Abstract
Ulva intestinalis (UI) is widely available edible seaweed and has potential to be introduced as functional food items in Bangladesh. However, potential health hazards of this seaweed with biotoxicity assays and its relation to heavy metal contents were not evaluated previously. With these objectives, toxic effects of UI collected from floating raft culture in Monkhali Beach was evaluated using various organisms such as Chlorella vulgaris, Artemia salina, Daphnia magna, and Lactuca sativa. In relation to this effects, heavy metal concentrations (Fe, Cr, Ni, Cu, Zn, Pb, Cd, and As) and its potential health hazards were subsequently analyzed. The results showed that UI water extract had positive effects on the survivability and growth of the all-test organisms over different time periods, with minimal LC50 values, indicating no toxic to tested organisms. However, increased levels of total dissolved solids and electrical conductivity were observed as extract concentrations increased but considered to be safe below 5 mg kg−1, as compared to control. Moreover, Fe, Cr, Ni, Cu and Zn (43.60, 0.10, 0.44, 0.07, 0.27 and 0.13 mg kg−1 of dry weight, respectively) in UI were found to be low levels compared to previous studies, in addition, Cd and As remained not detected. No significant health risk (HQ < 1) and target carcinogenic risk were found. Therefore, UI could be utilized as functional foods or nutraceuticals for health-conscious consumers of Bangladesh without having potential risks.
Similar content being viewed by others
Introduction
Seaweed has historically been consumed by those living around the coast, and due to its many health benefits, there has recently been a rise in interest in eating seaweeds or seaweed extract1. One special functional diet that includes seaweed in the European Union regulation (2015/2283). In addition to providing needed nutrients, functional foods may have positive health effects2. Seaweeds rich in bioactive chemicals exhibit strong antibacterial, antifungal, anti-inflammatory, antioxidant, and memory enhancing properties3,4,5. However, consuming seaweed may also increase the chance of humans being exposed to high-risk heavy metals or metalloids. According to studies6,7, seaweed has the capacity to bioaccumulate heavy metals at concentrations several orders of magnitude greater than ambient concentrations in seawater. Consequently, it can be considered a bio-indicator for determining oceanic contamination8.
The green seaweed Ulva intestinalis (UI), known as sea lettuce, is found across Bangladesh’s coast, including the coast of Cox’s Bazar, where it can flourish in a variety of environmental circumstances like rocks, mud, and even sand. Unlike more developed areas of the Cox’s Bazar coastline, Monkhali Beach is relatively less impacted by urbanization and industrial activities. This results in lower levels of pollutants, including heavy metals, in both the water and sediments, making it an ideal ___location for cultivating seaweeds like UI. According to a recent study, the approximate dry weight composition of UI was found to be 12.6% crude protein, 45.4% carbohydrates, 3.5% crude fat, 17.1% crude fibre, and 12.7% ash9. The total amounts of saturated, mono-unsaturated, omega-3, and omega-6 fatty acids were found to be 641.9, 563.7 (31.8%), 133.8 (7.6%), and 436.3 (24.6%) µg/g of dry weight of UI (4). Though UI seaweed has a high nutritional value and has the potential to be commercialized in Bangladesh, no research has been done to assess the biotoxicity of this seaweed in relation to quantities of heavy metals for consumer safety.
Within aquatic ecosystems, microalgae, which constitute the foundational component of the food web, play a vital role in the surveillance of water quality. Metrics such as cell density and daily cell growth are frequently employed to quantify microalgal proliferation, as evidenced by prior investigation on Chlorella vulgaris (Chlorophyta)10. Furthermore, the yield inhibition rate is commonly utilized as an integrative metric to assess the phytotoxicity of environmental contaminants on algal growth, thereby offering valuable insights into the inhibitory influences of pollutants on algal proliferation11. Artemia sp. (Arthropoda) represents one of the most frequently utilized invertebrate taxa for assessing seawater toxicity12, whereas Daphnia sp. (Arthropoda) is extensively employed in the evaluation of freshwater toxicity13. A multitude of factors underlie the selection of these species for toxicity investigations, encompassing their relatively brief observation durations, economical nature, well-characterized biological and ecological frameworks, ease of manipulation and maintenance within controlled laboratory environments, diminutive size which facilitates accommodation within small beakers or microplates, and significant adaptability to diverse experimental conditions14. Lettuce is a prevalent botanical specimen utilized in growth inhibition assays owing to its straightforward cultivation processes and rapid growth rates, rendering it an advantageous option for toxicological evaluations15. The pH, TDS (total dissolved solids), and EC (electrical conductivity) of seaweed water extracts are important because variations in these values can make hazardous compounds more mobile and bioavailable to organisms when consume16. When using seaweed extracts in applications, testing and adjusting these parameters might help reduce the possibility of toxicity.
In order to evaluate potential health concerns for adults, eight heavy metals (Pb, Cd, Zn, Cu, Ni, Mn, Cr, and Fe) were found in dried red seaweed (Hypnea musciformis) from the Bakkhali River estuary and Saint Martin’s Island, Bangladesh17. However, no significant health risk was found. Red seaweeds had the largest concentration of trace metals when compared to brown and green algae, and Asian seaweeds had a higher prevalence of numerous heavy metals than European seaweeds18. A comprehensive review of the literature reveals that numerous studies across the globe have reported elevated concentrations of heavy metals in seaweeds. For example, Pan et al.19 observed significant bioaccumulation of copper, chromium (Cr), nickel (Ni), zinc (Zn), lead (Pb), cadmium (Cd), and arsenic (As) in seaweeds collected from the Dongtou Islands in the East China Sea. Similarly, the high levels of Pb, Cd, Cu, Ni, Zn, and iron (Fe) in 11 dominant seaweed species from the Strait of Hormuz were reported20. Elevated concentrations of Fe and Pb in green (Chlorophyta) and brown (Phaeophyceae) seaweeds were found from the Antikyra Gulf in Viotia, Greece21.
But as far as the authors are aware, there have not been any prior research done on the biotoxicity of cultivated UI seaweed from Monkhali Beach on the Cox’s Bazar Coast, and how heavy metal levels relate to the seaweed safety for consumption. Keeping this objective in mind, various model organisms such as Chlorella vulgaris, Artemia salina, Daphnia magna, and Lactuca sativa (Angiosperm) seed were used in biotoxicity studies. Measurements of UI heavy metal concentrations (Fe, Cr, Ni, Cu, Zn, Pb, Cd, and As) were made concurrently using methods such as inductively coupled plasma mass spectrometry (ICP-MS) and atomic absorption spectroscopy (AAS). Subsequently, reference studies were used to compare the metal concentration in seaweed with safety standards in order to identify any possible health risks following ingestion.
Results
Effect of Ulva intestinalis water extract on freshwater microalgae Chlorella vulgaris
The impact of UI seaweed water extract on Chlorella sp. cell density over a period of seven days, as determined by absorbance at 680 nm, was depicted in Fig. 1A. The higher absorbance levels indicate greater cell growth, and the absorbance values are a good indicator of cell density. Increased cell density was frequently observed at higher concentrations of the extract, suggesting a possible growth-promoting action. The maximum cell density was significantly obtained at the highest concentration (5 mg mL−1), reaching 0.717 absorbance units by day 7. On the other hand, the cell density of the control group was the lowest and significantly decreased steadily from day 1 to day 7. This implies that the seaweed extract might promote Chlorella sp. growth rather than showing any phytotoxins.
Effect of UI water extract at different concentrations from 0.1 to 5 mg mL−1 on Chlorella vulgaris yield inhibition test from Day 1 to Day 7 by measuring (A) the culture absorbance (680 nm) and (B) cell density (106 Cells mL−1). Values are expressed as the mean ± standard error (SE) from measurements taken at least three times. Different letters denote statistically significant differences at p < 0.05.
Figure 1B shows the effect of UI seaweed water extract on Chlorella sp. cell density over seven days, expressed in ×106 cells mL−1. The data showed a dose-dependent increase in cell density with higher concentrations of the extract. The control group exhibited a decline in cell density, dropping from 2.48 million cells mL−1 on day 1 to 0.83 million cells mL−1 on day 7. In contrast, the highest concentration (5 mg mL−1) resulted in a significant increase, reaching 19.5 million cells mL−1 by day 7. This indicates that the seaweed extract promoted the growth of Chlorella sp. by acting as a source of nutrition and/or releasing growth-promoting phytochemicals in a concentration-dependent manner.
Effect of Ulva intestinalis water extract in Artemia cytotoxicity assay
Figure 2A shows the effects of water extract of UI seaweed on the survival of Artemia salina nauplii over 24, 48, and 72 h at various concentrations. The control group shows slight decreases in survival from 95% at 24 h- to 88.33% at 72 h, indicating baseline mortality. At the lowest concentration (0.001 mg mL−1), survival rates remain high, even slightly exceeding the control, suggesting negligible toxicity. Concentrations up to 1 mg mL−1 show similar trends, with survival rates close to or above the control values. However, at the highest concentration (10 mg mL−1), a noticeable decline in survival is observed, particularly at 48 and 72 h (81.67% and 78.33%, respectively), while after 24 h, survival rates (98.33%) interestingly above the control values. This indicates that while lower concentrations of the extract are relatively non-toxic, higher concentrations may cause some inhibitory effects, evidenced by reduced survival rates over time.
Effect of UI water extract at different concentrations from 0.001 to 10 mg mL−1 on Lactuta sativa germination inhibition test from 24 to 96 h by measuring (A) germination rate (%) (a) and its LC50 value (mg mL−1) (b), and (B) radicle length (mm) (a) and its LC50 value (mg mL−1) (b), at the respective time points. Values are expressed as the mean ± standard error (SE) from measurements taken at least three times. Different letters denote statistically significant differences at p < 0.05.
The LC50 values for Ulva sp. treatment on nauplii of Artemia salina over time are shown in Fig. 2B. Since no toxicity was seen after 24 h, the extract did not significantly increase mortality. The LC50 value at 48 h was roughly 33.37 mg mL−1, meaning that this quantity resulted in 50% of deaths. The LC50 value dropped to 30.87 mg mL−1 after 72 h, indicating gradually increasing mortality over time. However, accumulating results of LC50 value of Artemia lethality tests were concluded that UI water extract was considered as non-toxic to this assay.
Effect of Ulva intestinalis water extract on viability of Daphnia magna neonates
Figure 3A presents the survival rates of Daphnia exposed to various concentrations of water extract of UI seaweed over 24, 48, and 72 h. The control group showed a gradual decrease in survival from 90% at 24 h to 78.33% at 72 h, representing natural mortality. At the lowest concentration (0.001 mg mL−1), survival rates were slightly higher than the control, indicating no apparent toxicity. Concentrations up to 0.1 mg mL−1 also showed minimal impact, with survival rates comparable to the control. The LC50 values for UI treatment over time was shown in Fig. 3B. The LC50 value is 4.10 mg/mL after 24 h, dropping to 3.81 mg mL−1 after 48 h, and then to 3.41 mg mL−1 after 72 h, which regarded as no toxic effects in UI water extract on Daphnia acute toxicity assay.
Effect of UI water extract at different concentrations from 0.001 to 10 mg mL−1 on (A) survival of Artemia (%) and its (B) LC50 value (mg mL−1) at different time points from 24 h to 72 h. Values are expressed as the mean ± standard error (SE) from measurements taken at least three times. Different letters denote statistically significant differences at p < 0.05.
Effect of UI water extract on toxicity indices for Lactuca sativa seed
The effects of a 96 h seaweed water extract (UI) on the germination of Lactuca sativa (lettuce) seeds are shown in the table. After 24 h, the germination rate in the control group was 36.67%, and after 96 h, it reached 90%. In comparison to the control, lower concentrations (0.001–1 mg mL−1) usually increased germination rates; at 96 h, the highest rate (96.67%) was seen at 0.001 and 0.01 mg/mL. Only 26.67% of the seeds germinated after 96 h at the maximum dosage (10 mg mL−1), indicating significant inhibition of seed germination (Fig. 4A). This implies that while extract concentrations at lower levels can aid in seed germination, concentrations at greater levels are harmful to the seeds. The effect of UI seaweed water extract on Lactuca sativa seeds radicle length over a 96 h period is displayed in the table. The radicle length of the control group increased gradually over the course of 96 h, reaching 166.47 mm. In comparison to the control, lower dosages (0.001–1 mg mL−1) generally encouraged more radicle growth; the maximum was recorded at 0.001 mg mL−1 (194.14 mm at 96 h). In contrast, the radicles at the maximum dose (10 mg mL−1) only measured 50.52 mm in length after 96 h due to significant growth inhibition. This implies that large doses are inhibitory, but low quantities promote seed growth.
Effect of UI water extract at different concentrations from 0.001 to 10 mg mL−1 on (A) survival of Daphnia (%) and its (B) LC50 value (mg mL−1) at different time points from 24 h to 72 h. Values are expressed as the mean ± standard error (SE) from measurements taken at least three times. Different letters denote statistically significant differences at p < 0.05.
The germination of Lactuca sativa seeds increased over time, indicating a decreasing toxicity effect as exposure duration increases (Fig. 4B). The LC50 value at 24 h is 2.79 mg mL−1, indicating no toxicity. The LC50 increases to 6.71 mg mL−1 after 96 h, indicating that the growth of Lactuca sativa increased gradually and more seeds were able to survive and germinate. The effects of UI seaweed water extract on radicle length of Lactuca sativa seeds exhibited time-varying LC50 values. The LC50 at 24 h is 17.33 mg mL−1, indicating no inhibitory effects. On the other hand, LC50 value rises at 48 h (6.30 mg mL−1) and stays comparatively high at 72 h (7.14 mg mL−1). The LC50 value was further increased to 10.02 mg mL−1 after 96 h, showing growth-promoting effects. This variance reflects the growth of radicle length in time-dependent reaction to the effects of UI extract.
Chemical characteristics of UI water extract
The pH, TDS, and EC of UI water extracts (0.25 to 10 mg mL−1) provided insight on the possible toxicity when consumed. All concentrations of the UI extract showed to have decreasing trend of pH values (6.20–6.32) compared to control (6.95), whereas, values of TDS (38–675 ppm) and EC (77-1305 µS cm−1) were dose dependently increased compared to control (9.33 ppm and 19.66 µS cm−1, respectively). Moreover, at higher concentrations of UI (5–10 mg mL−1) showed increased levels of TDS and EC, indicating a greater load of dissolved ions and organic molecules (Table 1). The results suggested that moderate concentrations of the UI extract could be safer and non-toxic to organisms but higher concentrations might promote toxicity.
Heavy metal contents of Ulva intestinalis water extract
The analysis of heavy metals and metalloids content in UI was presented in Table 2 with other relevant studies. The findings of current study on Fe, Cr, Ni, Cu, Zn, Pb, Cd, and As concentrations in UI from Monkhali Beach, Cox’s Bazar revealed significantly lower levels compared to previous studies as follows: 43.60, 0.10, 0.44, 0.07, 0.27, 0.13, 0.00, and 0.00 mg/kg.
Health risk assessment of Ulva intestinalis for consumer
Table 3 presents an evaluation of the amounts of heavy metals in UI from the coast of Bangladesh. This analysis assessed the health hazards associated with consuming this seaweed, both non-carcinogenic and carcinogenic. The highest HQ value was 0.0032 for Fe, followed by 0.0022, 0.0011, 0.0009, 9.57 × 10−5 and 4.38 × 10−5 for lead (Pb), nickel (Ni), chromium (Cr), copper (Cu) and zinc (Zn), respectively; in which all were significantly below 1, indicating a very minimal risk of non-carcinogenic health impacts from these metals. The HQ values were combined to assess the Hazard Index (HI), which was 0.0077. This index further suggested that there was little cumulative non-carcinogenic risk from numerous metal exposures. In terms of CR, the values for CR for Cr, Ni, Cu, and Pb were all far less than 10^−6, falling within the range that health regulatory standards deemed insignificant or acceptable.
Discussion
Due to the availability of dietary fiber, omega-3 fatty acids, minerals, and vitamins, seaweed support better digestion, heart function, and metabolism. It has been demonstrated that the secondary metabolites of seaweed, such as antioxidants and anti-inflammatory compounds, lower the chance of developing chronic illnesses like diabetes, cancer, and cardiovascular disease2,3,22. However, for seaweed to be healthy for human consumption, it must be free of heavy metals and other toxic elements, as these harmful substances might counteract the health-promoting properties of seaweed. According to the results, UI extract helps Chlorella sp. grow, most likely because it contains important minerals or phytochemicals that stimulate growth4. This is consistent with previous research that has demonstrated the ability of seaweed extracts to promote microalgal development. For instance, Nash et al.23 used a model microalgal species for aquatic phytotoxicity assays, highlighting the potential of seaweed extracts to stimulate growth. Ouyang et al.24 showed that certain heavy metals at sub-lethal concentrations could impact the growth and photosynthesis of Chlorella sp. Similarly, Dauda et al.25 discovered that titanium dioxide nanoparticles affected the growth of Chlorella sp. The significant growth noted at increased doses suggests that UI extract may prove to be a feasible dietary supplement.
Tests on the lethality of Daphnia and Artemia revealed that UI water extract was deemed non-toxic for these particular assays. In support of this statement, Daphnia magna standard LC50 values can vary greatly, but in general, compounds with LC50 values less than 1 µg mL−1 are regarded as very toxic, and those with LC50 values between 1 and 10 µg mL−1 as moderately toxic; a value over 10 mg/L would suggest minimal toxicity10,26. A survey of the literature on the LC50 values of Artemia salina nauplii for toxicity tests showed considerable variation between scientific studies. Birrell et al.27 showed that the compounds found in seaweed can affect Artemia salina, while Brock et al.28 found the same thing for Fucus vesiculosus (Phaeophyceae) seaweed. When Ayesha et al.29 investigated the cytotoxicity of seaweeds from the Karachi Coast, they revealed that Artemia salina nauplii was subject to diverse harmful effects. The toxicity of phloroglucinol and crude phlorotannins was assessed by Harwanto et al.10, demonstrating the adaptability of Artemia salina as a model organism in toxicity tests. This is consistent with research by Choi and Choi30, who found that depending on the dose used, several marine algae extracts can either inhibit or stimulate germination. While high doses of the extract are inhibitory, lower concentrations encourage growth in phytotoxicity bioassay. With extracts from Padina gymnospora, González-Giro et al.15 reported similar findings. Furthermore, as noted by Bagur-González et al.31, the phytotoxicity of extracts may be influenced by the presence of heavy metals and other environmental conditions, which could account for the inhibitory effects observed at greater doses. Lower LC50 values (nearer to 1 µg mL−1) for Lactuca sativa are indicative of increased toxicity, as demonstrated by certain pharmaceuticals and pesticides32. Similar time-dependent toxicity variations were observed by Hofstätter et al. (2024)32 in their pesticide and water quality analysis. Considering the results from previous studies suggested that UI extract were not toxic to germination and radicle length of Lactuca sativa (lettuce) seeds in bioassay. The findings indicated that the release of dissolved solids and ions, as shown by the TDS and EC values, increased with the elevated concentration of UI water extracts. Osmotic and ionic imbalances as well as potential metal mobilization could make this rise potentially harmful to organisms, particularly at higher concentrations16. This statement was in agreement with the present findings, where UI water extract at 10 mg mL−1 showed potential toxic to the tested organisms. Additionally, the modest pH drop suggested increased acidity, which could be harmful in aquatic habitats with higher sensitivity.
Fe concentration in the present study was markedly lower than in Siddique et al.34 from Saint Martin’s Island (2290.26 mg/kg), Baghazadeh et al.33 from the southern coast of Iran (440.57 mg/kg), and Mohamed and Khaled35 from Alexandria, Egypt (118.65 mg/kg), suggesting regional differences in environmental contamination and industrial activities. Cr levels were also lower compared to 23.46 mg/kg by Siddique et al.34 and Rakib et al.36 from Cox’s Bazar (0.64 mg/kg), indicating reduced exposure to this metal in the current sampling area. Moreover, Ni concentration was significantly lower than that reported 13.75 mg/kg by Siddique et al.34 and 14.02 mg/kg by Baghazadeh et al.33, highlighting potential differences in local sources of contamination. Cu content was much lower compared to previous studies, including 11.96 mg/kg by Siddique et al.34 and 27.49 mg/kg by Baghazadeh et al.33, indicating minimal industrial discharges in the study area. Zn levels were also considerably lower than those reported in other regions, such as 13.03 mg/kg by Siddique et al.34 and 26.65 mg/kg by Baghazadeh et al.33. Similarly, Pb levels were lower than in previous reports of Siddique et al.34 noting 6.67 mg/kg and Rakib et al.36 reporting 2.76 mg/kg. Moreover, the current study detected no Cd and As in UI, unlike previous studies, who reported detectable levels of these metals34,36.
CR is generally seen as falling into an acceptable range if it is between 10−6 and 10−4, with below 10−6 being considered to be negligible and CR beyond 10−4 being unacceptable36,37. Overall CR value from UI seaweed was 1.09 × 10−8, indicating safe to eat because it presents little harm to health. The absence of data for arsenic (As) and cadmium (Cd) in UI was noteworthy; this could be because the amounts were below detectable levels. Using the USEPA37,38 approaches as a guide, the studies conducted by Rakib et al.36, Siddique et al.17, and Islam et al.39 evaluated health hazards from metal exposure using the Target HQ, HI, CR, and Total CR. After examining seaweed in the Bay of Bengal, Rakib et al.36 reported that certain metals have HQs above 1, which suggests non-carcinogenic risks to human health. Similar results were found when Siddique et al.17 looked at dried red seaweed. Islam et al.39 extended his evaluation to the northern part of Bangladesh to include fish and vegetables. They found that CR values occasionally above the permissible range, suggesting possible long-term carcinogenic risks, and that HQ and HI values were over the safety threshold. Comparing the results with those studies, UI possessed significantly decreased level of heavy metal concentrations, which was might be due to the collection of this seaweed from floating raft culture, indicating no possible health risks upon consumption. However, further studies are needed on the molecular and physiological processes underlying the decreased heavy metal bioaccumulation in seaweed grown on floating rafts. Comprehending these mechanisms may facilitate the development of seaweed cultivars or variants that are even more impervious to the absorption of heavy metals.
Based on the findings of current study, it is crucial to pay attention to some recommendations for reducing contamination in order to guarantee the safe intake of UI. Seaweed should be harvested from low-risk locations, away from cities, industrial zones, and areas that are prone to pollution. Harvesting should not be done when pollution is at its highest, such as right after an extensive amount of rain or when runoff is high. UI should be thoroughly rinsed with clean water after collection to get rid of sediments and contaminants. To lower the microbial load, it should also be sun-dried in a clean environment. Safety can be further ensured by routine testing for microplastics and heavy metals, and safer seaweed harvesting and processing can be promoted by teaching local communities for optimal practices. Therefore, these recommendations might help to improve the safety of Ulva intestinalis collected from Bangladesh’s coastal areas, encouraging people to adopt it as a low-risk and nutrient-dense functional food.
Methods
Study area
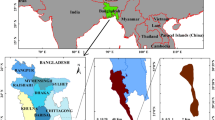
Located in Cox’s Bazar district on the southern coast of Bangladesh, Monkhali beach, positioned at approximately 20.9695 °N latitude and 92.3690 °E longitude, is part of the famous Cox’s Bazar beach (Fig. 5A). This beach stands out for its scenic beauty and relatively low level of commercial development compared to other areas of Cox’s Bazar. It stretches for miles, providing a natural setting ideal for marine explorations. Recently, two species of seaweed Ulva intestinalis and Gracilaria tenuistipitata have been included for floating raft culture in this area. Water quality parameters in the culture site were recorded. The observed salinity of 30.07 ± 0.20 ppt indicated stable saline conditions. With an average temperature of 23.53 ± 0.12 °C, the conditions were ideal for seaweed growth. With a transparency of 72.30 ± 0.12 cm, excellent light penetration was indicated. At 8.40 ± 0.17, the pH level was slightly alkaline, making it appropriate for the majority of seaweed species. Seaweed and related aquatic organisms require aerobic conditions, which were supported by the dissolved oxygen (DO) of 6.77 ± 0.09 mg/L. These metrics pointed to ideal growing conditions for seaweed at this ___location.
(A) Seaweed collection area of Monkhali beach, Cox’s Bazar district of Bangladesh (a) and floating raft culture plot (b). (B) Representative images of collected Ulva intestinalis (UI) seaweed (a), water ssssextraction of UI (b), biotoxicity tests of UI on different organisms such as Chlorella vulgaris (c), Artemia salina (d), Daphnia magna (e), and Lactuca sativa seed grown on petridish (f).
Collection of Ulva intestinalis seaweed
UI seaweed was collected from floating raft culture of Monkhali beach, Cox’s Bazar in March 2022 (Fig. 5B-a). Fifteen floating raft long-line culture plots were set up in the Monkhali study region. It was anticipated that the floating raft net long-line culture plots were 66 square meters in size (22 m long by 3 m wide). A floating raft long-line culture plot made up of 6 long-line ropes of 3 m long. An assembly of roughly 11 propagules, each weighing an estimated 6 g, was assigned to each long-line rope. UI seaweed was harvested every 20–25 days once the seaweed attained a length of 30–40 cm. The sample was collected and taken straight to the lab for additional processing. It was then put in a sizable plastic container filled to the full capacity with seawater.
Processing of Ulva intestinalis water extract
Considering the edibility study of UI, distilled water was used as solvent to prepare extract for toxicity study (Fig. 5B-b). The harvested UI was rinsed with distilled water after being properly cleansed with tap water to get removal of surface contaminants. The seaweed was cleaned, allowed to air dry at room temperature, and then extracted. In order to maintain uniformity and minimize possible impurities, we used distilled water in every extraction procedure. A 4 g of finely ground seaweed was steeped in 100 mL of solvent. To expedite extraction, the sample was spun on and off for 24 h at 40 °C and 120 rpm in a shaking incubator (JSSI-100T JSR South Korea). After incubation, the mixture was passed through Whatman filter paper No. 4 (20–25 μm). To guarantee the highest possible extraction, this procedure was carried out twice more. After filtering, the material was dried in a freeze drier for 48 h at – 110 °C (VC-2200, LABOGENE, GYROZEN Co. Ltd.) until dry completely, and it was kept at 4 °C until the experiment was finished. The extraction yield of UI water extract was 29.54% (w/w %). In the end, 5 mg mL−1 solutions were prepared for every extract for further experiments.
Freshwater Chlorella vulgaris yield inhibition test
The experimental procedure was modified compared to prior methodology40. In the experimental setup, Chlorella sp. was introduced into 5 mL eppendorf conical tubes containing 2 mL BBM medium and various concentrations of UI water extract (0.1, 0.5, 1, 2.5, and 5 mg mL−1) were added (Fig. 5B-c). A control group without UI was used at the same time; each experimental condition involving Chlorella sp. experiment was repeated three times. All tubes were maintained in an incubator under consistent environmental conditions similar to those used for biological maintenance. Aseptic technique was strictly followed to reduce the risk of contamination. The total exposure time was 7 days. The optical density (OD) of algal suspensions was recorded at intervals of each day at a wavelength of 680 nm (OD680) using a SPECTROstar® Nano spectrophotometer (BMG Labtech). To determine the correlation between OD680 and cell density (CD, cells mL−1), high-density algal culture medium was diluted to four different concentrations. The OD680 values corresponding to each concentration were then evaluated. CD was then quantified using a hemocytometer under a microscope according to the Neubauer counting method; a linear correlation was established by comparing OD values with Chlorella sp. cell volume, yielding the following equation:
Artemia and Daphnia lethal test
The lethality assessment methods employed for Artemia salina nauplii and Daphnia magna were slightly modified compared to previous reports10,41. This experiment was conducted to determine the toxicological effects of UI water extract on Artemia (Fig. 5B-d) and Daphnia (Fig. 5B-e). In summary, 1000 L of seawater sterilized with 0.22 mm filters (for Artemia) or distilled water (for Daphnia) was carefully dispensed into 24-well plates. A solution containing Artemia or Daphnia larvae (400 µL, containing 20 larvae) was introduced into each well. UI water extract was administered at concentrations of 0.001, 0.01, 0.1, 1, and 10.0 mg mL−1 for Artemia and Daphnia. Artemia and Daphnia in sterile seawater or freshwater was then quickly added to bring the final well volume to 2.0 mL. Toxicity was evaluated by counting the number of surviving larvae and calculating mortality after 24, 48, and 72 h of exposure. A vehicle control consisting of 0.22 μm filtered sterile seawater (for Artemia) or distilled freshwater for Daphnia was conducted. Mortality was assessed according to established criteria10, with slight modifications. Larvae were considered dead if they did not show motility throughout the observation period. If mortality was less than 50%, the extract concentration was classified as nonlethal; if mortality was between 50% and 75%, the extract concentration was considered mild larvicidal; if mortality is greater than 75%, the extract concentration is considered highly larvicidal; and finally, if complete larval mortality is 100%, the extract is classified as very larvicidal10.
Bioassays for Lactuta sativa germination
One mL of each concentration of UI water extract (0.001, 0.01, 0.1, 1, and 10.0 mg mL−1) was added to filter paper (55 mm diameter; Toyo Filter PaperCo., Tokyo, Japan) placed in a sterile Petri dish (6 cm diameter; SPL Life Science, Pocheon, Korea). The filter paper containing extract or vehicle was then dried completely on a clean bench to ensure complete absorption of the sample into the filter paper. Ten sterilized L. sativa seeds were placed evenly on the filter paper containing extract in each petri dish (Fig. 5B-f). They were then moistened with 1.2 mL of polyoxyethylene sorbitan monolaurate (Tween 20, L33109, Duksan, Korea) at 1% concentration10. Tween 20 was used as a surfactant that was considered non-toxic30. All petri dishes were placed in an incubator in the dark at 25 ± 1 °C. Germination rate was determined by counting the number of germinated seeds at 24 h intervals for 4 days. Germination was defined as when the rhizobial leaf protruded more than 1 mm30. Root length was measured manually using a digital caliper under aseptic conditions. Germination percentage in each treatment was calculated and compared to the control with 1% Tween 20 without phlorotannin or phloroglucinol. Germination rate (%) was calculated as n/N × 100. where n is the number of seeds germinated and N is the number of seeds sown30.
Chemical analyses of UI water extract
Different concentrations of UI water extracts (0.25, 0.5, 1, 5 and 10 mg mL−1) were prepared for chemical analyses of pH, total dissolved solids (TDS), and electrical conductivity (EC). Digital multi-parameter probe (HI98129, Hanna Instruments Ltd, Eden Way, UK) was used for chemical analyses in the laboratory.
Determination of minerals and heavy metals
Using an ICP-OES optima 2000 DV (PerkinElmer, Inc., Waltham, USA) equipped with winLab32 software, version 6.0 (PerkinElmer, Inc., Waltham, USA; https://www.perkinelmer.com), the minerals and heavy metal content of the UI sample was determined. The working conditions of the instrument were preserved with minor adjustments, as previously mentioned42. Initially, 1 g of each sample was placed into a muffle furnace and heated at 600 °C for 6 h to convert it into ash. Following this, 4 mL of nitric acid and 1 mL of peroxide were added to the ashed residue, with distilled water being added to reach a final volume of 60 mL. The resulting solution was then heated on a hot plate until it reduced to 30 mL. At this stage, 4 mL of distilled water and 1 mL of peroxide were added, and the solution was heated again until it was reduced to 17.5 mL. After this second reduction, additional distilled water was added to bring the total volume back to 50 mL. The solution was then filtered using 125 mm filter paper, with the filtering process being performed twice. Once filtering was completed, the solution was prepared for mineral and heavy metal analysis. Mineral and heavy metal concentrations were measured on the basis of mg/100 g of dry weight.
Health risk assessment
The evaluation of health risks (non-carcinogenic) was performed using exposure dose, the Hazard Quotient (HQ), and the Hazard Index (HI), as outlined by the US EPA (1989, 2010)37,38 and Anandkumar et al.43. The HI was derived from the average metal concentrations found in the seaweeds of this study, compared with the reference values provided by US EPA guidelines37,38. The HI represents the total of all individual Hazard Quotients (HQs) for each metal. The calculation of chronic daily intake (CDI) was based on the following formula:
Where, Cmetal = Mean concentration of metal, CoR = Consumption rate [5.2 g–0.0052 kg/person/day], EF = Exposure frequency (250 days), ED = Exposure duration (70 years), BW = Average body weight (70 kg), and AT = Averaging time (70 × 365 days).
Increased HQ values indicate a greater likelihood of long-term non-carcinogenic health consequences39.
Where, RfD = Reference dose (mg/kg/day), values were given for each metal in Table 4.
According to the USEPA38 guidelines, an HI value of less than 1 indicates that no significant health risk is anticipated. Conversely, an HI value exceeding 1 suggests a moderate to high risk of negative effects on human health.
The increased chance that an individual will get cancer during their lifetime as a result of being exposed to the possible carcinogen was determined to be the target carcinogenic risk38,39. The following equation was used to estimate the target carcinogenic risk (1):
Where, FIR = Food ingestion rate (5.2 g/person/day), CSFo = Carcinogenic potency slope (mg/kg body weight/day). Table 4 showed the CSFo oral carcinogenic slope factor of metals derived from prior reports36,38,44,45.
Statistical analysis
The data was shown as mean ± SE (n = 3). One-way analysis of variance (ANOVA) with post hoc Duncan multiple comparisons will be used for statistical comparisons using SPSS software, version 25.0 (IBM Software, Inc., Chicago, IL, USA; https://www.ibm.com/spss). A predetermined threshold of p ≤ 0.05 will be used to define statistical significance. Using linear regression analysis, which analyzes the link between concentration and mortality proportion, median lethal concentrations (LC50) of the extract were determined. In order to make sure the regression line fits the data correctly; the data were finally confirmed using the R-squared value.
Data availability
Upon reasonable request, data can be obtained from the corresponding author.
References
Morais, T. et al. Seaweed potential in the animal feed: a review. J. Mar. Sci. Eng. 8, 559 (2020).
Mohamed, S., Hashim, S. N. & Rahman, H. A. Seaweeds: a sustainable functional food for complementary and alternative therapy. Trends Food Sci. Technol. 23, 83–96 (2012).
Cox, S., Abu-Ghannam, N. & Gupta, S. An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds (2010).
Mohibbullah, M. et al. The edible seaweed Gelidium amansii promotes structural plasticity of hippocampal neurons and improves scopolamine-induced learning and memory impairment in mice. CNS Neurol. Disord Drug Targets (Formerly Curr. Drug Targets-CNS Neurol. Disorders) 22, 1391–1402 (2023).
Mohibbullah, M. et al. A systematic review on marine algae-derived fucoxanthin: an update of pharmacological insights. Mar. Drugs 20, 279 (2022).
Ma, Z. et al. Total and inorganic arsenic contents in seaweeds: absorption, accumulation, transformation and toxicity. Aquaculture 497, 49–55 (2018).
Olsson, J., Toth, G. B. & Albers, E. Biochemical composition of red, green and brown seaweeds on the Swedish west coast. J. Appl. Phycol. 32, 3305–3317 (2020).
Paz, S. et al. Toxic metals (Al, cd, pb and hg) in the most consumed edible seaweeds in Europe. Chemosphere 218, 879–884 (2019).
Hossain, M. T. et al. Nutritional value, phytochemical profile, antioxidant property and agar yielding potential of macroalgae from Coasts of Cox’s Bazar and St. Martin’s island of Bangladesh. J. Aquat. Food Prod. Technol. 30, 217–227 (2021).
Harwanto, D., Negara, B. F. S. P., Tirtawijaya, G., Meinita, M. D. N. & Choi, J. S. Evaluation of toxicity of crude phlorotannins and phloroglucinol using different model organisms. Toxins 14, 312 (2022).
Qian, L. et al. Toxic effects of boscalid on the growth, photosynthesis, antioxidant system and metabolism of Chlorella vulgaris. Environ. Pollut. 242, 171–181 (2018).
Manfra, L., Savorelli, F., Pisapia, M., Magaletti, E. & Cicero, A. M. Long-term lethal toxicity test with the crustacean Artemia franciscana. J. Vis. Exp. : JoVE (2012).
Tkaczyk, A., Bownik, A., Dudka, J., Kowal, K. & Ślaska, B. Daphnia magna model in the toxicity assessment of pharmaceuticals: a review. Sci. Total Environ. 763, 143038, https://doi.org/10.1016/j.scitotenv.2020.143038
Nunes, B. S., Carvalho, F. D., Guilhermino, L. M. & Van Stappen, G. Use of the genus Artemia in ecotoxicity testing. Environ. Pollut. 144, 453–462 (2006).
González-Giro, Z., Batista-Corbal, P. L., González-Pérez, Y. & Rodríguez-Leblanch, E. & Marcos-Albear, E. Phytotoxicity evaluation of an aqueous extract of Padina gymnospora seaweed on Lactuca sativa L. seeds (2018).
Fouad, M. S., Mustafa, E. F., Hellal, M. S. & Mwaheb, M. A. A comprehensive assessment of water quality in Fayoum depression, Egypt: identifying contaminants, antibiotic pollution, and adsorption treatability study for remediation. Sci. Rep. 14, 18849 (2024).
Siddique, M. A. M. et al. Metal and metalloid bioaccumulation in dried red seaweed Hypnea musciformis and health risk assessment for consumers. Mar. Pollut. Bull. 194, 115302. https://doi.org/10.1016/j.marpolbul.2023.115302 (2023).
Filippini, M. et al. Heavy metals and potential risks in edible seaweed on the market in Italy. Chemosphere 263, 127983 (2021).
Pan, Y. et al. Screening of seaweeds in the East China Sea as potential bio-monitors of heavy metals. Environ. Sci. Pollut Res. 25, 16640–16651 (2018).
Dadolahi-Sohrab, A., Mohamad, S. A., Nabavi, B., Safahyeh, A. & Ketal-Mohseni, M. Environmental Monitoring of Heavy Metals in Seaweed and Associated Sediment from the Strait of Hormuz, I.R. Iran (2011).
Malea, P., Haritonidis, S. & Kevrekidis, T. Metal content of some green and brown seaweeds from Antikyra Gulf (Greece). Hydrobiologia 310, 19–31 (1995).
Gade, R., Tulasi, M. S. & Bhai, V. A. Seaweeds: a novel biomaterial. Int. J. Pharm. Pharm. Sci. 5, 975–1491 (2013).
Nash, S. M. B., Quayle, P. A., Schreiber, U. & Müller, J. F. The selection of a model microalgal species as biomaterial for a novel aquatic phytotoxicity assay. Aquat. Toxicol. 72, 315–326 (2005).
Ouyang, H. et al. Effects of five heavy metals at sub-lethal concentrations on the growth and photosynthesis of Chlorella vulgaris. Chin. Sci. Bull. 57, 3363–3370 (2012).
Dauda, S., Chia, M. A. & Bako, S. P. Toxicity of titanium dioxide nanoparticles to Chlorella vulgaris Beyerinck (Beijerinck) 1890 (Trebouxiophyceae, Chlorophyta) under changing nitrogen conditions. Aquat. Toxicol. 187, 108–114 (2017).
Biesinger, K. E., Williams, L. R. & van der Schalie, W. H. Procedures for conducting Daphnia magna toxicity bioassays. User’s guide (1987).
Birrell, C. L., McCook, L. J., Willis, B. L. & Harrington, L. Chemical effects of macroalgae on larval settlement of the broadcast spawning coral Acropora millepora. Mar. Ecol. Prog. Ser. 362, 129–137 (2008).
Brock, E., Nylund, G. M. & Pavia, H. Chemical inhibition of barnacle larval settlement by the brown alga Fucus vesiculosus. Mar. Ecol. Prog. Ser. 337, 165–174 (2007).
Ayesha, H., Sultana, V., Ara, J. & Ehteshamul-Haque In vitro cytotoxicity of seaweeds from Karachi coast on brine shrimp. Pak J. Bot. 42, 3555–3560 (2010).
Choi, J. S. & Choi, I. S. Inhibitory effect of marine green algal extracts on germination of Lactuca sativa seeds. J. Environ. Biol. 37, 207 (2016).
Bagur-González, M. G., Estepa-Molina, C., Martín-Peinado, F. & Morales-Ruano, S. Toxicity assessment using Lactuca sativa L. bioassay of the metal (loid) s as, Cu, Mn, Pb and Zn in soluble-in-water saturated soil extracts from an abandoned mining site. J. Soil. Sediment. 11, 281–289 (2011).
Hofstätter, K. et al. Water quality, pesticides, and phytotoxicity in seeds of Lactuca sativa, Solanum lycopersicum, and Eruca sativa. Int. J. Environ. Sci. Technol. 21, 5971–5980 (2024).
Baghazadeh Daryaii, L., Samsampour, D., Bgheri, A. & Sohrabipour, J. The high content of heavy metals in seaweed species: a case study in the Persian Gulf and the Gulf of Oman in the southern coast of Iran. Plant. Algae Env. 4, 544–560 (2020).
Siddique, M. A. M., Hossain, M. S., Islam, M. M., Rahman, M. & Kibria, G. Heavy metals and metalloids in edible seaweeds of Saint Martin’s Island, Bay of Bengal, and their potential health risks. Mar. Pollut. Bull. 181, 113866. https://doi.org/10.1016/j.marpolbul.2022.113866 (2022).
Mohamed, L. A. & Khaled, A. Comparative study of heavy metal distribution in some coastal seaweeds of Alexandria, Egypt. Chem. Ecol. 21, 181–189 (2005).
Rakib, M. R. J. et al. Macroalgae in biomonitoring of metal pollution in the Bay of Bengal coastal waters of Cox’s Bazar and surrounding areas. Sci. Rep. 11, 20999. https://doi.org/10.1038/s41598-021-99750-7 (2021).
USEPA. Risk assessment guidance for superfund: Human health evaluation manual part A, interim final. In Washington DC: United States Environmental Protection Agency; EPA/540/1–89/002 vol. I (1989).
USEPA. Risk-Based Concentration Table. http://www.epa.gov/reg3hwmd/risk/human/index.htm (2010).
Islam, M. S. et al. Health risk assessment due to heavy metal exposure from commonly consumed fish and vegetables. Environ. Syst. Decis. 36, 253–265 (2016).
Xiong, J. Q., Kurade, M. B. & Jeon, B. H. Biodegradation of levofloxacin by an acclimated freshwater microalga, Chlorella vulgaris. Chem. Eng. J. 313, 1251–1257. https://doi.org/10.1016/j.cej.2016.11.017 (2023).
Kim, Y. D. & Choi, J. S. Larvicidal effects of Korean seaweed extracts on brine shrimp Artemia salina. J. Anim. Plant. Sci. 27, 1039–1046 (2017).
Kumaravel, S. & Alagusundaram, K. Determination of mineral content in Indian spices by ICP-OES. Orient. J. Chem. 30, 631–636 (2014).
Anandkumar, A. et al. Bioaccumulation of trace metals in the coastal Borneo (Malaysia) and health risk assessment. Mar. Pollut. Bull. 145, 56–66. https://doi.org/10.1016/j.marpolbul.2019.05.002 (2019).
Soumaoro, I., Pitala, W., Gnandi, K. & Kokou, T. Health risk assessment of heavy metal accumulation in broiler chickens and heavy metal removal in drinking water using Moringa Oleifera seeds in Lomé, Togo. J. Health Pollut. 11, 210911.https://doi.org/10.5696/2156-9614-11.31.210911 (2023).
Ali, A. Y. A., Idris, A. M., Eltayeb, M. A. H., El-Zahhar, A. A. & Ashraf, I. M. Bioaccumulation and health risk assessment of toxic metals in red algae in Sudanese Red Sea coast. Toxin Rev. 40, 1327–1337 (2021).
Acknowledgements
This research work was funded by Sustainable Coastal and Marine Fisheries Project, Department of Fisheries, Matshya Bhaban, Ramna, Dhaka-1000, Bangladesh (Project ID Number: P161568_2022-23).
Author information
Authors and Affiliations
Contributions
M.M.: Conceptualization, Methodology, Validation, Sample analysis, Data curation, Supervision, Investigation, Project administration, Writing-Original Draft, Writing-Review and Editing. M.A.H.; M.A.M.: Methodology, Data Curation, Sample analysis. M.M.I.; M.S.A.: Investigation, Project administration; M.N.A.K; J.-S.C: Methodology, Sample analysis, Validation, Writing, Review and Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mohibbullah, M., Hossain, M.A., Mithu, M.A. et al. Edibility of cultivated green seaweed Ulva intestinalis from Monkhali Beach, Cox’s Bazar coast of Bangladesh: bio-toxicity and heavy metal contents. Sci Rep 14, 32124 (2024). https://doi.org/10.1038/s41598-024-83909-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-83909-z