Abstract
The aim of this study is to evaluate the real-world safety of brodalumab by analyzing adverse events (AEs) associated with the drug. The AE reports related to brodalumab from the FAERS database from 2017 Q1 to 2023 Q4 were collected. Subsequently, we employed four disproportionality analysis methods to identify positive signals among AEs associated with brodalumab, including Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Multi-Item Gamma Poisson Shrinker (MGPS). In 1480 AE reports with brodalumab as the primary suspected drug, 168 preferred terms (PTs) exhibiting positive signals were identified. This study confirmed several known positive AEs, such as injection site vesicles and injection site hemorrhage. In addition, the study identified several positive AEs not listed in the drug product information, including palmoplantar pustulosis and extranodal marginal zone b-cell lymphoma (malt type). This study evaluated the real-world safety profile of brodalumab and identified several unexpected AEs, such as palmoplantar pustulosis and extranodal marginal zone b-cell lymphoma (malt type). These findings provide new safety insights for clinicians and may contribute to the safer and more rational use of brodalumab in clinical practice.
Similar content being viewed by others
Introduction
Psoriasis is a chronic, relapsing, inflammatory, systemic disease that affects approximately 125 million people worldwide1. Psoriasis is characterized by distinct erythematous, scaly plaques on the skin that are unsightly, itchy, and painful2. Research has demonstrated that interleukin (IL)-17 is crucial in the pathogenesis of psoriasis. Moreover, therapies targeting IL-17 have proven to be effective in managing this condition.
Brodalumab (marketed as Siliq® by Valeant Pharmaceuticals in the United States and Canada, as Kyntheum® by LEO Pharma A/S in Europe, and as Lumicef® by Kyowa Kirin Co., Ltd. in Japan) is a human interleukin-17 receptor A(IL-17RA) IgG2 monoclonal antibody. Brodalumab binds to IL-17RA, thereby inhibiting downstream pro-inflammatory signaling pathways and alleviating clinical manifestations such as erythema and scaling3,4. Although multiple clinical trials have demonstrated the efficacy of brodalumab in improving the clinical symptoms of psoriasis, its use has also been associated with several known adverse events(AEs), including injection site reactions, myalgia, and headache5,6. With the widespread post-marketing use of brodalumab globally, increasing attention has been directed toward its real-world safety profile. Some researchers have suggested a potential association between brodalumab and the development of colitis7,8. Mantovani et al. reported that brodalumab may induce ichthyosis9, and Okazaki et al. documented a case of brodalumab-induced autoimmune hepatitis10. However, these studies are limited by small sample sizes, short follow-up durations, a focus on isolated safety events, or a lack of statistical analysis. Therefore, the overall safety profile of brodalumab in real-world settings remains to be further investigated.
The aim of this study is to analyze the AEs associated with brodalumab in the FAERS database using multiple disproportionality analysis methods, providing new insights into the real-world safety of brodalumab to support patient medication safety.
Methods
Data source
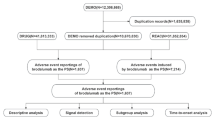
The FAERS database collects spontaneously submitted AE reports from clinicians, pharmacists, nurses, and consumers, and plays a critical role in post-marketing drug safety surveillance. Reports in the FAERS database primarily contain seven major categories of information: demographic and administrative information (DEMO), drug information (DRUG), adverse drug reaction information (REAC), patient outcome information (OUTC), reporting source information (RPSR), date of treatment initiation and end date of reported medication (THER), and medication administration indications (INDI). We extracted AE reports that listed brodalumab as the primary suspect (PS) drug from the FAERS database, covering the period from the first quarter of 2017 to the fourth quarter of 2023. The seriousness of AEs was classified according to the Council for International Organizations of Medical Sciences (CIOMS) criteria, which include: Death (DE), Life-Threatening (LT), Hospitalization (HO), Disability (DS), and Other Serious Outcomes (OT).
Data process
We primarily performed deduplication of AE reports and standardization of AE terms. Deduplication followed the principles established by the U.S. Food and Drug Administration (FDA). Specifically, the process was based on case identifiers (CASEIDs), FDA receipt date (FDA_DT), and primary identifiers (PRIMARYID). When CASEIDs were identical, the report with the most recent FDA_DT was retained; if both CASEID and FDA_DT were the same, the report with the largest PRIMARYID was preserved. Subsequently, AEs were standardized using the Medical Dictionary for Regulatory Activities (MedDRA, version 26.1) and mapped to both the preferred term (PT) and system organ class (SOC) levels.
Data analysis
In this study, four disproportionality analysis methods were employed to assess statistical associations between brodalumab and AEs, including the reporting odds ratio (ROR)11,12, proportional reporting ratio (PRR)13, Bayesian confidence propagation neural network (BCPNN)12,14, and multi-item gamma Poisson shrinker (MGPS)15. All the above methods are based on the parameters of the signal detection 2 × 2 table, the 2 × 2 table is shown in Table 1, and the equations and criteria for the four algorithms are listed in Table 2. In this study, an AE was defined as a positive signal if it met the criteria of at least one disproportionality analysis method.
Results
Basic characteristics of brodalumab-related AEs
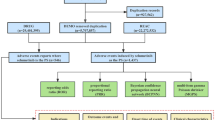
From the third quarter of 2018 to the fourth quarter of 2023, this study obtained a total of 1480 AE reports from the FAERS database. The basic characteristics were presented in Table 3. A total of 55.5% of AE reports were submitted by males, 42.4% by females, and 2.0% of reports did not specify gender information. The majority of reports involved individuals aged 18 to 65 years (54.5%), followed by elderly patients aged over 65 years (25.7%). Only one report involved a patient under the age of 18. The most reported indication was psoriasis (76.5%), followed by psoriatic arthropathy (3.6%), pustular psoriasis (2.1%), hidradenitis (0.6%), erythrodermic psoriasis (0.5%) and ankylosing spondylitis (0.4%). Regarding the reporting countries, United States (28.3%) submitted most reports, followed by Canada (27.8%), Japan (14.3%), Germany (7.8%), and United Kingdom (6.8%), respectively. The most frequently reported severe outcomes were OS (57.6%) and HO (31.3%).
Brodalumab signal mining
AEs related to brodalumab involved 23 SOCs. The study showed that the three most frequent SOCs were skin and subcutaneous tissue disorders (n = 662, ROR 2.06, PRR 1.93, IC 0.95, EBGM 1.93), infections and infestations (n = 583, ROR 1.93, PRR 1.83, IC 0.87, EBGM 1.83), and musculoskeletal and connective tissue disorders (n = 534, ROR 1.9, PRR 1.81, IC 0.86, EBGM 1.81). Details can be found in Table 4.
At the PT level, a total of 168 positive signals were identified in this study. Ranked based on the strictest EBGM algorithm, the top 30 PTs are displayed in Table 5. Results revealed PTs with high signal strength, such as injection site vesicles (n = 4, ROR 2291.56, PRR 2289.91, IC 10.58, EBGM 1526.94), injection site haemorrhage (n = 4, ROR 632.15, PRR 631.7, IC 9.12, EBGM 555.25), and calcium metabolism disorder (n = 3, ROR 259.37, PRR 259.23, IC 7.94, EBGM 245.4). Screening and excluding product problems, various types of injuries, poisoning and surgical complications, and possible clinical manifestations of the disease itself, the top 5 most frequent PTs are headache, depression, suicidal ideation, cellulitis, blood pressure increased. This study identified several unexpected AEs, including palmoplantar pustulosis, extranodal marginal zone b-cell lymphoma (malt type), and calcium metabolism disorders. Despite their rarity, the signal strength of these AEs was notably high. Details can be found in Table 5.
Discussion
Despite the well-documented benefits of biological agents for psoriasis relief, unexpected AEs also have been reported16. Thus, there is a need to monitor their actual use and AEs to ensure their safety and efficacy. By delving the FAERS database from the first quarter of 2017 to the fourth quarter of 2023, this study systematically assessed the AEs associated with brodalumab, reaffirming known safety data while identifying new potential risks. These findings provide novel safety information that may inform clinical practice and public health decision-making.
This study identified several known AEs associated with brodalumab, including injection site vesicles and injection site haemorrhage, both of which are classified as injection site reactions. In addition, some unexpected AEs were observed, such as palmoplantar pustulosis, extranodal marginal zone b-cell lymphoma (malt type), and calcium metabolism disorder.
A phase II clinical trial review of IL-17 inhibitors for the treatment of psoriasis indicated that injection site reactions are among the most frequently reported AEs17. Furthermore, a separate phase III clinical trial review also confirmed that injection site reactions are common across IL-17 targeted therapies17. Consistent with these findings, our study demonstrated that injection site reactions are frequently associated with brodalumab use. These reactions may present as erythema, pain, pruritus, bleeding, or vesicle formation at the site of administration. To reduce injection site reactions associated with injectable biologics, pharmaceutical manufacturers have improved injection delivery systems, including the use of finer-gauge needles and the development of citrate-free formulations, both of which have been shown to alleviate injection-related discomfort18. In addition, standardized injection techniques and patient education by clinicians may further help patients with psoriasis reduce injection pain and improve adherence to treatment.
Palmoplantar pustulosis is a chronic inflammatory disease characterized by aseptic pustules on the palms and soles. It shares many clinical features with pustular psoriasis, leading some researchers to classify palmoplantar pustulosis as an acral variant of pustular psoriasis, while others regard it as a distinct clinical entity19. Consistent with our findings, cases of palmoplantar pustulosis induced by brodalumab have been reported in the literature20. It is speculated that blockade of IL-17A might lead to an increased production of inflammatory cytokines upstream of IL-17A or that the Th1 pathway for cytokines such as IL-12 and TNF is upregulated and may mediate this paradoxical response21. In light of this, we recommend close monitoring for the potential onset of palmoplantar pustulosis during brodalumab treatment.
Marginal zone lymphomas (MZL) represent the second most prevalent subtype of indolent lymphomas, constituting 7% of all non-Hodgkin lymphomas (NHL). In 2016, there were 7,460 new cases diagnosed in the United States22,23. Extranodal marginal zone b-cell lymphoma is one of the MZL subtypes24. Psoriasis is associated with an increased incidence of certain cancers, and patients with psoriasis have an increased risk of developing lymphohematopoietic and pancreatic cancers and other malignant tumors, and the risk is further elevated in patients with a long history of the disease25, which may be related to impaired immune function26. The results of our study suggest that brodalumab does not have any clear link with the incidence of benign, malignant, and tumors of undetermined nature, but has a higher signal intensity with the development of extranodal marginal zone b-cell lymphomas. However, the association between brodalumab and extranodal marginal zone b-cell lymphomas requires further investigation for confirmation.
Recent studies have increasingly highlighted that individuals with psoriasis may face a heightened risk of pathologic fractures and osteoporosis27. Numerous studies have reported that patients with psoriasis commonly exhibit serum vitamin D deficiency or insufficiency28,29. In addition, several clinical case–control studies have demonstrated that serum 25-hydroxyvitamin D [25(OH)D] levels are significantly lower in patients with psoriasis compared to healthy controls30,31. Furthermore, a negative correlation between serum 25(OH)D levels and disease severity has been reported32. Vitamin D has a regulatory effect on calcium metabolism, promoting intestinal absorption of calcium, calcium deposition in osteoblasts, and renal reabsorption of calcium33. Therefore, our potential signal of calcium metabolism disorders appears questionable.
This study has several limitations. First, AE reports in the FAERS database are submitted voluntarily by clinicians, pharmacists, nurses, and consumers, which may introduce reporting bias and affect the reliability of the results. Second, the submitted reports may be subject to underreporting, overreporting, misreporting, or missing data, and often lack critical clinical details such as dosage, duration of treatment, comorbidities, and concomitant medications. These missing data hinder further exploration of factors influencing AEs and may compromise the accuracy of the study findings. Therefore, the results should be interpreted with caution. In addition, although we applied four disproportionality analysis methods, including two Bayesian approaches that improve robustness and help mitigate masking effects, some potential safety signals may still have been missed. Finally, disproportionality analysis reveals only statistical associations between brodalumab and AEs, without establishing causality.
Conclusion
This study assessed the real-world safety profile of brodalumab using four disproportionality analysis methods. Several known AEs were confirmed, such as injection site vesicles and injection site hemorrhage. In addition, unexpected AEs were identified, including palmoplantar pustulosis and extranodal marginal zone b-cell lymphoma (malt type). These findings provide new insights into the safety profile of brodalumab. Further large-scale prospective studies are needed to validate these observations.
Data availability
The dataset generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Armstrong, A. W. & Read, C. Pathophysiology, clinical presentation, and treatment of psoriasis: A review. JAMA 323(19), 1945–1960. https://doi.org/10.1001/jama.2020.4006 (2020).
Boehncke, W. H. & Schon, M. P. Psoriasis. Lancet 386(9997), 983–994. https://doi.org/10.1016/S0140-6736(14)61909-7 (2015).
Nakagawa, H., Niiro, H., Ootaki, K., Japanese brodalumab study group. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: Efficacy and safety results from a phase II randomized controlled study. J. Dermatol. Sci. 81(1), 44–52. https://doi.org/10.1016/j.jdermsci.2015.10.009 (2016).
Gottlieb, A. et al. Improvement in itch and other psoriasis symptoms with brodalumab in phase 3 randomized controlled trials. J. Eur. Acad. Dermatol. Venereol. 32, 1305–1313. https://doi.org/10.1111/jdv.14913 (2018).
Papp, K. A. et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N. Engl. J. Med. 366(13), 1181–1189. https://doi.org/10.1056/NEJMoa1109017 (2012).
Papp, K. et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J. Am. Acad. Dermatol. 71(6), 1183-1190.e3. https://doi.org/10.1016/j.jaad.2014.08.039 (2014).
Deng, Z., Wang, S., Wu, C. & Wang, C. IL-17 inhibitor-associated inflammatory bowel disease: A study based on literature and database analysis. Front Pharmacol. 14, 1124628. https://doi.org/10.3389/fphar.2023.1124628 (2023).
Petitpain, N. et al. IL-17 inhibitors and inflammatory bowel diseases: A postmarketing study in vigibase. Clin. Pharmacol. Ther. 110(1), 159–168. https://doi.org/10.1002/cpt.2155 (2021).
Mantovani, L. et al. A possible case of brodalumab-induced ichthyosis. J. Dtsch. Dermatol. Ges. 21(3), 288–290. https://doi.org/10.1111/ddg.14976 (2023).
Okazaki, S., Hoashi, T., Saeki, H. & Kanda, N. A case of autoimmune hepatitis/primary biliary cholangitis overlap syndrome during treatment with brodalumab for generalized pustular psoriasis. J. Nippon Med. Sch. 88(6), 569–573. https://doi.org/10.1272/jnms.JNMS.2021_88-517 (2021).
Van Puijenbroek, E., Diemont, W. & van Grootheest, K. Application of quantitative signal detection in the Dutch spontaneous reporting system for adverse drug reactions. Drug Saf. 26(5), 293–301. https://doi.org/10.2165/00002018-200326050-00001 (2003).
Van Puijenbroek, E. P. et al. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol. Drug Saf. 11(1), 3–10. https://doi.org/10.1002/pds.668 (2002).
Evans, S. J., Waller, P. C. & Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 10(6), 483–486. https://doi.org/10.1002/pds.677 (2001).
Bate, A. et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 54(4), 315–321. https://doi.org/10.1007/s002280050466 (1998).
Dumouchel, W. Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system. Am. Stat. 53(3), 177–190. https://doi.org/10.1080/00031305.1999.10474456 (1999).
Boyman, O., Comte, D. & Spertini, F. Adverse reactions to biologic agents and their medical management. Nat. Rev. Rheumatol. 10(10), 612–627. https://doi.org/10.1038/nrrheum.2014.123 (2014).
Brown, G., Malakouti, M., Wang, E., Koo, J. Y. & Levin, E. Anti-IL-17 phase II data for psoriasis: A review. J. Dermatolog. Treat. 26(1), 32–36. https://doi.org/10.3109/09546634.2013.878448 (2015).
Battista, T. et al. Injection site reactions resulting from the use of biological therapy in the treatment of moderate-to-severe plaque psoriasis. Expert Opin. Drug Saf. 23(9), 1115–1126. https://doi.org/10.1080/14740338.2024.2392007 (2024).
Yamamoto, T. Similarity and difference between palmoplantar pustulosis and pustular psoriasis. J. Dermatol. 48, 1346–8138. https://doi.org/10.1111/1346-8138.15826 (2021).
Mössner, R. & Pinter, A. Paradoxical palmoplantar pustulosis induced by secukinumab and brodalumab: A report of three cases. Eur. J. Dermatol. 30(2), 177–209. https://doi.org/10.1684/ejd.2020.3702 (2020).
Dogra, S., Bishnoi, A., Narang, T. & Handa, S. Secukinumab-induced paradoxical pustular psoriasis. Clin. Exp. Dermatol. 44(1), 72–73. https://doi.org/10.1111/ced.13731 (2019).
Reid, R. & Friedberg, J. W. Management of marginal zone lymphoma. Oncology (Williston Park) 27(840–842), 844 (2013).
Sriskandarajah, P. & Dearden, C. E. Epidemiology and environmental aspects of marginal zone lymphomas. Best Pract. Res. Clin. Haematol. 30, 84–91. https://doi.org/10.1016/j.beha.2016.07.002 (2017).
Cheah, C. Y. & Seymour, J. F. Marginal zone lymphoma: 2023 update on diagnosis and management. Am. J. Hematol. 98(10), 1645–1657. https://doi.org/10.1002/ajh.27058 (2023).
Brauchli, Y. B. et al. Psoriasis and risk of incident cancer: An inception cohort study with a nested case-control analysis. J. Invest. Dermatol. 129(11), 2604–2612. https://doi.org/10.1038/jid.2009.113 (2009).
Pouplard, C. et al. Risk of cancer in psoriasis: A systematic review and meta-analysis of epidemiological studies. J. Eur. Acad. Dermatol. Venereol. 27(s3), 36–46. https://doi.org/10.1111/jdv.12165 (2013).
Muñoz-Torres, M., Aguado, P., Daudén, E., Carrascosa, J. M. & Rivera, R. Osteoporosis and psoriasis. Actas Dermosifiliogr (Engl Ed) 110(8), 642–652. https://doi.org/10.1016/j.ad.2019.02.005 (2019).
Moussa, S., Khaled, H., Elshishtawy, H. & Elnaggar, H. Subclinical atherosclerosis in psoriatic patients: Relation with vitamin D. QJM: Int. J. Med. https://doi.org/10.1093/qjmed/hcab116.011 (2021).
Moosazadeh, M. et al. Comparing vitamin D level between patients with psoriasis and healthy individuals: A systematic review and meta-analysis. J. Evid.-Based Integrat. Med. https://doi.org/10.1177/2515690X231211663 (2023).
Gupta, S., Garg, P., & Gupta, N. Serum 25-hydroxyvitamin D level in psoriasis patients: A case-control study. International Journal of Research in Dermatology. 2019 https://doi.org/10.18203/ISSN.2455-4529.INTJRESDERMATOL20185500.
Formisano, E., Proietti, E., Borgarelli, C., & Pisciotta, L. Psoriasis and Vitamin D: A Systematic Review and Meta-Analysis. Nutrients. 2023; 15. https://doi.org/10.3390/nu15153387 .
Pokharel, R., Agrawal, S., Pandey, P. & Lamsal, M. Assessment of vitamin D level in patients with psoriasis and its correlation with disease severity: A case–control study (2022).
Kulda, V. Metabolizmus vitaminu D [Vitamin D metabolism]. Vnitr. Lek. 58(5), 400–404 (2012).
Acknowledgements
Funding for this study was provided by Beijing Natural Science Foundation (grant number 7232288).
Author information
Authors and Affiliations
Contributions
Yue Wan and Yang Zhou wrote the manuscript and generated figures. Wenjin Chen, Huanhuan Li and Jingjing Huang contributed to editing the manuscript. Jianhong Li supervised the study and approved the submission of this article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wan, Y., Zhou, Y., Chen, W. et al. Analysis and mining of brodalumab adverse events based on FAERS database. Sci Rep 15, 18175 (2025). https://doi.org/10.1038/s41598-025-03192-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-03192-4