Abstract
De-regulated protein expression contributes to tumor growth and progression in medulloblastoma (MB), the most common malignant brain tumor in children. MB is associated with impaired differentiation of specific neural progenitors, suggesting that the deregulation of proteins involved in neural physiology could contribute to the transformed phenotype in MB. Calsynthenin 1 (CLSTN1) is a neuronal protein involved in cell-cell interaction, vesicle trafficking, and synaptic signaling. We previously identified CLSTN1 as a putative target of the pro-invasive kinase MAP4K4, which we found to reduce CLSTN1 surface expression. Herein, we explored the expression and functional significance of CLSTN1 in MB. We found that CLSTN1 expression is decreased in primary MB tumors compared to tumor-free cerebellum or brain tissues. CLSTN1 is expressed in laboratory-established MB cell lines, where it localized to the plasma membrane, intracellular vesicular structures, and regions of cell-cell contact. The reduction of CLSTN1 expression significantly increased growth factor-driven invasiveness. Pharmacological inhibition of pro-migratory MAP4 kinases caused increased CLSTN1 expression and CLSTN1 accumulation in cell-cell contacts. Co-culture of tumor cells with astrocytes increased CLSTN1 localization in cell-cell contacts, which was further enhanced by MAP4K inhibition. Our study revealed a repressive function of CLSTN1 in growth-factor-driven invasiveness in MB, identified MAP4 kinases as repressors of CLSTN1 recruitment to cell-cell contacts, and points towards CLSTN1 implication in the kinase-controlled regulation of tumor-microenvironment interaction.
Similar content being viewed by others
Introduction
Medulloblastoma (MB) is the most common malignant pediatric brain tumor; it is subgrouped into WNT, SHH, Group 3 (Grp3), and Group 4 (Grp4) MB1. Despite considerably improved molecular diagnostics, prognosis remains dismal for patients with disseminated disease2. A commonality of MB across subgroups is impaired differentiation of specific neural progenitors as a mechanism associated with tumor initiation3,4,5,6,7,8. Neuronal-like, undifferentiated, and differentiating malignant populations of cells are the originating cells of WNT, SHH, and Grp3 tumors4. SHH tumors closely resembled granule neurons of varying differentiation states that correlated with patient age, whereas Grp3 tumors exhibit a developmental trajectory from primitive progenitor-like to more mature neuronal-like cells4. It follows that common to all MB are likely some neuronal traits, which may include neuron-specific Ca2+signal transmission as well as the capability to form synapse-like cell-cell interactions. Additionally, OLIG2-expressing glial cells are highly enriched in therapy-resistant and recurrent medulloblastomas and display highly up-regulated gene sets of the neuronal and astrocyte lineages5. However, the contribution of neural-specific proteins involved in cell-cell interaction regulation and Ca2+ signaling to MB growth and tissue invasion remains poorly understood.
In a previous surface proteomic analysis in medulloblastoma cells, we found that knock-down of the Ser/Thr kinase MAP4K4 increased the plasma membrane association of the kinesin 1 adaptor protein Calsyntenin-1 (CLSTN1)9. CLSTN1 was originally identified as a proteolytically cleaved protein in postsynaptic membranes of both excitatory and inhibitory neurons, and through its C-terminal Ca2+ binding capabilities, it was suggested to control synaptic Ca2+ signaling10. CLSTN2 and CLSTN3 were subsequently identified based on high sequence similarities, whereby CLSTN1 was found to be ubiquitously expressed in all CNS neurons and CLSTN2 and 3 showed highest expression in GABAergic neurons11. CLSTN1 binds to the light chain of kinesin 1 (KLC1) via two conserved L/M-E/D-W-D-D-S motifs and is involved in anterograde tubulovesicular transport in neurons12,13. CLSTN1 recruits KLC1 to the trans-Golgi network (TGN), and overexpression of CLSTN1 causes ER and Golgi stacking14. This phenotype is explained by a disturbed stoichiometry of the physiological interaction of two CLSTN1 C-termini with one KLC1 molecule, which mediates vesicle cargo coupling to kinesin motors for anterograde transport13. CLSTN1 binding to KLC1 can be negatively regulated by phosphorylation of S460 in KLC115. The repression of ERK was shown to reduce S460 phosphorylation and increase CLSTN1-KLC1 binding, suggesting that CLSTN1 trafficking could be controlled by reversible KLC1 S460 phosphorylation downstream of MAPK signaling15. Increased KLC1 S460 phosphorylation was observed in Alzheimer’s disease (AD), which suggested that reduced CLSTN1-KCL1 interaction may contribute to AD onset or progression16. CLSTN1 controls N-methyl-D-aspartate (NMDA) receptor subunit trafficking in pyramidal neurons during postnatal development in mice, and CLSTN1 knock-out (KO) impaired neuronal arborization and maturation17. CLSTN1 also regulates the transport of Rab5-positive endosomes towards specific compartments in developing axons, and it was suggested that this directed transport contributes to axon branching18. CLSTN1 also restricts the surface expression of ICAM5 in cultured neurons, which is necessary for proper spine maturation in dendrites. Reduced CLSTN1 and increased ICAM5 membrane localization further correlate with excessive formation of filopodia-like spines and impaired spine formation19.
Collectively, these studies on CLSTN1 indicate a critical function of CLSTN1 in the anterograde transport of cargo from ER/Golgi toward specific peripheral compartments in neuronal cells. Little is known about CLSTN1 function in cancer. ESRP1-mediated exon 11 skip splicing of CLSTN1 has been implicated as an anti-metastatic mechanism in gastric cancer, whereby the alternatively spliced, shortened CLSTN1 restricts migration and invasion by stabilizing E-cadherin-β-catenin binding20. The RNA binding protein AKAP8 was also implicated in CLSTN1 exon 11 skip splicing, yielding a short CLSTN1 isoform involved in maintaining an epithelial state and restricting metastasis in breast cancer21.
MAP4K4 is overexpressed in MB tissue compared to normal cerebellum and contributes to tissue invasion downstream of growth factor signaling in MB22,23. However, how MAP4K4 is connected to CLSTN1 and whether an interaction of MAP4K4 and CLSTN1 could be functionally relevant in MB is not known. Here, we explored whether CLSTN1 could contribute to the cancerous phenotype in MB and whether MAP4 kinase modulation of CLSTN1 expression could constitute an alternative mechanism to exon 11 skip splicing control of migration and invasion.
Materials and methods
Cells and tissue culture
Cell lines used
All cell lines used are described in the supplementary methods file. All media, reagents, and solutions used were filtered using 0.45 μm filters. The standard cell culture media were prepared using 10% heat-inactivated Fetal Bovine Serum (FBS), 1% Penicillin Streptomycin (P/S), and 1% L-glutamine. Serum-free media (SFM) were supplemented with 1% P/S and 1% L-glutamine only.
Generation of stable cell lines
Stable MB cell lines over-expressing mNeonGreen(mNG)-tagged CLSTN1 (CLSTN1-mNG) and V5-tagged CLSTN1 (CLSTN1-V5) were generated using lentiviral transduction and drug selection. Lentivirus was produced in HEK293-T cells transfected with 4.5 µg pPAX2, 3 µg pVSVG and 7.5 µg of either pLV-Bsd-CMV-hCLSTN1-3xGGGGS-mNeonGreen or pLV-Bsd-CMV-hCLSTN1-3xGGGGS-V5. Medium was replaced after 18 h and virus-containing supernatant was collected 30 h after transfection. The supernatant was centrifuged (300xg, 5 min) and filtered using a 5 μm pore filter. 1 ml of filtered supernatant was added to the recipient cells in 1 ml complete medium with 10 µg/ml polybrene. Filtered supernatants were stored at −80 °C until further use. Two days after transduction, the MB cells were expanded, and the following day, antibiotic selection was started using 5 µg/ml blasticidine dissolved in fresh complete medium. The selection was continued for two weeks and the medium containing blasticidine was changed every second day. Ectopic protein expression levels of CLSTN1-mNG and CLSTN1-V5 were confirmed using IB or flow cytometry (FC) analysis, and uniformity of expression by microscopy imaging. UW228 LA-EGFP_mCherry-Nuc9 cells were produced by lentiviral transduction of UW228 with pLenti-LA-EGFP and Nuc9mCherry. Two days after transduction, cells were transferred to selection medium containing 2 µg/ml puromycin for at least three passages. LA-EGFP expression was confirmed by microscopy imaging.
Gene expression analyses in human primary samples
mRNA expression analysis across tumor samples and healthy tissues was performed using the R2 Genomic Analysis & Visualization Platform. The following control datasets were used: Diseased brain: GSE9770; Brain regions: GSE11882; Cerebellum (ages of postmortem donors in years: 25 (m), 38 (m), 39 (f), 30 (m), 35 (m), 52 (m), 50 (f), 48 (f), 53 (f), 23 (f)): GSE3526. The following tumor datasets were used: Kool – 62: GSE10327; Delattre – 57; Pfister – 73: GSE49243; Gilbertson _ 76: GSE37418; Pfister – 223: PubMed 28,726,821; Hsieh – 31: GSE67851; denBoer – 51: GSE74195; Cavalli – 763: GSE85217. For genomic analysis across datasets, the Affymetrix Human Genome U133 Plus 2.0 Arrays were used.
Spheroid invasion assay (SIA)
SIA was performed and quantified according to24. In brief: 24 h after siRNA transfection, 2’500 cells were seeded per well in 100 µl of complete medium in a 96-Well Clear Round Bottom Ultra-Low Mount Microplate (Corning™ Costar™ Cat#7007) and incubated overnight at 37° C, 5% CO2 conditions. After 24 h, the formation of compact and uniform spheroids was confirmed by light microscopy. 70 µl medium was then removed and replaced with 70 µl of a solution containing 2.7 mg/ml PureCol® bovine collagen (CellSystems, Cat#5005). Polymerized collagen hydrogels were overlaid with 100 µl of SFM containing growth factors and/or inhibitors 2X concentrated. The cells were allowed to invade the collagen matrix for 24 h, after which they were stained with Hoechst (Sigma Aldrich, Cat#B2883, 1:2000) for 3–4 h. The image acquisition was performed using an Operetta CLS High-Content Analysis System (PerkinElmer, Cat# HH16000000) using the 405 nm channel. Spheroids and the invading cells were defined based on the fluorescence threshold using Harmony software. For each tumor cell nucleus, the distance from the center of the spheroid was calculated. The total distance of invasion from the center of the spheroid was calculated by summing up the individual invasion distances of each cell using Harmony (Perkin Elmer). The data was displayed and statistically analyzed using Prism 9.3.1 software (GraphPad).
Immunoblot (IB)
Cells were lysed using RIPA buffer supplemented with cOmplete™, Mini Protease Inhibitor Cocktail (Roche, Cat#11836153001) and phosphatase inhibitors PhosSTOP™ (Roche, Cat#4906837001), and cleared by centrifugation (13’000xg, 5 min at 4 °C in table top centrifuge). Protein concentration was determined using the Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, Cat# 23225). Gel electrophoresis was performed with 4–20% Mini-PROTEAN® TGX™ Precast Protein Gels (Bio-Rad Laboratories AG, Cat#4561094/4561096). Proteins were transferred to Trans-Blot Turbo 0.2 μm Nitrocellulose Transfer Membrane (Bio-Rad, Cat#1704158/1704159) and membranes blocked with 5% non-fat milk for 1 h. For primary antibodies used see supplementary file 1, Table 2. HRP-linked secondary antibodies were used with Western Blotting Substrate (Thermo Fisher Scientific, Cat#32209) or SuperSignal™ West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, Cat#34095). The integrated density of immuno-reactive bands was quantified using Image Lab (Version 5.2.1, Bio-Rad Laboratories).
Subcellular protein fractionation
6 × 105 cells/well were seeded in 6 cm dishes and incubated at 37 °C. After 24 h, 3 ml of media were removed, and the cells were collected by scraping into the remaining medium. The cells were washed twice in PBS and the cellular contents from the cell pellets was fractionated using the subcellular protein fractionation kit for cultured cells (ThermoFisher Scientific, Cat# 78840) exactly according to the manufacturer’s protocol. This protocol enabled the stepwise extraction of the proteins from the cytoplasm (CEB), membranes (MEB), nuclear soluble (NEB), chromatin-bound regions (ChrB), and cytoskeleton (Ck). The subcellular protein extracts were analyzed by IB as described above.
siRNA transfection
1.5 × 105 DAOY, UW228, and ONS-76 cells, or 3 × 105 D425, HD-MBO3, and D283 cells were seeded per well in 6-well plates. After 24 h, cells were transfected with 6 nM siRNA mixed with OptiMEM reduced serum medium (Thermo Fisher Scientific, Cat# 31985062) using Lipofectamine RNAiMAX transfection reagent (Invitrogen, Cat# 13778075). 6 h after adding the transfection mixture, the transfection media was replaced with complete fresh medium, and the cells were cultured for another 24–72 h for further analysis.
Flow cytometry (FC) analysis
The cells were collected on ice by gentle scraping. PBS-washed cells were fixed in 4% PFA in PBS for 20 min at room temperature (RT). Cells were then either processed immediately or stored overnight at 4 °C before staining. Biotin-tagged anti-CLSTN1 antibody diluted (1:100) in FC buffer was used for CLSTN1 detection. PE-labelled IgG1 antibody (1:300) in PBS containing 2% FBS (FC buffer) was used as the negative control. Samples were incubated at 4 °C in the dark for 1 h, and then washed once with 300 µl FC buffer. Anti-CLSTN1 labeled samples were subsequently incubated with PE-coupled streptavidin diluted (1:200) in FC buffer at 4 °C in the dark for 30 min and washed 3 × 5 min with 300 µl FC buffer at RT. All samples were then filtered into 5 ml polystyrene round-bottom tubes (Falcon/Brunschwig, Cat# 352235) and stored at 4 °C in dark until data acquisition. Data was acquired using a BD Fortessa™ Cell Analyzer, analyzed using FlowJo 10.8.0 software, and plotted using GraphPad Prism 9.3.1 software.
Immunofluorescence analysis (IFA)
200 µl of a suspension of 4 × 103 LA-EGFP mCherry-Nuc9 cells/ml were seeded per well in an ibidi 8-well plate and incubated overnight. Cells were then fixed in complete medium containing 4% PFA for 20 min at 37 °C. Cells were washed 2 × 5 min in PBS, permeabilized with 0.1% Triton X100 in PBS for 20 min at RT and washed again 3 × 5 min in PBS. Cells were blocked with PBS supplemented with 5% FBS for 1 h at RT. Primary antibodies were diluted in PBS + 5% FBS and incubated overnight at 4 °C in a humidified chamber. Cells were then washed 3 × 5 min in PBS and nucleic acids stained with DAPI (1:5000) diluted in PBS for 5 min at RT. One drop of pre-warmed (37 °C) DAKO Glycergel mounting medium (DAKO, Cat#C0563) was added to each well. Image acquisition was performed either on a NikonTi2 widefield or an SP8 Leica confocal microscope.
Quantification of intercellular CLSTN1 localization in IFA images
Cell-cell contacts were localized using the LA-EGFP signal. A 10 μm long line with a width of 100 pixels was drawn across cell-cell contacts in the CLSTN1 fluorescent channel in ImageJ. The line was drawn such that it was oriented exactly perpendicular to the cell-cell contact and centered in the middle of the contacts. The profile of the line was plotted, and the grey values were determined every 0.09 μm. The sum of all grey values was calculated, and the percentage of total grey values calculated every 0.09 μm was plotted. An unpaired T-test with individual variance for each row was performed. The false discovery rate was calculated using a two-stage step-up (Benjamini, Krieger, an Yekutieli).
Tumor co-culture with immortalized normal human astrocyte (iNHA)
2 × 103 UW228 LA-EGFP mCherry-Nuc9 cells and 2 × 103 iNHA25 or primary mouse cerebellar astrocytes were cultured together per well in an 8-well ibidi plate in complete DMEM medium supplemented with 1% sodium pyruvate. DMSO (control) or 0.5 µM Prostetin/12k (P/12k)26 treatments were performed for 24 h after which cells were fixed and labelled with anti-CLSTN1 and anti-GFAP as described for IFA.
[Ca2+]i detection using GcaMP6 calcium indicators
1.5 × 105 GcAMP6-expressing UW228 cells were seeded per well in a 6-well plate and transfected with either si1-CLSTN1 or siRNA Ctrl 24 h after seeding as described above. After 24 h, 1 × 104 transfected cells were seeded per well in an 8-well ibidi plate in 200 µl complete DMEM. After 48 h, the medium was replaced with freshly prepared artificial cerebrospinal fluid (aCSF) solution without phenol red. Image acquisition was performed on a Nikon Ti2 widefield fluorescence microscope using a 20x objective. For each imaging series, 601 frames were recorded at 2-second intervals for 20 min. The image analysis was performed with Fiji 2.0.0 software (ImageJ2) and the frame rate was set up as 10 fps. Overlay images and calcium traces were generated with phyton programming.
Live imaging of CLSTN1-mNG intracellular dynamics
6 × 103 UW228 CLSTN1-mNG cells were seeded per well in an 8-well ibidi plate in 200 µl complete DMEM overnight. Cells were treated with 0.5 µM P/12k or an equivalent volume of the solvent DMSO (control) and incubated for 24 h. 24 h after the start of the treatment, the cells were monitored using a Leica SP8 Confocal Microscope with a 63X objective using the 488-laser line. Images were recorded every 30 s for 10 min. The analysis was performed using Fiji 2.0.0 software (ImageJ2).
Cell proliferation analysis
1.5 × 105 UW228 cells were seeded in 6-well plate for siRNA transfection. After 24 h, cells were transfected with 6 nM siCtrl and si1-CLSTN. 24 h after transfection, 5 × 104 cells/well of each condition were seeded in 6-well plates and cultured overnight. Cells were counted 48 h and 72 h post-transfection using Trypan blue in combination with an automated cell counter (NanoEnTek).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 9.3.1 software. Unpaired Student’s t-test was used to determine the statistical significance of the difference between two groups, and One-way ANOVA repeated measures test was used for multiple comparisons. Results with p-values ≤ 0.05 were considered as significant (* = P < 0.05, ** = P < 0.01, *** = P < 0.001, **** = P < 0.0001, ns = not significant).
Results
Reduced expression of CLSTN1 in medulloblastoma
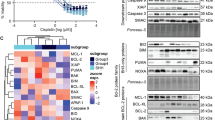
The Calsyntenin (CLSTN) family of proteins consists of three members, CLSTN1, CLSTN2 and CLSTN311, with relatively low protein sequence identity scores (CLSTN1:CLSTN2 = 51.81%, CLSTN1:CLSTN3 = 55.20%, CLSTN2:CLSTN3 = 49.02%). By surface proteome analysis, we only detected CLSTN1 in MB cells9. CLSTN1 belonged to a small set of proteins that we found to be upregulated in the plasma membrane-proximal proteome by MAP4K4 depletion (Fig. 1A). To more broadly assess expression levels of the three CLSTNs across human MB samples in comparison to normal brain regions and the cerebellum, we analyzed CLSTN1, CLSTN2, and CLSTN3 mRNA expression across five publicly available MB datasets as well as an atypical teratoid rhabdoid tumor (ATRT) and an ependymoma dataset (Fig. 1B). This analysis revealed significantly lower levels of CLSTN1 and CLSTN3 in the MB samples in comparison to normal brain regions or cerebellum samples. In contrast, CLSTN2 expression is significantly increased in MB compared to the cerebellum controls. We next assessed differential expression of CLSTNs across the 12 MB subtypes using the Cavalli dataset1, which comprises 763 primary MB tumor samples (Fig. 1C). We observed some variations in the expression levels between the subtypes, with increased CLSTN1 and CLSTN3 in SHH-γ and Grp3-γ, and decreased CLSTN1 and CLSTN3 in SHH-δ and Grp3-δ samples. Interestingly, the expression levels of CLSTN2 within each subgroup vary considerably more compared to CLSTN1, which may indicate a tighter control of CLSTN1 transcription. The expression of CLSTN1 and CLSTN3 correlates negatively with MAP4K4 expression, whereas CLSTN2 correlates positively (Fig. 1D), further indicating differential regulation of CLSTN1 and CLSTN3 expression compared to CLSTN2. CLSTN1 (Fig. 1E) and CLSTN3 (Fig. S1B) expression increase sharply in the cerebellum after birth27, unlike CLSTN2, where expression drops at birth (Fig. S1A). Consistent with a potential co-regulatory mechanism, expression of MAP4K4 in the cerebellum also drops sharply after birth28. In the developing human cerebellum29, CLSTN1 (Fig. 1F, S2) and CLSTN3 (Fig. S1D, S2) are strongly expressed in granule cells. CLSTN3 is additionally expressed in glutamatergic deep nuclei neurons and unipolar brush cells. In contrast, CLSTN2 is highly expressed in Purkinje cells (Fig. S1C) and a subset of granule cells. Interestingly, MAP4K4 expression is lowest in granule cells, where CLSTN1 and CLSTN3 are highly expressed, and highest in oligodendrocytes, where CLSTN1 and CLSTN3 are not expressed (Fig. 1G). CLSTN1 expression shows a strong positive correlation with the postsynaptic neurexins 1–3 across all MB subgroups (Fig. S1E).
CLSTN1 expression is reduced in primary MB tumor samples. (A) Differential protein expression in the plasma membrane-associated proteome between sgCTRL and sgMAP4K4 cells assessed by spatial mass-spectrometry analysis identified CLSTN1 as one of several specifically upregulated proteins9. The mean Z-score and SD of up-regulated proteins in sgMAP4K4 cells are shown. N = 3–4 biological replicas. P values of multiple unpaired T-tests are shown when P = < 0.05. (B) mRNA expression analysis in three controls (diseased brain, normal brain regions (white), and cerebellum (blue)), five primary MB (orange), one ATRT, and one ependymoma dataset (grey). Significant differences between CLSTN expression in tumor samples compared to cerebellum controls were calculated using ANOVA multiple comparisons and Kruskal-Wallis test and are indicated by asterisks: * p = < 0.05, ** p = < 0.01, **** p = < 0.0001. (C) CLSTN mRNA expression analysis across the 12 MB subtypes. ANOVA multiple comparisons and Kruskal-Wallis test were used. * p = < 0.05, ** p = < 0.01, *** p = < 0.001, **** p = < 0.0001. (D) XY plots showing the correlation between CLSTN and MAP4K4 expression across the four MB subgroups WNT, SHH, Grp3 and Grp4. Interrupted line indicates best fit of linear regression analysis. The slope and p-value of the slope are indicated. (E) Analysis of CLSTN1 expression across human organ development. (F, G) snRNAseq analysis of CLSTN1 (F) and MAP4K4 (G) expression across cerebellar cell types. Upper: Manifold Approximation and Projection (UMAP) of 180,956 human cells colored by cell type (from29). Lower: Expression levels of CLSTN1 across projected cell types. CPM, Counts per million; GABA, Gamma-aminobutyric acid, DN, Dentate nucleus; GC, Granule cells; UBC, Unipolar brush cells; Glut_DN, Glutamatergic deep nuclei neurons; Isth_N, Isthmic nuclei neurons; NTZ, Nuclear transitory zone; VZ, Ventricular zone.
Taken together, this data indicates a reduced expression of CLSTN1 in MB tumor cells and points towards tight control of CLSTNs during cerebellar development. The negative correlation of MAP4K4 and CLSTN1 expression in the developing cerebellum further indicates a potential mechanism of mutual control of these two genes.
CLSTN1 is associated with membranes in MB cells and detected at the cell surface
To explore expression levels of CLSTN1 in different established human MB tumor cell lines, we probed lysates of three SHH and three Grp3 MB cell lines with anti-CLSTN1 antibodies by immunoblotting (IB). We detected similar expression levels across all tested cell lines, with slightly higher expression in HD-MB03 (Fig. 2A, left). As we expected biological relevance from cell surface expressed CLSTN1 specifically, we also assessed CLSTN1 expression by flow cytometry (FC) analysis. We detected CLSTN1 expression on all six cell lines (Fig. 2B). Except for ONS-76, all cell lines displayed a small population of CLSTN1-low or -negative cells. The highest levels of CLSTN1 expression were detected in ONS-76 cells. To further confirm CLSTN1 membrane localization, we performed cell fractionation analysis in one SHH (UW228) and one Grp3 (HD-MB03) MB cell line. We detected CLSTN1 in the membrane fraction of both cell lines. Somewhat surprisingly, however, we detected more than 50% of CLSTN1 in the cytosolic fraction in HD-MB03 cells (Fig. 2C). Immunofluorescence analysis revealed that a majority of CLSTN1 protein under regular growth conditions is localized diffusely in the cytoplasm with some enrichment in perinuclear regions (Fig. 2D). However, we also clearly detected CLSTN1 at the plasma membrane (arrowheads), in lamellipodia-like protrusions and filamentous cell-cell contacts (arrows).
Subcellular distribution of CLSTN1 in cultured MB cells. (A) Immunoblot (IB) analysis of CLSTN1 expression in six different MB cell lines. GAPDH expression was used as the loading control. (B) Surface expression analysis of CLSTN1 in six different MB cell lines by FC. The bar plot depicts mean fluorescence intensities in the six different cell lines from different experiments. (C) Subcellular fractionation analysis of CLSTN1 expression in the SHH MB cell line UW228 and the Grp3 MB cell line HD-MB03. Integrin-a5 and PRPF9 expression were used as fractionation controls for the membrane and the nuclear fractions, respectively. CE: cytoplasmic extraction; ME: membrane extraction; NE: nuclear extraction. UW228: n = 3, HD-MB03: n = 2 independent experiments. (D) IFA of endogenous CLSTN1 protein in UW228 LA-EGFP-mCherry-Nuc cells. Inverted greyscale images of the green-fluorescent signal (LA-EGFP, middle) and the red-fluorescent signal (CLSTN1, right) are shown. Scale bar is 20 µm. Magnifications a, b and c depict CLSTN1 localization in filamentous cell-cell connections and lamellipodia-like protrusions, respectively. Arrowheads indicate CLSTN1 localization in the plasma membrane, arrows accumulations of CLSTN1 in the filamentous cell-cell connections.
Reduced CLSTN1 expression increased growth factor-driven invasiveness
To explore the potential functional significance of CLSTN1 in MB cells, we tested several siRNAs against CLSTN1 (Fig. S3A). For subsequent experiments, we depleted CLSTN1 using si1CLSTN1 and si2CSLTN1, which effectively reduced CLSTN1 protein in all tested cell lines (Fig. 3A). By flow cytometry, we could still detect CLSTN1 in siCLSTN1-treated cells. However, the expression levels in siCLSTN1-transfected cells were significantly lower compared to control siRNA-transfected cells (Fig. 3B). To assess the potential implication of CLSTN1 in cell invasion control, we compared the invasiveness of CLSTN1-depleted DAOY cells compared to control using the spheroid invasion assay (SIA24). Interestingly, the reduction of CLSTN1 causes a significant increase in bFGF-induced but not basal invasion (Fig. 3C). Both si1-CLSTN1 and si2-CLSTN1 increased invasiveness to an extent, which correlates with the depletion efficacy (Fig. S3B). As cells for the SIA are grown in low adhesion plates, we tested whether suspension culture could influence CLSTN1 protein levels. We found that cells grown as free-floating cell clusters expressed higher CLSTN1 protein levels (Fig. 3D). Depletion of CLSTN1 caused some increase in focal connexin 43 (Cx43) staining in regions of cell-cell contact (Fig. S4A), but did not affect [Ca2+]i fluctuations in the tumor cells (Fig. S4B). To assess the effect of CLSTN1 overexpression, we generated stable cell lines overexpressing CLSTN1-mNeonGreen (CLSTN1-mNG). A marked increase of CLSTN1 was noted in cells expressing CLSTN1-mNG (Fig. 3E). The band of the overexpressed CLSTN1-mNG was detected as expected at a higher molecular weight by IB (Fig. 3E, upper). We observed no significant difference in 2D culture growth between cells with increased (CLSTN1-mNG overexpression) or decreased (siCLSTN1) CLSTN1 expression (Fig. 3F). This indicates that CLSTN1 is not critical for cell proliferation in UW228 cells and that CLSTN1-mNG overexpression is not impacting cell viability. To confirm that the overexpressed CLSTN1-mNG was localized similarly to the endogenous protein, we visualized CLSTN1-mNG localization in the cells by fluorescence microscopy (Fig. 3G). CLSTN1-mNG primarily accumulated around the nucleus. Comparable to the endogenous CLSTN1 (Fig. 2D), we also detected CLSTN1-mNG in the plasma membrane, in lamellipodia-like structures, and cell-cell connections (Fig. 3G).
CLSTN1 expression levels determine the invasive phenotype of MB cells. (A) IB analysis of CLSTN1 expression after siRNA-mediated depletion in DAOY, UW228, ONS-76, and HD-MB03 cell lines. Two different siRNAs targeting CLSTN1 were used. GAPDH expression was used as loading control. (B) Surface expression analysis by FC of CLSTN1 after siRNA-mediated depletion in DAOY, UW228, ONS-76, and HD-MB03 cell lines. (C) SIA of bFGF stimulated (100 ng/ml) control (siCtrl) and si1-CLSTN1-transfected cells treated with 100 ng/ml bFGF for 24h. Upper left panel shows representative images of Hoechst-stained cultures at the endpoint. UT: Untreated. Right panel box-dot plot depicts the median cumulated invasion distances of n = 13–18 samples. ANOVA **** p = < 0.0001. (D) IB analysis of CLSTN1 expression in D425 and DAOY cells grown either as adherent or suspension cultures. (E) IB (upper) and FC analysis (lower) of endogenous (light purple) and ectopically overexpressed (light green) CLSTN1 in UW228, DAOY and D425 cells. (F) Proliferation analysis of UW228 cells transfected with siCLSTN1 or of UW228 cells overexpressing CLSTN1-mNG. (G) Fluorescence imaging of UW228 cells expressing CLSTN1-mNG. a, b, and c depict magnifications of areas highlighted with white rectangles. Arrowheads indicate CLSTN1-mNG localization at the plasma membrane, arrows localization of CLSTN1-mNG in lamellipodia-like structures. Magnification c depicts CLSTN1-mNG accumulation in filamentous cell-cell connection. Scale bar is 20 µm.
These data indicate that CLSTN1 is associated with cellular membranes and the plasma membrane. Depletion of CLSTN1 increases growth factor-induced invasion, suggesting that the expression levels of CLSTN1 may determine growth factor-driven invasive capabilities of the MB tumor cells.
Visualization of CLSTN1 trafficking dynamics in living MB tumor cells
We used confocal live cell imaging of CLSTN1-mNG expressing UW228 cells to determine dynamic alterations in CLSTN1 localization in cells (Fig. 4A, movie 1). We detected CLSTN1-mNG accumulated in lamellipodia (a, movie 2), in a Golgi-like structure near the nucleus (b, movie 3), in small vesicles trafficking from the cell periphery towards the cell body (c, movie 4) and on filamentous structures, moving anterogradely relative to the nucleus (d, movies 5,6,7). Large endocytic vesicles in lamellipodia near the leading-edge membranes were devoid of fluorescence (movies 2 and 5), indicating that plasma membrane-localized CLSTN1 is internalized through a different endocytic mechanism. Rather, we observed considerably smaller, strongly fluorescent spots emanating from the lamellipodial membranes and trafficking inwards (Fig. 4A, movie 4). Figure 4B and S5A depict time series of still images of an enlarged area (movie 7) from the original movie 6 (Fig. 4B). The filamentous, anterogradely moving structures are indicated by red arrowheads. Within the 10 min observation window, three consecutive passages of CLSTN1-mNG from the perinuclear region towards the cell periphery were observed (Fig. 4B).
CLSTN1-mNG internalization and trafficking of CLSTN1-mNG. (A) Still image of CLSTN1-mNG overexpressing UW228 cell (Movie 1). An inverted greyscale of the green fluorescent signal is shown. Squares a – d depict different subcellular localization of CLSTN1-mNG. a: Lamellipodia-like structure at cell leading edge (Movie 2); b: Perinuclear near Golgi-like structure (Movie 3); c: in small vesicles at cell trailing edge; d: on filamentous structures connecting the cell core with the periphery (Movie 5. (B) Still image series of Movies 6 and 7 showing anterograde trafficking of CLSTN1-mNG in UW228 cell. Inverted greyscale images of the green fluorescent signals are shown. Red arrowheads indicate a filamentous structure progressing from the perinuclear region toward the cell periphery. Three passages of such structures are observed in the 10 min observation period. Red asterisks indicate the position of the front of filamentous structures at the indicated time point.
These observations point towards a rapid turnover of CLSTN1 at peripheral membranes. Delivery may occur through microtubule (+)-end-directed transport as was already suggested previously14, whereas internalization is likely mediated through a specific endocytic mechanism to be determined.
CLSTN1 localizes to tumor-tumor and tumor-astrocytic cell contacts
Using IFA, we next investigated the localization of endogenous CLSTN1 in tumor cells seeded as monocultures or seeded in co-culture with immortalized normal human astrocytes (iNHAs25). In monoculture, most cell-cell contacts displayed some CLSTN1 signal (Fig. 5A). However, this signal was weak and difficult to detect. In contrast, when cells were grown in co-culture with iNHAs, the signal in tumor-tumor cell contacts increased (Fig. 5B). We detected a CLSTN1 signal in nearly every cell-cell contact (Fig. 5B, S6A), and the signal was significantly higher in this region compared to cell-cell contacts in monoculture (Fig. 5C). In regions of tumor-iNHA cell interactions, we also detected CLSTN1 accumulations, indicating that CLSTN1 is localized to both homotypic and heterotypic cell interactions. We confirmed CLSTN1 localization to heterotypic tumor-astrocyte interactions using primary mouse cerebellar astrocytes (Fig. S6B). In homotypic cell-cell interactions between UW228 cells co-cultured with iNHA, CLSTN1 aligns in parallel linear structures of approximately 1 μm in length (Fig. 5C). By IB analysis we confirmed CLSTN1 expression in primary murine cerebellar astrocyte culture, which in expression levels is considerably higher than in UW228 cells (Fig. 5D). In the cerebellar astrocyte culture, CLSTN1 migrates at a higher molecular weight in the SDS-PAGE, indicating either alternative splicing or differential posttranslational modifications.
Increased CLSTN1 localization to cell-cell contacts in tumor-astrocyte co-cultures. (A) IFA analysis of CLSTN1 expression in UW228-LA-EGFP-mCherry-Nuc cells. Red: CLSTN1, green: actin (LA-EGFP), blue: Nuclei (mCherry-Nuc). Arrows indicate CLSTN1 localization at the cell cortex, and arrowheads in cell-cell contacts. (B) IFA analysis of CLSTN1 expression in UW228-LA-EGFP-mCherry-Nuc co-cultured with iNHAs. iNHAs do not express LA-EGFP or mCherry-Nuc. Red dotted line indicates the circumference of iNHA cell clusters, and arrowheads indicate CLSTN1-positive cell-cell contacts. Magnifications of zones indicated with white rectangles show homotypic (a) and heterotypic (b) cell-cell contatcs. (C) Plot of the % total grey values of a 10 µm long line across all detected cell-cell contacts in representative images. Mean and 95% confidence intervals of measured values at indicated positions are shown. Monoculture: N = 17, co-culture: N = 31. P values were calculated using multiple unpaired t-tests. (D) IB analysis and quantification of CLSTN1 expression in primary murine cerebellar astrocytes and UW228 MB cells.
Collectively, these data indicate that co-culture of UW228 tumor cells with iNHAs increased CLSTN1 localization to cell-cell contacts.
Increased CLSTN1 in cell-cell contacts upon treatment with the MAP4K inhibitor prostetin/12k
Surface proteomic analysis in DAOY MB cells indicated that CLSTN1 surface expression is negatively regulated by the Ser/Thr kinase MAP4K49. MAP4K4 is a pro-invasive kinase with pleiotropic functions in health and disease28, highly expressed in MB, where it drives growth factor-induced cell invasion22,23. To test whether MAP4K4 kinase activity could be involved in the control of CLSTN1 plasma membrane expression, we treated cells with a novel pan MAP4K inhibitor prostetin/12K (P/12K)26 for 24 h and determined CLSTN1 expression and subcellular distribution by cell fractionation analysis (Fig. 6A). CLSTN1 was detectable exclusively in the total membrane fraction in UW228 cells. We observed no significant difference in the proportion of membrane-associated CLSTN1 in cells treated with P/12k. To test whether P/12k treatment alters cell surface expression of CLSTN1, we probed P/12k-treated cells by FC analysis. We found that P/12k treatment increased the mean fluorescence intensity of CLSTN1 in UW228 by 46% (n = 3 independent experiments, SD = 7.3%, Fig. 6B, C) and by 47% in DAOY cells (Fig. S5B). siRNA-mediated depletion of MAP4K4 also causes a small increase in CLSTN1 surface expression by 23% (n = 3 independent experiments, SD = 4,0.2%, Fig. S5C, D). We next compared P/12k impact on subcellular CLSTN1 distribution by IFA. In DMSO-treated samples, we detected CLSTN1 in homotypic cell-cell contacts (Fig. 6D). P/12k treatment caused an increase in cell size and led to the more pronounced accumulation of CLSTN1 in regions of cell-cell contact (Fig. 6E, S6C). CLSTN1 accumulation was particularly evident in contacts between tumor cells and iNHA after P/12k treatment (Fig. 6F), where it focally accumulated specifically in contacts between UW228 and iNHA (Fig. 6G, S7A, B). Increased CLSTN1 expression is not a consequence of increased CLSTN1 transcription as we did not observe a significant increase in CLSTN1 mRNA after 6 and 24 h P/12k treatment (Fig. S5E).
Pharmacological inhibition of MAP4 kinases with P/12k increases CLSTN1 surface expression and accumulation in cell-cell contacts. (A) Upper: IB of cell fractionation analysis of CLSTN1 subcellular localization in UW228 cells. Antibodies against proteins indicated to the right of the panels were used. CE: cytoplasmic extraction; ME: membrane extraction; NE: nuclear extraction. Lower: Quantification of relative protein expression levels in the different compartments. (B) FC analysis of CLSTN1 surface expression on UW228 cells treated with 0.5 µM P/12k for 24h. (C) Quantification of relative CLSTN1 surface expression from FC analysis shown in B and estimation plot of n = 3 independent experiments. (D) IFA analysis of endogenous CLSTN1 expression in DMSO-treated (24h) UW228-LA-EGFP-mCherry-Nuc cells co-cultured with iNHA. Red: CLSTNA1, green: actin (LA-EGFP), blue: Nuclei (mCherry-Nuc). Inverted greyscale images of the green-fluorescent signal (LA-EGFP, middle) and the red-fluorescent signal (CLSTN1, right) are shown. The scale bar is 20 µm. The letter “A” indicates the positions of iNHA cells. Magnifications in a) and b) indicate cell-cell contacts between tumor cells. (E) IFA analysis of CLSTN1 expression of UW228-LA-EGFP-mCherry-Nuc cells co-cultured with iNHAs and treated for 24 h with 0.5 µM P/12k. Red: CLSTN1, green: actin (LA-EGFP), blue: Nuclei (mCherry-Nuc). The letters “A” indicate the positions of iNHAs. Arrows indicate tumor-iNHA contacts. Magnifications in c) and d) indicate cell-cell contacts between tumor cells. (F) Magnifications of tumor-iNHA cell-cell contacts in DMSO- (e and f) or – P/12k- treated samples (g and h). The letter “A” indicates the positions of iNHA cells. (G) Left IFA images: Representative images of heterotypic cell-cell contacts. Dotted squares indicate representative measuring zones across cell-cell contacts. Four representative plots of the calculated % of the total measured grey value of the whole area at the positions indicated on the x-axis are shown to the right of the IFA images. Right: Plot of the % total grey values of a 10 µm long line across all detected cell-cell contacts in representative images. Mean and 95% confidence intervals of measured values at indicated positions are shown. Monoculture: N = 16, co-culture: N = 16. P values were calculated using multiple unpaired t-tests.
These data indicate that the MAP4Ks including MAP4K4 targeted by P/12k could be involved in CLTSN1 expression and function in MB by repressing localization of CLSTN1 to cell-cell contacts.
Discussion
Localization, regulation, and functions of the neuronal postsynaptic adhesion protein CLSTN1 have been mostly investigated in the context of physiological cell functions. Herein we addressed the expression, subcellular localization, and function of CLSTN1 in the embryonal brain tumor medulloblastoma and further explored its previously observed regulation by the pro-invasive kinase MAP4K4. We provide evidence that CLSTN1 may exert tumor-suppressive functions by repressing invasiveness and enabling homo- and heterotypic cell-cell interactions. These functions of CLSTN1 are in part controlled by MAP4 kinases, as P/12k, a neuroprotective, novel selective inhibitor of this kinase family, increased plasma membrane-associated CLSTN1 expression in MB tumor cells and enhanced CLSTN1 localization in tumor-astrocyte interactions. Depletion of CLSTN1 on the other hand caused a significant increase in growth factor-induced invasion. Together, our study provides novel insights into the subcellular expression and function of CLSTN1 in the context of a malignant brain tumor. Specifically, it sheds light on kinase regulation of CLSTN1 subcellular localization, which we found to be of potential importance in mediating microenvironment interactions.
In the differentiating chick cerebellum, CLSTN1 was found to be strongly expressed in granule cells in the internal granule layer (IGC), whereas CLSTN2 and CLSTN3 are specifically expressed in Purkinje cells30. This finding was recently confirmed in the human cerebellum, where CLSTN1 is predominately expressed in a subset of granule cells (GCs)29. In contrast, CLSTN2 was found localized to a subset of Purkinje cells, unipolar brush cells, and a subset of granule cells. CLSTN3 was predominately detected in a subset of granule cells and dispersed in some Purkinje cell populations. High CLSTN1 expression in cerebellar GCs is contrasted by low MAP4K4 expression in the same cellular compartment29, which corroborates the negative regulatory function of MAP4K4 on CLSTN1 expression we observed in vitro. The overall negative correlation of MAP4K4 and CLSTN1 expression in vivo and our findings of increased CLSTN1 cell surface protein levels after P/12k treatment indicate that MAP4 kinases could negatively regulate CLSTN1 expression. We observed no increase in CLSTN1transcripts after 6–24 h of P/12k treatment, suggesting that CLSTN1 protein expression is controlled by MAP4Ks via a posttranscriptional mechanism. A study in whole mice and lung adenocarcinoma cells found that CLSTN1 expression is decreased in response to 4 Gy gamma irradiation31. We previously observed an increased MAP4K4-dependent invasiveness in SHH MB tumor cells exposed to 1–2 Gy22, suggesting that the irradiation-induced increase in cell invasion observed could in part be mediated by CLSTN1 downregulation via increased MAP4K4 activity.
Our live-cell imaging analysis of the subcellular dynamics of CLSTN1 confirms anterograde trafficking in MB tumor cells. This observation could indicate that CLSTN1 links organelles to kinesin-mediated transport processes and cargo delivery in tumor cells, which was observed in healthy neuronal axons12,13. Consequently, the increased accumulation of CLSTN1 in homo- and heterotypic cell-cell interactions upon treatment may indicate that MAP4Ks either prevent proper trafficking of CLSTN1 and associated cargo or cause increased turnover at adhesion sites by controlling proteins involved in CLSTN1 trafficking, such as ERK, which negatively regulates CLSTN1-KLC via KLC1S460 phosphorylation15. The repression of ERK was shown to reduce S460 phosphorylation and increase CLSTN1-KLC1 binding, suggesting that CLSTN1 trafficking could be controlled by reversible KLC1 S460 phosphorylation downstream of MAPK signaling15. MAP4K4 activates ERK through repression of PP2A in lung adenocarcinoma32; hence, P/12k treatment may increase CLSTN1 interaction with KCL1 through the reduction of S460 phosphorylation in KLC1.
We observed signals of the gap junction protein Cx43 in cell-cell contact zones after CLSTN1 depletion. However, since we observed no changes in [Ca2+]i, we do not think that CLSTN1 expression and localization impacts [Ca2+]ipropagation in MB tumor cells under monoculture conditions. Thus, the functional significance of CLSTN1 recruitment and accumulation in regions of cell-cell contacts between tumor cells and astrocytes remains to be determined. The high number of astrocytes in the cerebellum, which outnumber Purkinje cells and provide critical functions that support the proliferation and migration of cerebellar granule neuron precursors33, argues for the relevance of direct tumor-astrocyte interaction in the control of tumor growth and progression. Indeed, in MB with extensive nodularity (MBEN), astrocytic cells were found associated with malignant and migratory cells in the internodular space, which was suggested to provide a source of neoplastic cells that then migrate to the nodular compartment while losing the proliferative potential34. Additionally, MB tumor-associated astrocytes display even distribution in small SHH tumors or accumulation at the tumor margin in large tumors, forming a glial scar in mouse models in vivo35. Importantly, several studies identified SHH MB tumor-promoting activities of astrocytes via the induction of sustained hedgehog signaling35,36,37,38. Loss of Patched is sufficient for the induction of SHH medulloblastoma from granule neuronal precursor cells39, but astrocyte secretion of SHH is necessary to support MB growth, and astrocyte ablation prevented progression of SHH tumors in Ptch1-/-mice38. Astrocyte-secreted fibronectin supports the outgrowth and survival of primary SHH-derived tumoroids40, suggesting that both soluble and physical components secreted by astrocytes contribute to MB tumor growth. Future studies should thus address how the tumor-astrocyte coupling via CLSNT1 in cells treated with the MAP4K inhibitor could influence hedgehog signaling as well as astrocyte biology and function in the context of MB.
In conclusion, our study provides a first insight into potential tumor-restrictive interactions between tumor cells and astrocytes, which involve CLSTN1 and its regulation by the MAP4 kinase family.
Data availability
Data and material of this study can be made available upon reasonable request to: [email protected].
References
Cavalli, F. M. G. et al. Intertumoral heterogeneity within Medulloblastoma subgroups. Cancer Cell. 31, 737–754e6 (2017).
Fults, D. W., Taylor, M. D. & Garzia, L. Leptomeningeal dissemination: a sinister pattern of medulloblastoma growth. J. Neurosurg. Pediatr. 23, 613–621 (2019).
Vladoiu, M. C. et al. Childhood cerebellar tumours mirror conserved fetal transcriptional programs. Nature 572, 67–73 (2019).
Hovestadt, V. et al. Resolving medulloblastoma cellular architecture by single-cell genomics. Nature 572, 74–79 (2019).
Zhang, L. et al. Single-cell transcriptomics in Medulloblastoma reveals tumor-initiating progenitors and Oncogenic Cascades during Tumorigenesis and relapse article single-cell transcriptomics in Medulloblastoma reveals tumor-initiating progenitors and oncogenic cascades. Cancer Cell. 36, 302–318e7 (2019).
Ocasio, J. et al. scRNA-seq in medulloblastoma shows cellular heterogeneity and lineage expansion support resistance to SHH inhibitor therapy. Nat. Commun. 10, 1–17 (2019).
Gojo, J. et al. Single-cell RNA-Seq reveals Cellular hierarchies and impaired developmental trajectories in Pediatric Ependymoma. Cancer Cell. 38, 44–59e9 (2020).
Jessa, S. et al. Stalled Developmental Programs at the Root of Pediatric Brain Tumors. Nature Genetics vol. 51Springer US, (2019).
Capdeville, C. et al. Spatial proteomics finds CD155 and Endophilin-A1 as mediators of growth and invasion in medulloblastoma. Life Sci. Alliance. 5, e202201380 (2022).
Vogt, L. et al. Calsyntenin-1, a proteolytically processed postsynaptic membrane protein with a cytoplasmic calcium-binding ___domain. Mol. Cell. Neurosci. 17, 151–166 (2001).
Hintsch, G. et al. The calsyntenins - a family of postsynaptic membrane proteins with distinct neuronal expression patterns. Mol. Cell. Neurosci. 21, 393–409 (2002).
Filigheddu, N. et al. Calsyntenin-1 docks vesicular Cargo to Kinesin-1. Mol. Biol. Cell. 18, 986–994 (2007).
Araki, Y. et al. The novel cargo Alcadein induces vesicle association of kinesin-1 motor components and activates axonal transport. EMBO J. 26, 1475–1486 (2007).
Ludwig, A. et al. Calsyntenins mediate TGN exit of APP in a kinesin-1-dependent manner. Traffic 10, 572–589 (2009).
Vagnoni, A., Rodriguez, L., Manser, C., De Vos, K. J. & Miller, C. C. J. Phosphorylation of kinesin light chain 1 at serine 460 modulates binding and trafficking of calsyntenin-1. J. Cell. Sci. 124, 1032–1042 (2011).
Mórotz, G. M. et al. Kinesin light chain-1 serine-460 phosphorylation is altered in Alzheimer’s disease and regulates axonal transport and processing of the amyloid precursor protein. Acta Neuropathol. Commun. 7, 200 (2019).
Ster, J. et al. Calsyntenin-1 regulates targeting of dendritic NMDA receptors and dendritic spine maturation in CA1 hippocampal pyramidal cells during postnatal development. J. Neurosci. 34, 8716–8727 (2014).
Ponomareva, O. Y., Holmen, I. C., Sperry, A. J., Eliceiri, K. W. & Halloran, M. C. Calsyntenin-1 regulates axon branching and endosomal trafficking during sensory neuron development in vivo. J. Neurosci. 34, 9235–9248 (2014).
Cheng, K. et al. Calsyntenin-1 negatively regulates ICAM5 Accumulation in Postsynaptic Membrane and influences dendritic spine maturation in a mouse model of Fragile X Syndrome. Front. Neurosci. 13, 1–16 (2019).
Li, C. et al. ESRP1-driven alternative splicing of CLSTN1 inhibits the metastasis of gastric cancer. Cell. Death Discov 9, (2023).
Hu, X. et al. The RNA-binding protein AKAP8 suppresses tumor metastasis by antagonizing EMT-associated alternative splicing. Nat. Commun. 11, (2020).
Tripolitsioti, D. et al. MAP4K4 controlled integrin β1 activation and c-Met endocytosis are associated with invasive behavior of medulloblastoma cells. Oncotarget 9, 23220–23236 (2018).
Migliavacca, J., Züllig, B., Capdeville, C., Grotzer, M. A. & Baumgartner, M. Cooperation of Striatin 3 and MAP4K4 promotes growth and tissue invasion. Commun. Biol. 5, (2022).
Kumar, K. S. et al. Computer-assisted quantification of motile and invasive capabilities of cancer cells. Sci. Rep. 5, (2015).
Sonoda, Y. et al. Formation of intracranial tumors by genetically modified human astrocytes defines four pathways critical in the development of human anaplastic astrocytoma. Cancer Res. 61, 4956–4960 (2001).
Bos, P. H. et al. Development of MAP4 kinase inhibitors as motor neuron-protecting agents. Cell. Chem. Biol. 26, 1703–1715e37 (2019).
Cardoso-Moreira, M. et al. Gene expression across mammalian organ development. Nature 571, 505–509 (2019).
Jovanovic, D., Yan, S. & Baumgartner, M. The molecular basis of the dichotomous functionality of MAP4K4 in proliferation and cell motility control in cancer. Frontiers in Oncology vol. 12 1–18 Preprint at (2022). https://doi.org/10.3389/fonc.2022.1059513
Sepp, M. et al. Cellular development and evolution of the mammalian cerebellum. Nature 1–49. https://doi.org/10.1038/s41586-023-06884-x (2023).
De Ramon Francàs, G., Alther, T. & Stoeckli, E. T. Calsyntenins are expressed in a dynamic and partially overlapping manner during neural development. Front. Neuroanat. 11, 1–13 (2017).
Lee, E. S., Kim, W. T., Park, G. Y. & Lee, M. Gen Son, T. Calsyntenin 1 mRNA expression sensitivity to ionizing radiation in human hepatocytes and carcinoma cells and blood cells of BALB/c mice. J. Radiat. Res. Appl. Sci. 14, 44–50 (2021).
Gao, X. et al. MAP4K4 is a novel MAPK/ERK pathway regulator required for lung adenocarcinoma maintenance. Mol. Oncol. 11, 628–639 (2017).
De Zeeuw, C. I. & Hoogland, T. M. Reappraisal of Bergmann glial cells as modulators of cerebellar circuit function. Frontiers in Cellular Neuroscience vol. 9 1–8 Preprint at (2015). https://doi.org/10.3389/fncel.2015.00246
Ghasemi, D. R. et al. Compartments in medulloblastoma with extensive nodularity are connected through differentiation along the granular precursor lineage. Nat. Commun. 15, 1–20 (2024).
Li, H. et al. Tumor-associated astrocytes promote tumor progression of Sonic hedgehog medulloblastoma by secreting lipocalin-2. Brain Pathol. 1–19. https://doi.org/10.1111/bpa.13212 (2023).
Guo, D. et al. Tumor cells generate astrocyte-like cells that contribute to SHH-driven medulloblastoma relapse. J. Exp. Med. 218, (2021).
Cheng, Y. et al. Sustained hedgehog signaling in medulloblastoma tumoroids is attributed to stromal astrocytes and astrocyte-derived extracellular matrix. Lab. Invest. 100, 1208–1222 (2020).
Liu, Y. et al. Astrocytes promote medulloblastoma progression through hedgehog secretion. Cancer Res. 77, 6692–6703 (2017).
Yang, Z. J. et al. Medulloblastoma can be initiated by deletion of patched in lineage-restricted progenitors or stem cells. Cancer Cell. 14, 135–145 (2008).
Cheng, Y. et al. Sustained hedgehog signaling in medulloblastoma tumoroids is attributed to stromal astrocytes and astrocyte-derived extracellular matrix. Lab. Invest. https://doi.org/10.1038/s41374-020-0443-2 (2020).
Acknowledgements
The authors thank Dina Hochuli, Bernard Ciraulo, and Dejana Versamento for their technical support. This study was supported by the Swiss National Science Foundation (Project 31003A_165860, Sinergia_CRSII5_202245/1) and Innosuisse, the Swiss Innovation Agency (46812.1 IP-LS 0.1 IP-LS). Imaging was performed with equipment maintained by the Center for Microscopy and Image Analysis, University of Zurich.
Author information
Authors and Affiliations
Contributions
E.S. performed experiments, analyzed data, and contributed to figure preparation. S.Y. performed experiments, contributed to study design, analyzed data, and contributed to figure preparation. M.-S. L. contributed to essential primary cell model, performed experiments, analyzed data, and contributed to figure preparation. M.B. designed the study, analyzed data, prepared the figures, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary Material 2
Supplementary Material 3
Supplementary Material 4
Supplementary Material 5
Supplementary Material 6
Supplementary Material 7
Supplementary Material 10
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sönmez, E., Yan, S., Lin, MS. et al. MAP4 kinase-regulated reduced CLSTN1 expression in medulloblastoma is associated with increased invasiveness. Sci Rep 15, 946 (2025). https://doi.org/10.1038/s41598-024-84753-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84753-x
This article is cited by
-
Pea Peptides and Heavy Metal Neurotoxicity: Exploring Mechanisms and Mitigation Strategies in PC12 Cells
Plant Foods for Human Nutrition (2025)