Abstract
Few centers routinely report aortic arch calcification (AAC) due to the lack of an easy and effective evaluation method. The association between AAC and the clinical prognosis of patients who undergo transcatheter aortic valve replacement (TAVR) is unclear. We aimed to develop a rapid method to evaluate AAC in patients who underwent TAVR and to further assess their prognosis. We enrolled 464 consecutive patients with aortic stenosis who underwent TAVR. Patients with severe (11.2%), moderate (18.5%), mild (58.2%), and no (12.1%) AAC had an estimated 3-year all-cause mortality incidence of 39.6%, 20.8%, 13.4%, and 6.7% (log rank p < 0.001), respectively. Patients with severe AAC had a significantly higher incidence of both cardiovascular (log rank p = 0.002) and non-cardiovascular mortality (log rank p = 0.009), whereas patients with moderate AAC had a higher incidence of only non-cardiovascular mortality (p = 0.003) compared with patients with no/mild AAC. Moderate/severe AAC was an independent predictor of 3-year all-cause mortality in univariate (hazard ratio [HR]: 2.39, 95% confidence interval [CI]: 1.41–4.03; p = 0.001) and multivariate COX regression analyses (HR: 1.78, 95%CI: 1.04–3.06; p = 0.037). Our rapid semi-quantitative method to evaluate AAC is highly reproducible and can be used to assess AAC in patients who undergo TAVR.
Similar content being viewed by others
Introduction
The number of transcatheter aortic valve replacements (TAVR) has rapidly increased during the last decade1,2. Randomized controlled studies have provided favorable evidence for TAVR; therefore, it is recommended as the first-line therapy for severe aortic stenosis (AS) in older patients3. However, patients with extensively calcified aortic arch are generally excluded from randomized controlled trials because of embolism and vascular complications associated with the passing of delivery system through the heavily calcified aortic arch4,5. In fact, aortic arch calcification (AAC) can provide lots of information. It can reflect arterial stiffness, the magnitude of calcified change in the whole aorta, and even the frailty of the patients6,7,8. A large prospective population-based study demonstrated that a more severe AAC was associated with a higher risk of all-cause mortality and cardiovascular mortality in middle-aged and elderly persons7. Besides, AAC was found in over 60% of patients who underwent TAVR9. Therefore, AAC should be carefully evaluated in TAVR candidates.
Computed tomography (CT) is an essential examination to assess the anatomy of patients before TAVR and is routinely performed in most centers. Theoretically, AAC is easily evaluated; however, few centers routinely report AAC in their CT analysis because of the lack of an easy and reliable method. Accordingly, a limited number of studies focus on AAC. The association between AAC and the clinical prognosis of patients who undergo TAVR is also unclear. Recording AAC information and images is very important since these are necessary for further machine learning or deep learning study, which can provide valuable insights in prediction in cardiovascular contexts10,11. Therefore, we envisaged this study to propose a simple and rapid method to assess AAC and to assess the impact of AAC on the prognosis after TAVR.
Materials and methods
Study population
Consecutive severe AS patients who underwent TAVR between March 2016 and December 2020 were included in this study. The majority of patients were enrolled from the TORCH registry (Transcatheter Aortic Valve Replacement Single Center Registry in Chinese Population, NCT02803294); this is a prospective, ongoing, real-world registry, launched in June 2016. Patients who had a history of aortic valve replacement or did not have analyzable pre-operative multidetector CT (MDCT) images were excluded.
TAVR procedure and follow up
All TAVR procedures were decided by our multidisciplinary heart team. Self-expanding valves from VenusA (Venus Medtech, China), VitaFlow (Microport, China), TaurusOne (Peijia Medical, China), J-valve (Jiecheng, China) series were used in the majority of patients. The remaining patients were implanted with balloon-expandable (SAPIEN 3/XT, Edwards Lifesciences, California, USA) or mechanically-expandable (Lotus valve, Boston Scientific, Massachusetts, USA) valves. Patients with bicuspid aortic valve underwent TAVR using Hangzhou Solution strategy12,13. The detailed procedure has been previously described12,13,14,15,16. Our professional team subsequently followed up with patients at 30 days, 1 year, and yearly thereafter through face-to-face or telephonic consultation. We collected data during the 3-year follow-up to reduce the influence of limited sample size in the fourth/fifth-year follow-up (many patients did not reach the fourth or fifth follow-up window). The clinical events were defined according to the Valve Academic Research Consortium-2 consensus document17.
MDCT and its analyses
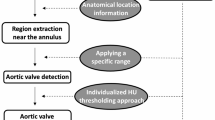
Contrast-enhanced MDCT was routinely performed on the second-generation dual-energy CT (SOMATOM Definition Flash, Siemens Medical Solutions, Germany) following our scan protocol. The anatomy of patients was assessed using 3mensio software (3mensio Medical Imaging BV, Bilthoven, The Netherlands). Aortic root anatomy was evaluated in a double-oblique reconstruction image using the best systolic phase. The severity of valve calcification was classified into no, mild, moderate, and severe, as previously described18. Maximal intensity projection image was used to assess AAC (Fig. 1). The aortic arch was defined as the segment between the ascending and descending aorta (between the innominate artery and the aortic isthmus)19, and AAC was visible as high-density portions in this region. Notably, the calcification in the innominate, left common carotid, and left subclavian arteries was distinguished and was not included in the AAC.
Representative images showing different degrees of AAC. Maximal intensity projection image was used to assess AAC. (A, C, E, G) Optimized images for aortic arch visualization. These images were used to assess the calcification involving the ratio of the aortic arch length. (B, D, F, H) Images that visually overlapped the vascular lumen of the aortic arch were assessed to identify the calcification involving the ratio of the vessel circumference; (A, B) no AAC; (C, D) mild AAC: calcification involving ≤ 1/3rd of aortic arch length or circumference; (E, F) moderate AAC: calcification involving > 1/3rd of aortic arch length and circumference, but not both > 2/3rd of aortic arch length and circumference; (G, H) severe AAC: calcification involving > 2/3rd of aortic arch length and circumference. Notably, the calcification in the innominate, left common carotid, and left subclavian arteries was not included as AAC. AAC = aortic arch calcification.
According to our protocol, the optimized image for aortic arch length visualization was used to assess the calcification involving the ratio of the aortic arch length. Then, an image that visually overlapped the vascular lumen of the aortic arch was assessed to identify the calcification involving the ratio of the vessel circumference. In this way, AAC was graded as no (free of calcification), mild (calcification involving ≤ 1/3rd of aortic arch length or circumference), moderate (calcification involving > 1/3rd of aortic arch length and circumference, but not both > 2/3rd of aortic arch length and circumference), and severe calcification (calcification involving > 2/3rd of aortic arch length and circumference). The typical images are shown in Fig. 1.
The analyses of AAC were performed by two authors (D.Z and H.Y.D) experienced in TAVR pre-operative CT imaging analyses. First, the two observers assessed the AAC in 20 patients together. Thereafter, CT images of 40 randomly selected consecutive patients were separately evaluated to measure interobserver variations. The two observers reassessed these cases independently at an interval of > 3 months to measure intraobserver variations in AAC analyses. The reproducibility of this method was confirmed, and the remaining images are evaluated independently. The two experts who analyzed the MDCT images were blinded to the procedural data and clinical outcomes.
Statistical analyses
Quantitative variables were expressed as means with standard deviation or median with interquartile ranges (IQR) according to the distribution as determined by Shapiro–Wilk test. Student’s t-test or Mann–Whitney U test was performed for continuous variables. All categorical data were presented as frequencies with percentages and were compared using a chi-square test or Fisher’s exact test, as appropriate. Cohen’s kappa coefficient was calculated to determine interobserver and intraobserver variations in the measurements of AAC grade. A kappa value of 0.81–1, 0.61–0.80, 0.41–0.60, 0.21–0.40, 0.01–0.20, and < 0 indicated almost perfect, substantial, fair, slight, and no agreements, respectively20,21. The cumulative all-cause mortality and cardiovascular/non-cardiovascular mortality rates were determined using the Kaplan–Meier survival analysis and were compared using the log-rank test. COX regression analyses were performed to evaluate the correlation of baseline characteristics and estimated 3-year all-cause mortality. Univariate COX regression analyses were performed for all baseline variables; variables with p-values < 0.05 in univariate COX regression analysis were entered in multivariate COX regression models using a forward likelihood-ratio method. A two-tailed p-value < 0.05 was considered to be statistically significant. All data analyses were performed using SPSS software version 20.0 (IBM, New York, USA).
Results
A total of 464 consecutive patients with AS who underwent TAVR were enrolled in our study, and 12.1%, 58.2%, 18.5%, and 11.2% of patients had no, mild, moderate, and severe AAC, respectively. Furthermore, we compared 138 (29.7%) patients with moderate or severe AAC and 326 (70.3%) patients with no or mild AAC. The baseline characteristics of the patients are shown in Table 1. Individuals in the moderate/severe AAC group were older (80.0 years [IQR: 73.8 to 84.0 years] vs.75.0 years [IQR: 70.0 to 79.0 years]; p < 0.001) and had higher Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) scores (7.7 [IQR:4.4 to 10.5] vs.4.8 [IQR:3.2 to 8.3]; p < 0.001) than those in no/mild AAC group. Higher prevalence of hypertension (69.6% vs. 48.8%; p < 0.001), chronic kidney disease (18.1% vs. 7.4%; p = 0.001), and history of stroke (8.7% vs. 3.4%; p = 0.016) were found in moderate/severe AAC group. The proportion of sex and smokers was not significantly different between the two groups. Further, the proportion of patients with hyperlipidemia, diabetes, chronic obstructive pulmonary diseases, prior myocardial infarction, prior percutaneous coronary intervention, or prior pacemaker implantation was not significantly different between the two groups.
The echocardiography and CT data are displayed in Table 2. The left ventricular ejection fraction, maximum velocity, mean gradient, and aortic valve area were comparable between the two groups. Approximately 18.1% and 15.1% of patients had low-gradient AS (severe AS with mean gradient lower than 40 mm Hg) in the moderate/severe AAC and no/mild AAC groups (p = 0.457), respectively. MDCT data revealed that patients with moderate/severe AAC had a smaller sinotubular junction, smaller ascending aorta diameter, and lower left main coronary height. The proportion of patients with moderate/severe aortic valve calcification was similar between the moderate/severe AAC and no/mild AAC groups (76.1% vs. 81.0%, p = 0.232).
The reproducibility of the proposed method was determined by repeated assessment of randomly selected consecutive 40 AAC cases independently; the kappa value for interobserver variability was 0.807. The kappa values for intraobserver variation were 0.962 (D.Z) and 0.922 (H.Y.D). Kappa values can be interpreted as: ≤ 0 (no agreement), 0.01–0.20 (none to slight), 0.21–0.40 (fair), 0.41–0.60 (moderate), 0.61–0.80 (substantial), and 0.81–1.00 (almost perfect agreement). Therefore, we achieved almost perfect interobserver and intraobserver agreements.
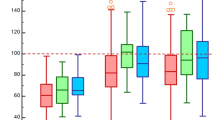
The procedural characteristics and 30-day clinical outcomes are mentioned in Table 3. Self-expandable valves were the most commonly used prosthesis in moderate/severe AAC (83.3%) and no/mild AAC (88.3%) groups. The transfemoral approach was the most frequently adopted access approach in the two groups (92.0% vs. 95.7%; p = 0.109). The proportion of patients who underwent balloon predilatation was similar between the two groups (97.8% vs. 97.2%, p = 0.965), while the proportion of those who underwent post-dilation was higher in the no/mild AAC group (47.8% vs. 59.8%; p = 0.017). All-cause mortality during 30 days of follow-up after TAVR was numerically more frequent in the moderate/severe AAC group than in the no/mild AAC group (3.6% vs. 1.5%); however, the difference was not statistically significant (p = 0.284). Moreover, the rates of myocardial infarction, stroke, bleeding, and permanent pacemaker implantation did not significantly differ between the two groups. Individuals with moderate or severe AAC had a statistically higher incidence of all-cause death than those with no or mild AAC (12.3% vs. 4.9%; p = 0.005) at 1-year follow-up; non-cardiovascular deaths were the major contributory factors (6.5% vs. 0.6%; p < 0.001; Fig. 2).
The median follow-up time was 25.1 months (IQR: 12.4–36.0). The time-to-event analysis determined that the patients with severe, moderate, mild, and no AAC had an estimated 3-year all-cause mortality of 39.6%, 20.8%, 13.4%, and 6.7% (log-rank p < 0.001, Figure S1). Figures 3 and 4 show that the patients with moderate/severe AAC had significantly higher 3-year all-cause mortality (27.7% vs. 12.3%, log-rank p = 0.001) and non-cardiovascular mortality (14.3% vs. 5.0%, log-rank p = 0.001) than those with no/mild AAC. Nonetheless, cardiovascular mortality was not significantly different between the two groups (15.6% vs. 7.7%, log-rank p = 0.123). Further, we evaluated patients with moderate or severe AAC separately and observed that patients with severe AAC had significantly higher incidences of cardiovascular and non-cardiovascular mortality compared with patients with no/mild AAC (cardiovascular: 31.5% vs. 7.7%, log-rank p = 0.002; non-cardiovascular: 13.1% vs. 5.0%, log-rank p = 0.009). In addition, patients with severe AAC had a higher rate of estimated 3-year cardiovascular mortality than those with moderate AAC (31.5% vs. 7.2%, log-rank p = 0.010). Patients with moderate AAC had a higher incidence of non-cardiovascular mortality but had a similar incidence of cardiovascular mortality compared to patients with no/mild AAC (cardiovascular: 7.2% vs. 7.7%, log-rank p = 0.726; non-cardiovascular: 14.7% vs. 5.0%, log-rank p = 0.003).
This rapid semi-quantitative method assesses AAC in maximal intensity projection images with good reproducibility. Among patients with AS who underwent TAVR, a considerable number of patients had a moderate (18.5%) or severe AAC (11.2%), which was associated with an increased incidence of 3-year all-cause mortality (moderate/severe AAC) and cardiovascular (severe AAC) or non-cardiovascular mortality (moderate or severe AAC). Multivariate Cox regression analysis showed that moderate/severe AAC was an independent predictor of 3-year all-cause mortality. AAC = aortic arch calcification; AS = aortic stenosis; CV = cardiovascular; CKD = chronic kidney disease; TAVR = transcatheter aortic valve replacement.
Table 4 shows that moderate/severe AAC was a risk factor for 3-year all-cause mortality in univariate (hazard ratio [HR]: 2.39, 95% confidence interval [CI]: 1.41–4.03; p = 0.001) and multivariate COX regression analyses (HR: 1.78, 95% CI: 1.04–3.06; p = 0.037). Higher STS-PROM strata (p = 0.011), stage 4 or 5 chronic kidney disease (p = 0.027), and low gradient AS (p = 0.018) were suggested as independent predictors in multivariate regression analyses. Furthermore, in another COX regression analyses model in which AAC was classified according to the grade of arch calcification, a more severe AAC grade was also an independent predictor of all-cause mortality (univariate: p < 0.001; multivariate: p = 0.025) and the above-mentioned independent predictors were still valid (Table S1).
Discussion
The main findings of our studies are as follows: (1) we have proposed a rapid and reproducible method to assess AAC; (2) among patients who underwent TAVR in our centers, 87.9% patients had AAC; of these, 18.5% and 11.2% had moderate and severe AAC, respectively; (3) patients with moderate/severe AAC had a higher incidence of 1-year all-cause mortality and non-cardiovascular mortality; (4) moderate/severe AAC was an independent predictor of 3-year all-cause mortality; (5) compared with no/mild AAC, severe AAC was associated with a higher incidence of 3-year cardiovascular and non-cardiovascular mortalities, whereas moderate AAC was associated with a higher incidence of non-cardiovascular mortality.
The assessment of aortic arch calcification is important for the operator since many manipulations during the procedure involve the aortic arch. The current main limiting factor in evaluating AAC is the lack of an easy, effective, and repeatable method. Although calcium volume score can provide a quantitative assessment of calcification, calculating the aortic arch calcium volume score is complicated. The analysts need to decide centerline from the aortic root to descending aorta, make a reconstruction, place a region of interest to measure average Hounsfield units, and manually adjust the best cut-off of Hounsfield units to optimize calcification detection. Few centers routinely do this analysis since clinicians usually focus more on the anatomy of the aortic root and access. In addition, simply reporting arch calcium volume score misses information regarding calcification distribution. AAC can be evaluated using another semiquantitative assessment method. Sagittal and reconstructed axial CT images are analyzed slice-by-slice, and the severity of arch calcification is determined by estimating the degree of calcification involving the vessel length and circumference22. This method is also relatively complicated and may have potentially large intraobserver and interobserver differences due to the slice-by-slice analyses. Therefore, we designed this study to provide a rapid and useful method to assess AAC.
Using our method, the AAC can be easily assessed during the analysis of access anatomy. During routinely analyzing aortic valve root structure before TAVR, it takes only 20 s to 1 min to finish the analyses and grading of AAC. Besides, adding typical imaging to the report of the anatomy of the patient is easy. The clinicians can also visualize the information of the AAC in patients just by reading the report. Severe AAC and porcelain aorta can be quickly identified, and then the operators can decide whether the patient should receive a transapical-TAVR rather than transfemoral-TAVR23. In addition, the application of cerebral protection devices has increased rapidly in recent years24,25. Some cerebral protection devices are placed in the aortic arch and information on the anatomy of the aortic arch is required. The routine use of this rapid assessment method can also provide information for evaluating the availability of cerebral protection devices.
In addition to providing important information for making clinical decision, using this approach to grade AAC and record images can also provide important data and information for conducting further research using machine learning techniques. Currently, more and more studies use machine learning techniques to enhance diagnostic accuracy and prediction by optimizing feature extraction and model performance. Related work in areas such as structural health monitoring and advanced imaging analysis has demonstrated the potential of these techniques11,26,27. Besides, novel algorithms have been proposed to enhance training performance and have been proven their predictive power in recent years28,29. However, the lack of an easy evaluate method of AAC results in this information being ignored in most center, restricting application of machine learning techniques to assess the predictive value of AAC in TAVR patients. Therefore, this method might help to conduct further machine learning studies.
Currently, the knowledge of AAC in old patients with severe AS is scarce. In our study, a considerable number of patients (29.7%) had moderate or severe AAC, suggesting that the occurrence of AAC should be critically considered. Intimal injury caused by hypertension and calcium and phosphorus metabolism disorder caused by renal insufficiency can lead to AAC. Thus, patients with moderate or severe AAC were older and had a higher proportion of hypertension and renal insufficiency, which were consistent with those of previous studies conducted in other populations6,8,30. Moreover, previous studies have indicated that prevalent stroke was associated with AAC30,31. Similarly, in our study, a history of stroke was more common in patients with moderate or severe AAC.
In a population-based cohort study including 2,408 participants from the Rotterdam study, AAC was found to be the strongest indicator of cardiovascular and non-cardiovascular mortalities among major vessel beds7. However, to the best of our knowledge, our study is the first one to illustrate the relationship between AAC and mid-to-long term clinical outcomes in patients who underwent TAVR. We also found that the patients with moderate or severe AAC had a higher incidence of estimated 3-year all-cause and non-cardiovascular mortalities, whereas patients with severe AAC had a higher incidence of 3-year all-cause, cardiovascular, and non-cardiovascular mortalities. Therefore, it is plausible that a better management of comorbidity after TAVR is needed in patients with moderate/severe AAC, especially in patients with severe AAC. For example, over 50% of patients can have an increased blood pressure after TAVR32,33,34, and management of hypertension in such patients may help delay further progression of aortic arch atherosclerosis and calcification.
We also found that higher STS-PROM risk strata, chronic kidney disease stage 4 or 5, and low gradient AS were independent predictors of the 3-year all-cause mortality. STS score includes several clinical characteristics (such as age, comorbidity, and medication) and is widely used in clinical practice. Low-gradient AS was associated with worse left heart function. All these three factors were classic strong risk factors for worse clinical outcomes. Although patients with moderate/severe AAC had higher STS scores and a higher proportion of chronic kidney disease stage 4 or 5 than those with no/mild AAC, moderate/severe AAC was still an independent predictor in multivariate COX regression analysis.
Limitation
Our study has some limitations. First, this method cannot identify low-density atherosclerosis. However, this rapid method can be combined with other assessment methods. Therefore, if obvious low-density atherosclerosis plaque or ulcers are present, we recommend that the analysts should further evaluate low-density atherosclerosis plaque or ulcers in the aortic arch in addition to the routine use of our method. Second, this method is only focused on assessing AAC. Nevertheless, the AAC can reflect the total burden of vascular calcification given its central ___location in the arterial system7,8. Third, since patients who had a history of aortic valve replacement or did not have analyzable MDCT images were excluded, patient selection biases may exist in this study. Fourth, although almost perfect agreement was achieved in our reproducibility test, there still existed some observer variability (especially interobserver variations). The variability were mainly found in analyzing AAC images with boundary values (such as 1/3rd and 2/3rd). Therefore, we suggest that clinicians should be trained using typical cases to reduce interobserver variability in clinical practice. Besides, some baseline characteristics differed between patients with moderate/severe and no/mild AAC (such as age, STS score, and renal function). Although moderate/severe AAC was an independent predictor of 3-year all-cause mortality in multivariate regression analysis, a well-designed study matching baseline characteristics well between different groups is needed to further verify our results. Finally, the sample size was relatively small and the follow-up was not long enough. Future large-scale studies with longer follow-ups are needed to validate our findings.
Conclusions
We developed a semi-quantitative method for evaluating patients undergoing TAVR and classified AAC into no, mild, moderate, and severe. The method had good reproducibility and can be routinely used to rapidly assess AAC in clinical practice. We found that approximately 30% of patients undergoing TAVR had a moderate/severe AAC, which was associated with a higher incidence of 1-year and 3-year all-cause mortalities. In addition, patients with moderate or severe AAC had an increased incidence of 3-year non-cardiovascular mortality, whereas only patients with severe AAC had a higher risk of 3-year cardiovascular mortality.
Data availability
Data Availability Statement: The data underlying this article will be shared on reasonable request to the corresponding author with permission of Second Affiliated Hospital of Zhejiang University, Hangzhou, China.
Abbreviations
- ACC:
-
aortic arch calcification
- TAVR:
-
transcatheter aortic valve replacement
- MDCT:
-
multidetector computed tomography
- AS:
-
severe aortic stenosis
- CT:
-
computed tomography
- IQR:
-
interquartile ranges
- STS-PROM:
-
Society of Thoracic Surgeons Predicted Risk of Mortality
References
Bowdish, M. E. et al. STS Adult Cardiac Surgery Database: 2021 Update on Outcomes, Quality, and Research. Ann. Thorac. Surg. 111, 1770–1780 (2021).
Lee, C. H., Inohara, T., Hayashida, K. & Park, D. W. Transcatheter Aortic Valve Replacement in Asia. JACC: Asia. 1, 279–293 (2021).
Otto, C. M. et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 77, 450–500 (2021).
Mack, M. J. et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 380, 1695–1705 (2019).
Smith, C. R. et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 364, 2187–2198 (2011).
Lee, S. Y., Chao, C. T., Huang, J. W. & Huang, K. C. Vascular Calcification as an Underrecognized Risk Factor for Frailty in 1783 Community-Dwelling Elderly Individuals. J. Am. Heart Association. 9, e017308 (2020).
Bos, D. et al., Comparison of Atherosclerotic Calcification in Major Vessel Beds on the Risk of All-Cause and Cause-Specific Mortality: The Rotterdam Study. Circ. Cardiovasc. Imaging 8 (2015).
Hashimoto, H. et al. Validity and usefulness of aortic arch calcification in chest X-ray. J. Atheroscler. Thromb. 16, 256–264 (2009).
Bapat, V. N., Attia, R. Q. & Thomas, M. Distribution of calcium in the ascending aorta in patients undergoing transcatheter aortic valve implantation and its relevance to the transaortic approach. JACC Cardiovasc. Intervent. 5, 470–476 (2012).
Topol, E. J. As artificial intelligence goes multimodal, medical applications multiply. Sci. (New York N Y). 381, adk6139 (2023).
Fahmy, A. S. et al. An Explainable Machine Learning Approach Reveals Prognostic Significance of Right Ventricular Dysfunction in Nonischemic Cardiomyopathy. JACC Cardiovasc. Imaging. 15, 766–779 (2022).
Liu, X. et al. Supra-annular structure assessment for self-expanding transcatheter heart valve size selection in patients with bicuspid aortic valve. Catheterization Cardiovasc. interventions: official J. Soc. Cardiac Angiography Interventions. 91, 986–994 (2018).
Liu, X. et al., Cerebral Ischemic Lesions after Transcatheter Aortic Valve Implantation in Patients with Non-Calcific Aortic Stenosis. J. Clin. Med. 11 (2022).
Fan, J. et al. Brain Injury After Transcatheter Replacement of Bicuspid Versus Tricuspid Aortic Valves. J. Am. Coll. Cardiol. 76, 2579–2590 (2020).
Liu, X. et al. Wearable Smartwatch Facilitated Remote Health Management for Patients Undergoing Transcatheter Aortic Valve Replacement. J. Am. Heart Association. 11, e023219 (2022).
Zhou, D. et al. Three-year outcomes of transcatheter aortic valve implantation for bicuspid versus tricuspid aortic stenosis. EuroIntervention: J. EuroPCR Collab. Working Group. Interventional Cardiol. Eur. Soc. Cardiol. 18, 193–202 (2022).
Kappetein, A. P. et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J. Am. Coll. Cardiol. 60, 1438–1454 (2012).
Tops, L. F. et al. Noninvasive evaluation of the aortic root with multislice computed tomography implications for transcatheter aortic valve replacement. JACC Cardiovasc. Imaging. 1, 321–330 (2008).
Kurra, V. et al. Extent of thoracic aortic atheroma burden and long-term mortality after cardiothoracic surgery: a computed tomography study. JACC Cardiovasc. Imaging. 3, 1020–1029 (2010).
Ginley, B. et al. Computational Segmentation and Classification of Diabetic Glomerulosclerosis. J. Am. Soc. Nephrology: JASN. 30, 1953–1967 (2019).
Landis, J. R. & Koch, G. G. The measurement of observer agreement for categorical data. Biometrics 33, 159–174 (1977).
Kandathil, A. et al. Atherosclerosis on CT Angiogram Predicts Acute Kidney Injury After Transcatheter Aortic Valve Replacement. AJR Am. J. Roentgenol. 211, 677–683 (2018).
Buz, S. et al. Trans-apical aortic valve implantation in patients with severe calcification of the ascending aorta. Eur. J. cardio-thoracic surgery: official J. Eur. Association Cardio-thoracic Surg. 40, 463–468 (2011).
Alkhouli, M., Alqahtani, F., Harris, A. H., Hohmann, S. F. & Rihal, C. S. Early Experience With Cerebral Embolic Protection During Transcatheter Aortic Valve Replacement in the United States. JAMA Intern. Med. 180, 783–784 (2020).
Butala, N. M. et al. Cerebral Embolic Protection and Outcomes of Transcatheter Aortic Valve Replacement: Results From the Transcatheter Valve Therapy Registry. Circulation 143, 2229–2240 (2021).
Khatir, A. et al. A new hybrid PSO-YUKI for double cracks identification in CFRP cantilever beam. Compos. Struct. 311, 116803 (2023).
Khatir, A. et al. Advancing structural integrity prediction with optimized neural network and vibration analysis. J. Struct. Integr. Maintenance. 9, 2390258 (2024).
Achouri, F., Khatir, A., Smahi, Z. & Capozucca, R. Ouled Brahim, A. Structural health monitoring of beam model based on swarm intelligence-based algorithms and neural networks employing FRF. J. Brazilian Soc. Mech. Sci. Eng. 45, 621 (2023).
Khatir, A., Capozucca, R., Khatir, S. & Magagnini, E. Vibration-based crack prediction on a beam model using hybrid butterfly optimization algorithm with artificial neural network. Front. Struct. Civil Eng. 16, 976–989 (2022).
Iribarren, C., Sidney, S., Sternfeld, B. & Browner, W. S. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. Jama 283, 2810–2815 (2000).
Elias-Smale, S. E. et al. Carotid, aortic arch and coronary calcification are related to history of stroke: the Rotterdam Study. Atherosclerosis 212, 656–660 (2010).
Michail, M. et al. Acute Effects of Transcatheter Aortic Valve Replacement on Central Aortic Hemodynamics in Patients With Severe Aortic Stenosis. Hypertens. (Dallas Tex. : 1979). 75, 1557–1564 (2020).
Perlman, G. Y. et al. Post-procedural hypertension following transcatheter aortic valve implantation: incidence and clinical significance. JACC Cardiovasc. Intervent. 6, 472–478 (2013).
Klinkhammer, B. J., Dyke, C. M. & Haldis, T. A. The development or worsening of hypertension after transcatheter aortic valve replacement (TAVR) improves short-term and long-term patient outcomes. Heart Asia. 10, e010994 (2018).
Acknowledgements
This work obtained funding support from the Second Affiliated Hospital Zhejiang University School of Medicine. Thanks to Hangzhou Qianjiang special expert, for the help and support.
Funding
This research was funded by State Key Laboratory of Transvascular Implantation Devices; The National Key R&D Program of China (2019YFA0110400 for JW); The National Natural Science Foundation of China (No. 81870292 for JW, No. 81570233, 81770252 for XBL, No. 82200552 for JQF); National Science Fund for Distinguished Young Scholars of China (No.82425007 for XBL); Zhejiang Province Science and Technology Department Key R&D Program (No.2024C03024, 2021C03097, 2018C03084 for JW, No.2022C03063 for XBL); Zhejiang Province Science and Technology Innovation Leading Talents Project (No.2023R5236 for XBL); Zhejiang Research Center of Cardiovascular Diagnosis and Treatment Technology (JBZX-202001 for JW); Natural Science Foundation of Zhejiang Province of China (LQ21H020005 for YCG).
Author information
Authors and Affiliations
Contributions
Conceptualization, D.Z., JQ.F. and XB.L.; methodology, D.Z., HY.D. and YC.G.; formal analysis and investigation, D.Z., HY.D., WJ.S., R.R.Z. and J.C.; data curation, A.Y., A.A. and YX.H.; writing—original draft preparation, D.Z., HY.D., WJ.S. and RR.Z; writing—review and editing, XB.L. and QF.Z.; visualization, D.Z., SS.Y; supervision, Q.L. and YX.W.; project Administration, XB.L. and JA.W. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Medical Ethics Committee of the Second Affiliated Hospital of Zhejiang University, Hangzhou, China.
Informed consent
All patients provided written informed consent for the TAVR procedures and the use of anonymous data for research.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, D., Dai, H., Sheng, W. et al. Evaluation of aortic arch calcification to predict prognosis after transcatheter aortic valve replacement. Sci Rep 15, 6396 (2025). https://doi.org/10.1038/s41598-024-83536-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-83536-8