Instead, regarding ROS levels, our data show that patients responded to the treatment differently. Cells from patients #2 and #20 significantly reduced reactive oxygen species with both PRP concentrations, whereas patient #94 only had the highest. Conversely, only patient #99 did not respond to PRP antioxidant activity. This different response may be due to the presence of a retractile testicle in pt #99, thus his testicular cells may not have been damaged as the other patients that were real cryptorchidic, in addition to other possible factors, such as the age of the patient, the position of the cryptorchid testicle, and the time it resides there before surgery. Moreover, our study is based on a specific time of 24 h in which we left the cells treated with PRP; future studies will consider extending the treatment session to evaluate whether the limiting factor is time-dependent or concentration-dependent. From the analysis of our results, considering four pediatric patients of different ages and with different cryptorchid testicles, we hypothesize that the result of the antioxidant and proliferative effect could be dependent on the patient himself. Therefore, the choice of firstly testing the adjuvant treatment with autologous PRP in vitro aims to evaluate its effectiveness, before moving towards clinical trials. Our results about the antioxidant effect of PRP on gubernacular cells strengthen the effectiveness of the treatment, aware of the fact that the PRP used in our study is of the heterologous and non-pediatric type. Finally, knowing the great impact that this data can have, some limitations are represented by: (i) the small number of patients: future studies will be directed toward expanding the case series to be able to categorize the behavior of various pathologies in response to PRP administration; (ii) the treatment of cells in vitro with only two dosages of PRP; (iii) the induction of oxidative damage through the use of the hypoxic chamber does not guarantee faithful reproduction of in vivo conditions at all times, especially with regard to the mode of onset of oxidative damage and its intensity; (iv) cell proliferation analysis may require a longer time of treatment, thus 24 h could be a short time window to study it.
Conclusion
This project regards the optimization of different techniques to improve the fertility outcome of patients with testicular torsion and cryptorchidism. In both cases, reactive oxygen species are the main molecular noxa causing gonadal cell dysfunction. Our study showed that in vitro administration of PRP results in a significant reduction in the oxidative state of testicular cells of patients affected by the previously mentioned diseases. Our result agrees with the literature data regarding the antioxidant properties of PRP, demonstrating its efficacy for use in pediatric andrological pathology.
This finding could open up new treatment perspectives. Since PRP is an autologous product that can be obtained safely and inexpensively, in the future, it could be proposed as an adjuvant treatment directly injected in the testicle after surgery to resolve the underlying acute or chronic pathology and, at the same time, ensure a better fertility outcome for patients. The best candidates for adjuvant therapy could be selected through autologous cell in vitro cultures and real-time proteomics to identify patients who may benefit from PRP injection.
Materials and methods
Patients
This study was approved by the Board of the “Pediatric-Adolescent Fertility Study Laboratory” and the Ethics Committee of the Azienda Ospedaliera Universitaria Integrata di Verona (Project Number 4159CESC; Protocol Code PRP 3.0). All experiments on patients’ derived tissues were performed in accordance with the relevant guidelines and regulations with the Declaration of Helsinki. Informed consent was obtained from all patients/parents involved in the study. Samples are collected in our laboratory from patients with primary azoospermia, genetic syndromes associated with azoospermia, cryptorchidism, testicular torsion, testicular trauma, and testicular neoplasms. Each patient is cataloged with a progressive identification number. In this study, we performed in vitro analysis on cells derived from two pediatric patients with testicular torsion and four with cryptorchidism. In addition, we performed the proteomic analysis by directly analyzing tissue biopsies from four individuals: two affected by testicular torsion and two who underwent surgery due to suspected torsion, as reported in Table 1.
Inclusion criteria
The inclusion criteria defined for patients enrolled in the study were:
-
inguinal cryptorchidism;
-
unilateral or bilateral orchidopexy;
-
true testicular torsion, exception for controls of the proteomic analysis;
-
no metabolic, genetic, or chronic inflammatory diseases;
-
only one surgery in life either of the genital organs or related to other districts (e.g., urinary system, gastrointestinal system) and without other associated diseases.
Exclusion criteria
We considered as exclusion criteria from the study:
-
patients with intraabdominal testis (to avoid interpretive bias about the true position of the testis);
-
patients undergoing orchidopexy surgery with the trans-scrotal technique, a surgical technique not uniformly approved by all surgeons and not standardized as the traditional technique;
-
conversion to laparoscopic technique: essential for intra-abdominal mobilization of sperm vessels.
Surgical intervention
Patients with cryptorchidism underwent traditional orchidopexy surgery with an inguinal and scrotal approach. Patients with testicular torsion underwent derotation and orchidopexy with a scrotal approach. During surgery, testicular or gubernacular biopsies have been taken according to the surgeon’s evaluation concerning the status of the testicle for each patient.
Processing and in vitro culture of testicular and gubernacular testis tissue
To study the effect of PRP on the ROS content and proliferation of primary cells, we preferentially considered gubernaculum biopsies as they well represent the testis due to the common embryonic origin and the expression of typical testicular markers, as previously demonstrated9. When gubernaculum testis biopsy was inaccessible, we picked up a testicular biopsy and cultured the cells in vitro.
We processed gubernacular testis9 or testicular30, as previously reported by our group. We cut the tissue with a scalpel on a petri dish with 500 µL of phosphate-buffered saline (PBS) in sterile conditions in a biological safety cabinet. Then, the tissue fragments were enzymatically disrupted with compounds diluted in cell culture medium: testicular biopsy with trypsin, type I DNase, type I collagenase, and hyaluronidase, whereas gubernacular testis only with type I collagenase and hyaluronidase. After this step, the cells were incubated at 37 °C and 5% CO2 until completely homogenized. Cell viability was assessed using the Trypan Blue assay. Ultimately, we cultured the cells with DMEM medium supplemented with 10% fetal bovine serum (FBS), 4.5 g/L glucose, and 50 g/mL gentamicin sulfate and maintained them in a 37 °C and 5% CO2 environment in cell culture flasks. Before treatment with PRP, we maintained cells for 16 h in an FBS-free medium so that the results obtained could not be altered by the presence of the growth factors normally present in serum31.
Hypoxia
To mimic the reduced blood flow during the torsion state of the testis, both tissues (gubernacular and testicular) derived from patients with testis torsion were grown under hypoxic conditions, i.e., 37 °C and 5% CO2 and 1% O2 for 6 h (Baker Ruskinn InvivO2® 300 Hypoxia Workstation). Then, cells were treated with PRP under normoxic conditions (21% O2) for 24 h using standard cell culture conditions (37 °C and 5% CO2). This situation mimics surgical derotation followed by oxygenated blood supply.
PRP
PRP collection and processing
Using a butterfly needle, we took 8 blood samples from an adult volunteer in 5 mL tubes with sodium citrate as an anticoagulant. The samples were processed according to the protocol of the Clinical Biochemistry Department of the Azienda Ospedaliera Universitaria Integrata di Verona. Centrifugation at 200×g for 10 min was performed without a rotor brake. Then, with a pipette, the supernatant PRP was withdrawn and placed in plastic tubes. The initial blood sample and the PRP obtained were quantified with a platelet counter to assess the platelet concentration, which was 2x of PRP compared with whole blood (176 × 109/L to 363 × 109/L). On average, leukocyte concentration was 1 × 109/L, while red blood cell concentration was 0.02 × 1012/L.
PRP storage
At a preliminary stage, we tested the effects on cell proliferation and oxidative stress of three different storage modes of PRP, namely: fresh; stored at -80 °C; and freeze-dried. Our experimental results are superimposable with all types of preservation, demonstrating the preservation of PRP functionality under freezing and freeze-drying conditions, as reported in literature data32,33. To study the unbiased effect of PRP on in vitro treated primary cells, we used the PRP obtained from one volunteer for all the analyses.
ROS production measurement
To assess intracellular ROS production, we used the DCF-DA (2′,7′-dichlorofluorescein diacetate) probe, which becomes fluorescent when oxidized by ROS. We placed cells in 96-well plates at a concentration of 7000 cells per well in DMEM + 10%FBS medium. After 24 h, we changed the culture medium, using one without FBS, and treated the cells with various concentrations of PRP for 24 h. At the end of treatment, we incubated the cells in a culture medium with 10 µM DCF-DA for 30 min at 37 °C. We washed with PBS and measured DCF probe fluorescence (Excitation 485 nm and Emission 535 nm) with a multimodal microplate reader (GENios Pro, Tecan). Emission values were normalized on cell viability by crystal violet assay. We performed at least three different experiments for each condition.
Cell proliferation assay
We seeded 7000 cells per well in 96-well plates. After 24 h we changed the culture medium and treated with different concentrations of PRP, leaving the plates in a 37 °C incubator for an additional 24 h. At the end of treatment, we assessed cell proliferation with crystal violet dye. We solubilized the dye with 1% sodium dodecyl sulfate (SDS) in PBS and measured it photometrically (A595 nm) to assess cell viability. We performed at least three different experiments for each condition.
Proteomics analysis
The proteomics data shown in this paper were extrapolated from a currently in-progress study on testicular biopsies obtained from 2 pediatric patients with testicular torsion (patient #12 and #13, both presenting necrotic tissue) and two controls (patient #61, surgically explored for suspected torsion; patient #68, operated for suspected torsion and infection of the left testicle) as reported in Table 1. Specifically, the reported proteome modulations were obtained by a label-free approach, as described previously34. Testicular tissue (0.5 mm3) was washed with PBS, cut into small fragments, and processed for total peptide extraction using the iST Sample Preparation Kit (Preomics). The peptides obtained were quantified by Pierce Quantitative Fluorometric Peptide Assay (Thermoscientific). To obtain quantitative analysis, an equal amount of peptides for each sample was analyzed by LC-MS/MS (Orbitrap). Identified proteins were selected based on significance (p-value < 0.05) and expression (fold change > 1.5 or < 0.6 between groups). Below is the GeneCards gene symbol and UNIPROT ID of the proteins modulated and discussed here: Catalase (CAT; P04040); Glutamate-cysteine ligase regulatory subunit (GCLM; P48507); Glutamate-cysteine ligase catalytic subunit (GCLC; P48506).
Statistical analysis
The presented results are the mean ± SE of at least three biological replicates for each condition. We determined statistically significant differences with the t-test. We analyzed the data using the GraphPad Prism program and reported statistical significance in: (*) p < 0.05; (**) p < 0.01; (***) p < 0.001.
Data availability
Data available on request from the authors by contacting [email protected].
References
Zhao, L. C., Lautz, T. B., Meeks, J. J. & Maizels, M. Pediatric testicular torsion epidemiology using a national database: incidence, risk of orchiectomy and possible measures toward improving the quality of care. J. Urol. 186, 2009–2013. https://doi.org/10.1016/j.juro.2011.07.024 (2011).
Choi, J. B. et al. The incidence of testicular torsion and testicular salvage rate in Korea over 10 years: a nationwide population-based study. Investig Clin. Urol. 63, 448–454. https://doi.org/10.4111/icu.20220122 (2022).
Uguz, S., Yilmaz, S., Guragac, A., Topuz, B. & Aydur, E. Association of torsion with testicular cancer: a retrospective study. Clin. Genitourin. Cancer. 14, e55–57. https://doi.org/10.1016/j.clgc.2015.09.014 (2016).
Zhong, H. & Bi, Y. Pediatric trauma-induced testicular torsion: a surgical emergency. Urol. Int. 105, 221–224. https://doi.org/10.1159/000511747 (2021).
Jung, J. E. et al. Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol. Neurobiol. 41, 172–179. https://doi.org/10.1007/s12035-010-8102-z (2010).
Ustün, H. et al. Effect of phospodiesterase 5 inhibitors on apoptosis and nitric oxide synthases in testis torsion: an experimental study. Pediatr. Surg. Int. 24, 205–211. https://doi.org/10.1007/s00383-007-2058-8 (2008).
Jacobsen, F. M. et al. The impact of testicular torsion on testicular function. World J. Mens Health 38, 298–307. https://doi.org/10.5534/wjmh.190037 (2020).
Gielchinsky, I. et al. Pregnancy rates after testicular torsion. J. Urol. 196, 852–855. https://doi.org/10.1016/j.juro.2016.04.066 (2016).
Errico, A., Camoglio, F. S., Zampieri, N. & Dando, I. Testicular torsion: preliminary results of in vitro cell stimulation using chorionic gonadotropin. Cells 11, 450. https://doi.org/10.3390/cells11030450 (2022).
Sijstermans, K., Hack, W. W. M., Meijer, R. W. & van der Voort-Doedens, L. M. The frequency of undescended testis from birth to adulthood: a review. Int. J. Androl. 31, 1–11. https://doi.org/10.1111/j.1365-2605.2007.00770.x (2008).
Hutson, J. M. & Hasthorpe, S. Testicular descent and cryptorchidism: the state of the art in 2004. J. Pediatr. Surg. 40, 297–302. https://doi.org/10.1016/j.jpedsurg.2004.10.033 (2005).
Park, K. H., Lee, J. H., Han, J. J., Lee, S. D. & Song, S. Y. Histological evidences suggest recommending orchiopexy within the first year of life for children with unilateral inguinal cryptorchid testis. Int. J. Urol. 14, 616–621. https://doi.org/10.1111/j.1442-2042.2007.01788.x (2007).
Kollin, C., Granholm, T., Nordenskjöld, A. & Ritzén, E. M. Growth of spontaneously descended and surgically treated testes during early childhood. Pediatrics 131, e1174–1180. https://doi.org/10.1542/peds.2012-2902 (2013).
Gao, Y. et al. The effects and molecular mechanism of heat stress on spermatogenesis and the mitigation measures. Syst. Biol. Reprod. Med. 68, 331–347. https://doi.org/10.1080/19396368.2022.2074325 (2022).
Ahotupa, M. & Huhtaniemi, I. Impaired detoxification of reactive oxygen and consequent oxidative stress in experimentally cryptorchid rat testis. Biol. Reprod. 46, 1114–1118. https://doi.org/10.1095/biolreprod46.6.1114 (1992).
Chaki, S. P., Misro, M. M., Ghosh, D., Gautam, D. K. & Srinivas, M. Apoptosis and cell removal in the cryptorchid rat testis. Apoptosis Int. J. Program. Cell. Death. 10, 395–405. https://doi.org/10.1007/s10495-005-0813-7 (2005).
Kral, J. B., Schrottmaier, W. C., Salzmann, M. & Assinger, A. Platelet Interaction with Innate Immune cells. Transfus. Med. Hemother. 43, 78–88. https://doi.org/10.1159/000444807 (2016).
Everts, P. A. et al. Exogenous application of platelet-leukocyte gel during open subacromial decompression contributes to improved patient outcome. A prospective randomized double-blind study. Eur. Surg. Res. Eur. Chir. Forsch. Rech Chir. Eur. 40, 203–210. https://doi.org/10.1159/000110862 (2008).
Mościcka, P. & Przylipiak, A. History of autologous platelet-rich plasma: a short review. J. Cosmet. Dermatol. 20, 2712–2714. https://doi.org/10.1111/jocd.14326 (2021).
Akhigbe, R. E., Odetayo, A. F., Akhigbe, T. M., Hamed, M. A. & Ashonibare, P. J. Pathophysiology and management of testicular ischemia/reperfusion injury: lessons from animal models. Heliyon. https://doi.org/10.1016/j.heliyon.2024.e27760 (2024).
Filho, D. W., Torres, M. A., Bordin, A. L. B., Crezcynski-Pasa, T. B. & Boveris, A. Spermatic cord torsion, reactive oxygen and nitrogen species and ischemia–reperfusion injury. Mol. Aspects Med. 25, 199–210. https://doi.org/10.1016/j.mam.2004.02.020 (2004).
Baker, A. et al. Catalase: a critical node in the regulation of cell fate. Free Radic. Biol. Med. 199, 56–66. https://doi.org/10.1016/j.freeradbiomed.2023.02.009 (2023).
Forman, H. J., Zhang, H., Rinna, A. & Glutathione, overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 30, 1–12. https://doi.org/10.1016/j.mam.2008.08.006 (2009).
Escobar, G. et al. Pure platelet-rich plasma and supernatant of calcium-activated P-PRP induce different phenotypes of human macrophages. Regen Med. 13, 427–441. https://doi.org/10.2217/rme-2017-0122 (2018).
Zhang, S., Li, P., Yuan, Z. & Tan, J. Effects of platelet-rich plasma on the activity of human menstrual blood-derived stromal cells in vitro. Stem Cell. Res. Ther. 9, 48. https://doi.org/10.1186/s13287-018-0795-3 (2018).
Winters, B. R. & Walsh, T. J. The epidemiology of male infertility. Urol. Clin. North. Am. 41, 195–204. https://doi.org/10.1016/j.ucl.2013.08.006 (2014).
Harrison, P. & Physiology, S. P. The use of platelets in regenerative medicine and proposal for a new classification system: guidance from the SSC of the ISTH. J. Thromb. Haemost JTH. 16, 1895–1900. https://doi.org/10.1111/jth.14223 (2018).
Tohidnezhad, M. et al. Role of platelet-released growth factors in detoxification of reactive oxygen species in osteoblasts. Bone 65, 9–17. https://doi.org/10.1016/j.bone.2014.04.029 (2014).
Aitken, R. J. & Roman, S. D. Antioxidant systems and oxidative stress in the testes. Oxid. Med. Cell. Longev. 1, 15–24 (2008).
Zampieri, N., Patanè, S. & Camoglio, F. S. Twenty-year experience with macro-area school screening for andrological disease in paediatric age. Andrologia 53, e14209. https://doi.org/10.1111/and.14209 (2021).
Lee, D. Y. et al. Review of the current research on fetal bovine serum and the development of cultured meat. Food Sci. Anim. Resour. 42, 775–799. https://doi.org/10.5851/kosfa.2022.e46 (2022).
Kim, J. I., Bae, H. C., Park, H. J., Lee, M. C. & Han, H. S. Effect of Storage conditions and activation on growth factor concentration in Platelet-Rich Plasma. J. Orthop. Res. 38, 777–784. https://doi.org/10.1002/jor.24520 (2020).
Andia, I., Perez-Valle, A., Del Amo, C. & Maffulli, N. Freeze-drying of platelet-rich plasma: the Quest for standardization. Int. J. Mol. Sci. 21, 6904. https://doi.org/10.3390/ijms21186904 (2020).
Brandi, J. et al. Investigating the Proteomic Profile of HT-29 Colon cancer cells after Lactobacillus kefiri SGL 13 exposure using the SWATH method. J. Am. Soc. Mass. Spectrom. 30, 1690–1699. https://doi.org/10.1007/s13361-019-02268-6 (2019).
Acknowledgements
We thank Lions Clubs International- Isola della Scala and Bovolone (Italy)- for supporting our research. We also thank “Centro Piattaforme Tecnologiche” of the University of Verona (Italy) and Prof. Daniela Cecconi for proteomics support.
Funding
This work was supported by Ministero dell’Università e della Ricerca (MUR), Rome, Italy. This study was also supported by the American Academy of Pediatrics (Surgery Section).
Author information
Authors and Affiliations
Contributions
S.V. and N.R. performed experiments; G.A., E.D.P., A.E., and N.M. contributed to data analysis; I.D. and S.V. conceived and designed the analysis; I.D., N.Z., and F.S.C. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Ethics Committee of the Azienda Ospedaliera Universitaria Integrata di Verona (Project Number 4159CESC; Protocol Code PRP 3.0). Informed consent was obtained from all patients/parents involved in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Vinco, S., Rinaldi, N., Errico, A. et al. Platelet-rich plasma effects on in vitro cells derived from pediatric patients with andrological diseases. Sci Rep 14, 31202 (2024). https://doi.org/10.1038/s41598-024-82459-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-82459-8