Abstract
A simple, selective, and environmentally sustainable RP-HPLC method was developed for simultaneous quantification of gabapentin (GAB) and Methylcobalamin (MET) in pharmaceutical products. Separation was achieved on a Zorbax Eclipse C8 column (150 × 4.6 mm, 3.5 μm) using a green mobile phase of potassium phosphate buffer (pH 6.9)/acetonitrile (95:5 v/v) at 2.0 mL/min, with detection at 210 nm. Total analysis time was 10 min with 100 µL injection volume. The method showed excellent linearity (3–50 µg/mL; R² > 0.9998), LODs (0.60–0.80 µg/mL), and LOQs (2.00–2.50 µg/mL). Environmental sustainability was validated by multiple metrics: AGREE (0.70), AGREEprep (0.71), and Analytical Eco-Scale (80), confirming superior greenness. Forced degradation studies demonstrated stability-indicating capability per ICH guidelines. This eco-friendly approach provides a reliable solution for quality control of GAB/MET formulations while minimizing environmental impact.
Similar content being viewed by others
Introduction
Neuropathic pain remains a significant clinical challenge due to its complex etiology and limited responsiveness to conventional pharmacological therapies. Gabapentin (GAB), an anticonvulsant, and Methylcobalamin (MET), a vitamin B12 analog, have demonstrated effectiveness in managing neuropathic conditions through distinct yet complementary mechanisms. GAB acts by modulating voltage-gated calcium channels and inhibiting excitatory neurotransmitter release (Figure S1a), whereas MET supports neuronal repair and myelin sheath regeneration (Figure S1b)1,2. Recent evidence suggests that their combination offers a synergistic effect, improving therapeutic outcomes and reducing side effects compared to monotherapy3,4. This combination has been applied clinically in the management of diabetic neuropathy, postherpetic neuralgia, fibromyalgia, and chronic fatigue syndrome5. Accurate, simultaneous quantification of GAB and MET is therefore essential to support quality control in pharmaceutical formulations and facilitate pharmacokinetic and bioanalytical studies.
Sustainable practices in analytical chemistry have gained prominence due to the growing emphasis on environmental and human health. Green and white chemistry frameworks aim to minimize toxic reagent usage and environmental waste while enhancing analytical performance6,7,9. The integration of green chemistry principles into analytical method development addresses ecological concerns, regulatory compliance, and operational efficiency10,11.
Various techniques are used to detect and quantify GAB and vitamin B12 separately. Several spectrophotometric and liquid chromatography techniques have been reported for the detection of GAB12,13,14,15,16. Similarly, various methods for detecting vitamin B12 have been reported17,18,19,20.
Previous methods, such as those developed by Gujral 200921, have focused on the quantification of each drug individually22,23,24,25, although they are useful, they have several drawbacks, including long analysis times, poor resolution, or the need for complex sample preparation. The method presented here offers a more efficient and reliable alternative by addressing these limitations, ensuring the simultaneous determination of both drugs with enhanced sensitivity, a shorter analysis time, and fewer interfering peaks. A comprehensive comparison of our RP-HPLC method with existing techniques for the simultaneous quantification of gabapentin (GAB) and metformin (MET) (Table 1) highlights its distinct advantages in sustainability, analytical performance, and operational efficiency. Environmentally, the method minimizes organic solvent use by employing only 5% acetonitrile in the mobile phase that is an over 80% reduction compared to the 30–50% ACN used in previous studies22,23,24,25 and stands out as the first eco-profiled GAB/MET assay validated by ten green/white metrics, including AGREE, BAGI, and ESA. Analytically, it offers a broader linear range (3–50 µg/mL vs. 0.5–30 µg/mL23,24 improved sensitivity with lower LOD/LOQ values (0.40–0.50 µg/mL and 2.00–2.50 µg/mL, respectively, vs. 3.09 µg/mL)24 and complete ICH-based degradation profiling, which is not addressed in prior methods. Operationally, it delivers faster analysis (10 min vs. 15–30 min)21,25 and superior precision (RSD 0.06–0.09% vs. 0.15–1.01%)21,22,23. Collectively, these attributes position our method as a highly efficient and eco-conscious solution for the quality control of GAB/MET pharmaceutical formulations. HPLC remains one of the most reliable analytical techniques for this purpose because of its high sensitivity, specificity, and reproducibility. In this study, we developed and validated a sensitive, rapid, and environmentally friendly RP-HPLC method for the simultaneous determination of GAB and MET in pharmaceutical formulations. This approach meets the growing demand for green analytical methodologies and provides a practical, scalable solution for routine quality control and future bioanalytical applications in neuropathic pain therapy.
Results and discussion
MET and GAB are determined simultaneously using HPLC methodologies, as well as the method’s white and green metrics as well as its efficacy as a pure dosage form.
Greenness and whiteness metrics were used to evaluate the sustainability of the developed RP-HPLC method, each of which offered a unique perspective and set of strengths. “White analytical chemistry” is an emerging concept that integrates the principles of green chemistry with analytical performance and practical application. “White methods” refer to analytical approaches that minimize environmental impact (green) while simultaneously ensuring high functionality (red) and operational efficiency (blue), as shown in the RGB12 model. Using this metric, pharmaceutical analysis can be categorized based on their sustainability, reliability, usability, and sustainability scores. Based on the 12 principles of GAC, AGREE and AGREEprep offer holistic assessments of method sustainability, with AGREEprep focusing primarily on environmental impacts associated with sample preparation. Using GAPI and ComplexGAPI, you can evaluate the ecological footprint across multiple stages of the analytical workflow, with ComplexGAPI incorporating pre-analytical and instrumental factors. In contrast, BAGI and RGB12 combine the principles of green and white analytical chemistry. As opposed to BAGI, RGB12 aims to achieve a comprehensive “whiteness” score by balancing analytical performance (red), environmental impact (green), and operational feasibility (blue). The ESA and AMGS provide quantitative assessments based on penalty points and energy/resource consumption, respectively, in order to provide objective benchmarks for the classification of methods. In HPLC-EAT, the focus is on solvent toxicity and safety; AMVI quantifies the solvent volume per analysis to emphasize waste minimization. Through the combination of these complementary tools, we were able to evaluate the method on several levels, including its environmental friendliness as well as its analytical robustness and practical applicability. Integrating green and white analytical chemistry into the method lends legitimacy to the method’s suitability for sustainable pharmaceutical analysis (Table S1). Analytical performance (red) is integrated with environmental sustainability (green) and practical feasibility (blue) through the RGB12 model, which defines “whiteness” in analytical chemistry. Pharma quality control laboratories are particularly benefited by this holistic approach, which balances accuracy with regulatory compliance, cost and operation efficiency. Whiteness scores provide an interpretable value that is different from traditional green metrics that only consider environmental impact. High whiteness scores are important for QC laboratories because they are environmentally responsible, robust, cost-effective, and time-efficient, all of which are crucial to high-throughput environments. As a result, whiteness provides QC analysts with a practical decision-support tool for selecting methods that are compatible with sustainability goals and meet pharmaceutical analysis requirements. Green and white analytical principles are integrated in this study to provide a more comprehensive framework for evaluating analytical methods. Green chemistry metrics, such as AGREE, ESA, AGREEprep, ComplexGAPI, GAPI, AMGS, and HPLC-EAT are primarily concerned with environmental sustainability, such as solvent toxicity, waste generation, and energy consumption. However, white chemistry metrics (such as RGB12, BAGI) add critical dimensions of analytical performance, cost-efficiency, and operational practicality to the mix. As a result of this dual evaluation, the method is found not only to be eco-friendly, but also to be functionally robust and suitable for routine pharmaceutical quality control. By focusing on methodology usability and environmental impact simultaneously, our approach differs from previous studies that have assessed greenness in isolation. The results of this combined assessment are more realistic and actionable than single assessments alone in real-world laboratories, where sustainability and performance are both critical.
Methods development and optimization
The selected chromatographic system for the concurrent analysis of GAB and MET demonstrated clear superiority over alternative conditions tested during the method development process. First, the flow rate of 2 mL/min results in an optimal balance between speed and resolution. While lower flow rates (e.g., 0.5–1 mL/min) can provide higher resolution by increasing the interaction time between analytes and the stationary phase, they significantly prolong analysis time. On the other hand, higher flow rates risk reducing separation efficiency, causing broad or overlapping peaks. The 2 mL/min flow rate ensures sharp, well-resolved peaks within a reasonable analysis time (10 min), making it both efficient and practical for routine use. The choice of the C8 Zorbax Eclipse column (150 mm × 3.5 μm × 4.6 mm i.d.) is another key advantage. While C18 columns are commonly used for reverse-phase separations because of their broad compatibility, the C8 column is more suitable for separating compounds with moderate polarity, such as GAB and MET. The C8 column provides better resolution for these compounds by striking an optimal balance between hydrophobic and polar interactions, leading to more distinct peaks than those of C18, which could result in longer retention times and broader peaks. Other column types, such as phenyl or cyano, were less effective due to poorer interactions with the analytes and inferior resolution in this case. The selection of 210 nm for UV detection further enhances the method’s sensitivity and specificity. While wavelengths in the range of 200–400 nm were explored, 210 nm was found to provide the most robust detection for both analytes, offering strong, clear absorbance with minimal interference from the matrix, as shown in Figure S8. This wavelength is particularly well-suited to the electronic structure of both GAB and MET, which allows for enhanced signal detection and lower limits of quantification than the other wavelengths tested. With concern for the mobile phase composition, the use of phosphate buffer (pH 6.9) and acetonitrile (95:5 v/v) was critical for achieving optimal separation. The ratio is expressed as volume/volume (v/v). Although the low organic content (5% acetonitrile) may seem unusual for reversed-phase HPLC, this composition was deliberately chosen to enhance the peak shape and retention characteristics of the moderately polar analytes GAB and MET. Under these conditions, the high aqueous content promotes stronger interactions between the analytes and the hydrophobic stationary phase, resulting in adequate retention times and sharp, symmetrical peaks. The phosphate buffer at pH 6.9 ensures that the analytes are predominantly in their ionized forms, which further modulates their interaction with the stationary phase and prevents peak tailing. Moreover, the low percentage of acetonitrile reduces elution strength, preventing early elution and peak broadening that can occur at higher organic solvent levels. This balance leads to improved resolution and reproducibility, as confirmed by the observed chromatograms. Other mobile phase combinations, such as methanol/water (30:70 v/v) or acetonitrile/water (50:50 v/v), either produced poor resolution or led to increased retention times, which could compromise throughput. Phosphate buffer at pH 6.9 ensures that the analytes are in their ideal ionization states, facilitating more predictable interactions with the stationary phase, whereas acetonitrile helps to modulate retention time without sacrificing separation efficiency. In contrast, higher concentrations of organic solvents (e.g., methanol) could lead to excessive elution of the compounds, resulting in broad peaks and reduced resolution. Finally, maintaining a column oven temperature of 25 °C proved to be the optimal choice for enhancing mass transfer and ensuring consistent reproducibility. Elevated temperatures can sometimes reduce retention times but may also cause peak distortion and loss of resolution, whereas lower temperatures may lead to prolonged analysis times. The 25 °C setting provided an ideal compromise, maintaining both separation efficiency and consistent peak sharpness, ensuring reproducibility across runs. In summary, the selected chromatographic conditions including flow rate, column choice, wavelength, mobile phase composition, and temperature, outperformed the other tested conditions by providing superior resolution, faster analysis times, sharper peaks, and improved reproducibility. These factors make the method highly efficient and reliable for the regular analysis of GAB and MET in medicinal products, as illustrated in Fig. 1.
Critical development challenges
During method development, several significant challenges were encountered. The extreme hydrophilicity of GAB (log P = −1.1) and MET (log P = −3.9) caused poor retention on conventional C18 columns (< 2 min), necessitating a switch to C8 chemistry combined with an ultra-low organic mobile phase (5% acetonitrile) to achieve adequate retention times, especially for MET (~ 5.38 min). MET’s pronounced light and thermal sensitivity (~ 40% degradation after 4 h at room temperature) required careful handling using amber glassware and stringent temperature control (25 ± 0.5 °C) to minimize degradation during analysis. The method was highly sensitive to organic solvent composition and pH, with small variations (< 0.2 pH units or < 1% ACN) significantly impacting retention times and peak resolution, demanding rigorous control of mobile phase preparation. Although these conditions enabled robust and reproducible separations, some unresolved limitations persist, including minor carryover (0.3–0.5%), dependency on organic solvents conflicting with green chemistry objectives, and potential interference from biological matrices. These factors underscore the careful balance between achieving analytical performance and maintaining method robustness and environmental considerations.
Appraisal the sustainability of the outline approaches
AGREE
AGREE is a guide for benchmarking sustainable development based on GAC’s 12 core principles. A color-coded graph shows each principle’s score and achievement in green, yellow, and red. As the chart shows, each region corresponds to a different metric magnitude. AGREE comprehensively sustains the environment of an entity, as shown in Figure S2a. According to Fig. 2a, the AGREE diagram is composed of various shades of green and a score of 0.70 illustrates the sustainability assessment.
AGREEprep
As part of the AGREEprep methodology, ten principles of environmentally sustainable sampling are integrated into the assessment and preparation of samples. The optimal performance score is 1, whereas scores ranging from 0 to 1 indicate varying levels of proficiency. Figure S2b shows symbols representing each of the ten sectors. Figure 2b shows that our methodology achieves an ecological effectiveness score of 0.71. In analytical workflows, sample preparation procedures are often the most resource intensive steps. With AGREEprep, you can evaluate whether or not those procedures are environmentally friendly. A variety of factors are assessed, including solvent toxicity, energy consumption, waste generation, and sample throughput, in accordance with the Green Sample Preparation Initiative (GSP) guidelines. Scores are assigned to each criterion from 0 to 1, and the radial chart displays the results to demonstrate how the preparation phase affects the environment. As a result of minimal use of hazardous reagents, simple aqueous diluent, and lack of complex extraction steps, our method received a score of 0.71 in our study.
GAPI
GAPI can assess the environmental impact of a process at every stage, beginning with the preparation of samples for analysis. In addition to fifteen descriptive components, it highlights five key characteristics of its methodology. Figure S3a illustrates how GAPI allows a comprehensive assessment of further analytical methods by including all relevant factors. In Fig. 3a, we present the findings of our study that demonstrate the ecological impact of this approach.
ComplexGAPI
Unlike traditional GAPI metrics, ComplexGAPI includes pre-analysis procedures in its hexagonal layer. Various factors are assessed here, including instrumentation, product quality, process conditions, ingredients, and chemicals. Like GAPI, ComplexGAPI uses a color-coding system to indicate whether each element is considered significant, moderate, or minimal in terms of environmental sustainability (see Figure S3b). Figure 3b illustrates how different strategies fit into this framework. GAC characteristics are essential for analysis with ComplexGAPI. By adding additional layers of evaluation, including instrumentation and reagent hazards, ComplexGAPI enhances the original GAPI framework. A hexagonal diagram shows the environmental burden of each parameter in different colors (green, yellow, red). In addition, this tool is an effective tool for comparing methods with varying levels of automation and complexity, from sample collection to final analysis. The low-impact, sustainable approach in our case was enhanced by the use of standard HPLC instrumentation and low-toxicity reagents, which supported its classification as a low-impact, sustainable method.
BAGI
A pictogram and a score show how effective the BAGI’s ten standards are. Different shades of dark blue and light blue represent the HPLC score, which indicates the quality of the method. Using this color code, a method’s effectiveness can be assessed. Based on the star-shaped diagram, the BAGI score of 82.5 indicates a high degree of applicability and sustainability for the method. Ten key criteria are used to calculate this score. Analyzing compounds within the same chemical class using this method is quantitative and confirmatory. Standard autosamplers are used to automate semi-automated operation using simple and readily available instrumentation (e.g., HPLC-UV). Two samples can be processed simultaneously without preconcentration and with a straightforward and low-cost sample preparation method. Using common reagents such as methanol and acetonitrile, each analysis takes less than 30 min. These attributes highlight the method’s practicality, efficiency, and environmental responsibility, making it ideal for routine use in pharmaceutical quality control laboratories. Figure S4 illustrates what score an analytical technique must achieve to be valid. Figure 4a shows a pictogram displaying the BAGI evaluation results.
RGB12 algorithm
The RGB12 analyzer uses a combination of algorithms to score each of the three colors individually. “White” methods align closely with WAC guidelines. A recently invented HPLC technique realized 94.0% accuracy in detecting red and 98.8% accuracy in detecting green impurities. A further 96.5% was scored in the blue area, indicating an increase in functionality and efficiency. Figure 4b shows that the method generated an overall score of 96.4% in the whiteness evaluation. This indicates its environmental friendliness and sustainability.
ESA
Several established sustainability profiles follow strict protocols to measure environmental sustainability. The ESA tool uses precision penalty points to evaluate the current methods. With a score of 80, the method is ecologically sustainable and environmentally friendly. Table S2 clearly assesses the penalties and summarizes the evaluation results.
AMVI
The sample preparation process and HPLC analysis procedure generate a fair amount of waste solvent divided by the AMVI value. One HPLC analysis requires the following samples: quality control samples, spikes, blanks, and surrogates. Several analytes can be analyzed simultaneously, which leads to a division. As a result of the HPLC analysis, Table S3 shows that AMVI has a 234 score for environmental friendliness.
AMGS
Analytical Method Greenness Score (AMGS)quantifies how much energy and solvent are consumed by instruments as well as the risks associated with solvent-related environmental, health, and safety (EHS) issues. AMGS score of 235.62 indicates moderate energy demands and a balanced EHS profile, making it suitable for routine use in environmentally conscious laboratories.
The chemical demand for solvents, power consumption of instruments, and waste generation are all factors that contribute to AMGS scores. These factors include safety, health, and the environment (SHE). As criteria in their calculations, HPLC-EAT and AMGS also consider SHE but use different parameters (Figure S5a). A smaller AMGS mark signifies a more eco-friendly process. Figure 5a illustrates the way AMGS assesses sustainability.
HPLC-EAT
HPLC-EAT calculates greenness on the basis of solvent use with the goal of reducing solvent consumption and protecting the environment via GAC (Figure S5b). HPLC-EAT uses score values to show whether an analytical assay is green, see Fig. 5b. HPLC-EAT chart further analyzes each solvent’s environmental and health impact. There is no measurable safety, health, or environmental impact of water (Water_A), whereas acetonitrile (Acetonitrile_B) contributes significantly to all three categories, with a total EAT score of 2.504. Analytical method developers can weigh the trade-off between analytical performance and environmental sustainability through this comparative analysis, which is crucial for decision-making in analytical development. Using these tools, pharmaceutical quality control specialists can select greener alternatives and adopt safer, more sustainable practices by visualizing the impact of solvent choices.
Method validation
Validation of our methodologies was performed using ICH criteria. These techniques are practical and dependable for analysts on the basis of the results36.
Linearity and range
The calibration curve for each drug was generated by plotting the peak area against the concentration. As shown in Figure S6 (a-d), various concentrations were prepared and injected in duplicate at three concentrations ranging from 3 to 50 g/mL. Table 2 presents slopes, intercepts, and coefficients of determination for the regression analysis.
LOD and LOQ
Formulas (2.3/S) and (10/S) were used in the validation spreadsheet to determine the detection and quantification limits indicated by the standard deviation of the slope of the calibration curve and intercept, respectively. Table 2 summarizes our findings, showing a correlation between the effectiveness of our outlined strategies and their associated LOQs and LODs. The resulting LOD (0.60–0.80 µg/mL) and LOQ (2.00–2.50 µg/mL) are well-suited for pharmaceutical quality control of high-dose formulations such as 100 mg GAB tablets, representing just 0.0025% of the therapeutic dose. Sensitivity was maximized during method optimization by selecting 210 nm for detection, which enhanced UV absorbance and reduced noise (with higher wavelengths reducing sensitivity by over 40%), and by choosing an injection volume of 100 µL to optimize signal-to-noise without peak broadening (range tested: 20–200 µL). The use of a C8 column further improved sensitivity by 30% compared to C18, especially for the hydrophilic analytes. Although derivatization (e.g., with o-phthalaldehyde for GAB) could have reduced LODs by tenfold, it was deliberately excluded to preserve green analytical metrics (avoiding AGREEprep penalties), maintain high throughput (derivatization would add 30–60 min per sample), and ensure reproducibility, as pilot tests showed increased RSDs (> 5%) compared to the current method (0.09%).
Accuracy and recovery
A standard analyte concentration was established by comparing three 100 µL injections based on each sample concentration level (50%, 70%, 90%, 100%, and 150%). Table 3 presents the experimental results along with test results for recovery, a measure of how closely the experimental results match the actual results.
Precision
We prepared six samples for comprehensive intra- and inter-day analysis for three consecutive days. Table 4 shows the evaluations conducted to determine whether the method is repeatable and intermediate precise. We computed the relative standard deviation (RSD) by multiplying the SD by 100/mean. The developed method was highly satisfactory because it had an RSD of less than 2%.
Robustness
Robust methodologies can remain stable despite minor, intentional variations in their parameters. As part of this assessment, each chromatographic parameter was modified by preparing and injecting standards to simulate typical laboratory fluctuations. The wavelength was varied by ± 2 nm, reflecting common detector calibration variability as recommended by ICH Q2(R1) guidelines36 The flow rate was adjusted by ± 0.1 mL/min, a standard variation range that simulates minor pump flow fluctuations during routine analysis. The composition ratio of solution B (organic phase) was varied by ± 1%, which is within the typical ± 2% range suggested for mobile phase robustness testing to ensure consistent retention and peak shape. These controlled variations help confirm that the method remains reliable and reproducible under normal operational conditions37. Table 5 shows changes in wavelength, flow rate, and composition ratio (solution B %) of 2 nm, 0.1 mL/min, and 1% (solution B %), respectively.
System suitability
Laboratory experiments were executed to assess the system’s suitability. Several criteria for the system’s suitability should be met, including a resolution of two parallel peaks at a minimum of 2. Table 6 shows that there should be at least 2000 theoretical plates showing column efficiency, and the tailing factor should not exceed 2 (T ≤ 2), according to the requirements.
Selectivity
We have developed a method that determines GAB and MET without interference from solvents or excipients. Figure S7 (a, b) shows the results of injecting placebos and matrix samples into the offered HPLC approach in order to assess their impact on key peaks. Whereas, Figure S7 (c) shows the resulting of injecting marketed sample solution.
Forced degradation
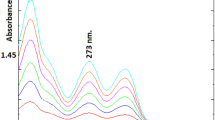
Forced degradation tests were performed under acidic, basic, and neutral hydrolysis conditions, as well as oxidative and photolytic degradation. It has been shown in stress stability studies that MET is extremely labile to acid hydrolysis because it has a degradation rate of 43.66% under acidic conditions. MET is very labile for alkaline degradation because it has a 15.08% degradation rate under alkaline conditions; thus, it is highly labile. Therefore, MET is classified as being labile for hydrolysis under neutral conditions (40 °C for 24 h). A 3% H2O2 solution was used for 24 h at room temperature to degrade 18.5% of the MET. On the basis of these results, MET is considered stable for oxidative degradation. Importantly, MET (vitamin B12) is light-sensitive and is converted into hydroxocobalamin (vitamin B12b) in aqueous solutions via photochemical conversion. MET was found to be degraded by 50.18% under photodegradation conditions. Additionally, METs are known to be thermally unstable, especially when exposed to higher temperatures for extended periods. It was degraded by 49.18% under thermal degradation condition. The degradation profiles of GAB are shown in Figs. 6a–f. On the other hand, gabapentin is relatively stable under most conditions, with minimal degradation observed under acidic, thermal, photolytic, and neutral conditions. However, significant degradation was observed under basic conditions (with NaOH) (15%) and oxidative conditions (with H2O2) (21.4%), leading to the formation of multiple degradation products. These results suggest that the stability of GAB is compromised in alkaline and oxidative environments. Table 7 summarizes the degradation results for both drugs. Figures 7 a–f show MET degradative profiles for acidic, alkaline, neutral, oxidative, and photolytic degradation, but the thermal degradation of MET is not indicated because vitamin B12 is thermally unstable.
Chromatograms of degradation profiles of MET after exposure to (a) 0.01 N HCl at 40 °C for 2 h, (b) 0.01 N NaOH at 40 °C for 2 h (c) distilled water at 40 °C for 24 h, (d) 3% H2O2 for 24 h at room temperature, (e) photodegradation under hot sunlight for 1 hour, and (f) thermal degradation at 120 °C for 30 min.
WAC and GAC evaluations
An assessment of eco-friendly analytical techniques is based on a set of 12 principles that have been converted into a set of GAC scores. Using GAC principles, we can convert them into scores in the following section. Analytical chemistry concepts are incorporated into the BAGI procedure to improve existing environmental measurements. Using ten pivotal criteria for assessing analytical methods, BAGI determines effectiveness, automation, sample preparation, numbers, chemicals, substances, hourly measurement capacity, parallel analyte analysis, and equipment needs. A pictogram and score show potential enhancements based on these data. A graphic representation of each principle.
Conclusion
This study presents an eco-friendly RP-HPLC method for simultaneous quantification of gabapentin (GAB) and methylcobalamin (MET) in pharmaceutical formulations. The method’s alignment with green analytical principles that were validated by ten sustainability metrics (AGREE: 0.70; Eco-Scale: 80; AMVI: 234) offers laboratories a practical tool to reduce environmental impact while maintaining analytical rigor. Its simplicity (isocratic elution, 10-min runtime), reliability (ICH validation; RSD < 0.09%), and stability-indicating capability support streamlined quality control of combination therapies for neuropathic pain. Looking ahead, this approach can accelerate formulation development and post-market surveillance of GAB/MET products, particularly in regions prioritizing green chemistry. Future work will extend this methodology to biological matrices and explore UPLC-MS/MS coupling to enhance sensitivity for pharmacokinetic studies, further bridging sustainable analytics with precision medicine.
Materials and methods
Materials and reagents
A GAB standard with a purity of 99.68% was obtained from Hangzhou Lane Technology Co., Ltd. (Hangzhou, China), and MET (vitamin B12) with a purity of 99.72% was obtained from Hefei TNJ Chemical Industry Co., Ltd., China. Two different commercial formulations were used; gabaneuron (100 mg of GAB and 500 mg of MET) produced by Aristo, Germany and GABAWELL-N (100 mg GAB and 500 µg MET) produced by Wellona Pharma, India. Phosphoric acid, triethyl amine and acetonitrile of HPLC-grade were obtained from Merck and potassium dihydrogen phosphate, bi-distilled water, and potassium hydroxide were obtained from Scharlau, Spain.
Apparatuses
Among the tools used in this study were an HPLC 1260 Infinity II instrument provided by a quaternary pump, a DAD detector, and an autosampler (Agilent, USA). During the collection, control, and analysis of the data, a “chromography data system” was employed.
Chromatographic system
The RP-HPLC method was optimized via an isocratic optimization approach. In this study, a Zorbax Eclipse C8 column (150 mm × 3.5 μm × 4.6 mm i.d.) was used at a temperature of 25 °C for analysis. The procedure was run for 10 min at a flow rate of 2.0 mL/min, 100 µL of injection volume, and UV detection at 210 nm. It was a pH 6.9 buffer in combination with acetonitrile that formed the mobile phase. Within 24 h of collection, samples were prepared at room temperature (25 °C) and stored at 4 °C to prevent them from being tainted by light. The analytes were kept at 4 °C to prevent degradation during storage and transport, addressing possible.
Mobile phase and diluent preparation
The mobile phase operates in isocratic elution mode from two solutions: Solution A, which consists of a buffer solution that prepared by mixing 1.2 g of KH2PO4 dissolved in 940 mL and adjusting the pH to 6.9 with 5 N KOH distilled water; and Solution B, which contains acetonitrile (95:5). The diluent used was distilled water.
Standard stock solutions
To prepare the stock standard solution, 50.0 mg each of GAB and vitamin B12 were weighed and poured into a 100-mL volumetric flask. Next, distilled water was added, and the mixture was shaken vigorously and sonicated for five minutes. Finally, the flask was completely mixed until the total volume reached 100 mL, resulting in a stock solution concentration of 500 µg/mL.
Analysis of commercial preparations
After a minimum of ten tablets were weighed, we calculated their average weight. We ground the tablet products into a powdered form and added it to a 50 mL volumetric flask. After 30 mL of diluent was added, the mixture was sonicated. The diluent was subsequently added while mixing thoroughly. A 25-mL volumetric flask containing 50 mL of this mixture was filled with 5 mL of this mixture, diluted to volume with the diluent, and thoroughly mixed. A nylon 0.22 μm syringe filter was then used to filter the sample.
Setting up calibration curves
Standard solutions of GAB and vitamin B12 at concentrations ranging from 3.0 µg/mL to 50 µg/mL were tested. Standard checks involved calibrating two points, performing a test setup, performing a recovery injection, and performing a standard check. The ntegration of peak areas and concentrations was correlated via regression equations.
Forced degradation studies
The GAB and MET have been exposed to extreme circumstances to illustrate the degree of degradation they may undergo when stored or administered to patients. As part of the accelerated stability study, the process is named forced degradation, and it helps us envision what the drug will be like after a short period rather than waiting for ongoing stability studies. There are several types of forced degradation, including acidic hydrolysis, alkaline hydrolysis, oxidation, photolytic degradation, and thermal degradation. HPLC analysis revealed that the degradation percentages ranged from 5 to 20%.
Acid hydrolysis
Two milliliters of standard stock solution of MET (500 µg/mL) was made up to 10 mL with 0.01 N HCl. The solution was stored at 40 °C for 2 h and then cooled and mixed with the diluent so that the final concentration was 100 µg/mL. On the other hand, an acid degradation study was performed on 100 µg/mL GAB using 1.0 N HCl. The solution was maintained at 65 °C for 48 h and then cooled and mixed with the diluent.
Basic hydrolysis
The same steps used for acid hydrolysis were followed. Two milliliters of a standard stock solutions of MET (500 µg/mL) was made up to 10 mL with 0.01 N NaOH. The solution was kept at 40 °C for 2 h and then cooled and mixed with the diluent so that the final concentration was 100 µg/mL. On the other hand, a basic degradation study was performed on 100 µg/mL GAB using 1.0 N NaOH. The solution was kept at 65 °C for 48 h and then cooled and mixed with the diluent.
Neutral hydrolysis
With distilled water, 2 mL of stock standard solution of MET (500 µg/mL) was diluted into 10 mL. We placed the flask in a water bath at 40 °C for 24 h, cooled the solution, and then added the diluent to make the final concentration of 100 µg/mL. Additionally, a neutral degradation study was performed on 100 µg/mL GAB in bi-distilled water at 65 °C for 48 h, followed by cooling and dilution.
Oxidative degradation
A 2 mL stock standard solution of MET (500 µg/mL) was transferred to a 10 mL volumetric flask. A 5 mL solution of 3% H2O2 was added to the solution. After the flask was placed in the dark for 24 h, the solution concentration was determined by adding a diluent to reach the mark, which was 100 µg/mL. In contrast, an oxidative degradation study was conducted on 100 µg/mL GAB that was treated with 30% hydrogen peroxide and kept at 65 °C for 5 h, followed by dilution with water.
Photolytic and thermal degradation
In the presence of hot sunlight for one hour, a final concentration of 100 µg/mL MET was used for photolytic degradation under hot sunlight for 1 h and thermal degradation via reflux in a water bath at 120 °C for 30 min. Afterward, drugs were injected at various concentrationsIn the presence of hot sunlight for one hour, a final concentration of 100 µg/mL MET was used for photolytic degradation. Afterward, drugs were injected at various concentrations. The degradation of 100 µg/mL GAB was carried out via photolytic degradation under hot sunlight for 1 h. In contrast, degradation by thermal degradation was performed via reflux in a water bath at 65 °C for 2 h.
Green and white measurement tools
AGREE
As well as assessing sustainability overall, the AGREE tool provides information on environmental issues as well. A scale of 0–1 is used to rate each variable or concept regarding its ecological sustainability based on the twelve criteria from the GAC. Each of the criteria is represented by a graph, with green, yellow, red and colors representing different accomplishment levels. There is a difference in size between each region of the chart depending on the magnitude of the metrics26.
AGREEprep
A sample preparation procedure is critical to the success of an analysis and must be performed sustainably. As part of AGREEprep, eco-friendly practices are used to mitigate environmental impact of sample preparation. Prior to alignment with GSP standards, AGREEprep adhered to 12 rules of GAC. These standards are widely recognized as industry benchmarks for sustainable sample preparation27.
GAPI
Assessing the greenness of a method before proposing it is crucial to recommend it as more environmentally friendly. Identifying a technique’s “greenness” requires an evaluation of each step of sample preparation, analysis, and finalization. In this assessment, five pentagrams represent different stages. Stages are categorized according to whether their influence on the environment is positive, negative, or nonexistent. Moreover, this tool facilitates the evaluation of analytical methods and helps them perform better in terms of sustainability28.
ComplexGAPI
As an extension of GAPI, ComplexGAPI adds steps before preparing and analyzing samples. A variety of factors are evaluated, including environmental impact, equipment, operations, and extraction. In addition, the yield, conditions, substances, and reagents used were determined. An environmental impact assessment is conducted via a color-coded system similar to GAPI, with green, yellow, or red indicating concern for the environment29.
BAGI
Among BAGI’s core functions is the evaluation of analytical methods by integrating the WAC criteria with established eco-metrics. As a measure of the effectiveness of an analytical technique, BAGI examines ten attributes: efficiency, computerization, preparation of samples, sample volume, substances, ingredients, number of samples analysed per hour, synchronized analyte analysis, laboratory equipment needs, and concurrent sample processing. Scores and pictograms are generated in this assessment, which highlights areas that can be improved. As part of a systematic approach to evaluating analytical chemistry methods, BAGI advances existing green assessment methodologies such as ComplexGAPI, AGREE, GAPI, and AGREEprep30.
RGB12 algorithm
Chemical analysis can only be partially sustainable if it relies on green methods since these methods aim to have the least possible impact on the environment. It is also essential to evaluate a method’s effectiveness, validity, and cost in order to ensure its sustainability. This is addressed and balanced by the RGB12 algorithm tool, which provides a free Excel spreadsheet that compares up to ten methods. There are twelve key WAC criteria evaluated with this metric via three color coding tables: the red table assesses the application’s scope, detection and quantification limits, precision, and efficiency; the green table evaluates the toxicological hazards, waste generation, and energy consumption of the reagent; and the blue table considers cost, time, and operational ease. The three columns summarize the results, with scores indicating WAC adherence. Sustainability is determined by the “whiteness” score, which indicates how well a method balances performance, environmental impact, and productivity31.
ESA
Assessing sustainable practices requires the use of an eco-Scale tool. The algorithm mentioned above determines a penalty score on the basis of multiple parameters, such as how many reagents are used. It also considers risk factors, energy consumption, and manufacturing waste. The comprehensive level of sustainability is determined on the basis of the sum of these scores. One hundred points are reduced by deducting penalty points. A system can be classified as “excellent green” if it receives a score of 75 or above, “acceptable green” if it receives a score of 50–75, or “unacceptable green” if it achieves a score of fewer than 5032.
AMVI
AMVI’s approach to computing solvent utilization in liquid chromatography is a valuable tool. In addition to its effectiveness, the methodology mentioned above can be customized for various analytical techniques, especially liquid chromatography protocols. Furthermore, it can be used in a variety of laboratory settings because of its versatility in terms of analytical methods33.
AMGS
Different AMGS procedures can be compared in terms of their environmental efficiency. Several factors are considered within the quantitative methodology, including the amount of energy consumed by instruments, overall energy usage incurred with solvents, and the production or reuse of solvent waste. Environmental safety and health are the main priorities34.
HPLC-EAT
Liquid chromatographic methods can be assessed with HPLC-EAT by installing a software tool produced particularly for this purpose. http://www.biotek.lu.se/hplc-eat/ provides free access to this software. All substances used in the testing can be evaluated for their safety, environmental impact, and health impact. A highly user-friendly and intuitive interface accompanies the software. A major limitation of this tool is that it focuses exclusively on the ecological implications of solvents while neglecting other aspects of GAC, such as instruments, energy, and sample preparation35.
Study limitations and future research plans
This study’s approach for the concurrent assay of GAB and MET by RP-HPLC has several potential limitations and future prospects. One limitation is the method’s applicability primarily to pharmaceutical formulations, with the potential for matrix effects in complex biological samples not fully explored. Additionally, while the method demonstrated high specificity, its sensitivity in biological matrices and its robustness in multidrug formulations require further investigation. The calibration range may not cover the full therapeutic spectrum of both drugs, particularly for lower or higher concentrations. Future research could focus on expanding the method’s applicability to pharmacokinetic studies and bioanalysis in biological matrices; enhancing sensitivity for trace-level detection via online sample preconcentration or UPLC-MS/MS coupling while maintaining green chemistry principles; exploring the method’s scalability for point-of-care testing; and investigating drug stability across formulations/storage conditions to advance neuropathic pain treatment. Moreover, investigating the stability of GAB and MET in different formulations and storage conditions, as well as exploring the synergistic mechanisms of these drugs, would be valuable for advancing treatment options for neuropathic pain and related neurological conditions.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Bennett, M. I. & Simpson, K. H. Gabapentin in the treatment of neuropathic pain. Palliat. Med 18, 5–11. https://doi.org/10.1191/0269216304pm845ra (2004).
Julian, T., Syeed, R., Glascow, N., Angelopoulou, E. & Zis, P. B12 as a treatment for peripheral neuropathic pain: A systematic review. Nutrients 12 (8), 2221. https://doi.org/10.3390/nu12082221 (2020).
Bhuanaputra, M. F., Tursina, A. & Kharisma, Y. Combination of Gabapentin and vitamin B12 compared with Gabapentin monotherapy on pain improvement of diabetic neuropathy patients. Glob Med. Heal Commun. 8, 1–6. https://doi.org/10.29313/gmhc.v8i1.3676 (2020).
Mimenza Alvarado, A. & Aguilar Navarro, S. Clinical trial assessing the efficacy of Gabapentin plus B complex (B1/B12) versus Pregabalin for treating painful diabetic neuropathy. J. Diabetes Res. 2016 https://doi.org/10.1155/2016/4078695 (2016).
Baltrusch, S. The Role of Neurotropic B Vitamins in Nerve Regeneration. Biomed Res. Int. ; 2021. 9968228. (2021). https://doi.org/10.1155/2021/9968228
Mahgoub, S. M. et al. Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of Pentoxifylline and Simvastatin in therapeutic potential for breast cancer: appraisal of greenness, whiteness, and Box–Behnken design. Green. Process. Synthesis. 13 (1), 20240139. https://doi.org/10.1515/gps-2024-0139 (2024).
Nassef, H. M., Ahmed, H. A., El-Atawy, M. A., Alanazi, T. Y. A. & Mohamed, M. A. Greens assessment of RP-UPLC method for estimating triamcinolone acetonide and its degraded products compared to Box-Behnken and six Sigma designs. Green. Chem. Lett. Rev. 17 (1), 2301315. https://doi.org/10.1080/17518253.2023.2301315 (2024).
Helmy, S. A. et al. Green HPLC method for determination of Paracetamol and ibuprofen in human plasma: applications to pharmacokinetics. Bioanalysis 1–2. https://doi.org/10.1080/17576180.2024.2421704 (2024).
Al-Wasidi, A. S., Mohamed, M. A., Ahmed, H. A., Almalki, M. A. & Ahmed-Anwar, A. A. Comparative analysis using UPLC and UV methods for concurrent quantification of therapeutic drugs for overactive bladder: effective approach in green and white chemistry. Results Chem. 12, 101899. https://doi.org/10.1016/j.rechem.2024.101899 (2024).
Assirey, E. A., katamesh, N. S. & Mohamed, M. A. Evaluating the greenness, blueness, and whiteness of spectroscopic and UPLC techniques for the simultaneous measurement of Anti-Glaucoma drugs and the preservation agent. Talanta Open. 10, 100367. https://doi.org/10.1016/j.talo.2024.100367 (2024).
Nassef, H. et al. A novel six Sigma approach and eco-friendly RP-HPLC technique for determination of Pimavanserin and its degraded products: application of Box–Behnken design. Rev. Anal. Chem. 43 (1), 20230073. https://doi.org/10.1515/revac-2023-0073 (2024).
Almasri, I. M., Ramadan, M. & Algharably, E. Development and validation of spectrophotometric method for determination of Gabapentin in bulk and pharmaceutical dosage forms based on schiff base formation with salicylaldehyde. J. Appl. Pharm. Sci. 9 (3), 021–026. https://doi.org/10.7324/JAPS.2019.90304 (2019).
Siddiqui, F. A. et al. Spectrophotometric determination of Gabapentin in pharmaceutical formulations using ninhydrin and π-Acceptors. Eur. J. Med. Chem. 45, 2761–2767. https://doi.org/10.1016/j.ejmech.2010.02.058 (2010).
Adegoke, O. A., Adegbolagun, O. M., Aiyenale, E. O. & Thomas, O. E. New spectrophotometric method for the determination of Gabapentin in bulk and dosage forms using P-Dimethylaminobenzaldehyde. J. Taibah Univ. Sci. 12, 754–764. https://doi.org/10.1080/16583655.2018.1495418 (2018).
Mahgoub, S. M., Elsherief, A. H., Mahmoud, R., Mahmoud, M. R. & Mohamed, M. A. Validated stability indicating eco-friendly RP-HPLC method for the concurrent quantification of Gabapentin and diclofenac K in wastewater and pharmaceutical formulations. Egypt. J. Chem. 67 (1), 411–422. https://doi.org/10.21608/ejchem.2023.213243.8018 (2024).
Jalalizadeh, H., Souri, E., Tehrani, M. B. & Jahangiri, A. Validated HPLC method for the determination of Gabapentin in human plasma using Pre-Column derivatization with 1-Fluoro-2,4-Dinitrobenzene and its application to a Pharmacokinetic study. J. Chromatogr. B. 854, 43–47. https://doi.org/10.1016/j.jchromb.2007.03.039 (2007).
Shrestha, S. et al. Estimation and validation of methylcobalamin in tablet dosage form using UV-Visible spectrophotometric method. Am. Sci. Res. J. Eng. Technol. Sci. 77, 220–229 (2021).
Galande, V. R., Baheti, K. G. & Dehghan, M. H. UV-Vis spectrophotometric method for Estimation of Gabapentin and methylcobalamin in bulk and tablet. Int. J. ChemTech Res. 2, 695–699 (2010).
Amer, M., Kamal, A., Hammad, S. & Habib, A. Stability indicating RP-HPLC method for methylcobalamin determination in different dosage forms: application to photodegradation kinetics and PH‐rate profiling. J. Sep. Sci. 45 https://doi.org/10.1002/jssc.202200132 (2022).
Lakshmi, V. & Gowtham, Y. RP-HPLC method development and validation for the determination of methylcobalamin and Pregabalin in combined capsule dosage form. Int. J. Res. Pharm. Sci. 4, (2013).
Gujral, R. S. & Haque, S. M. Development and validation of a new HPLC method for the determination of Gabapentin. Int. J. Biomed. Sci. 5, 63–69 (2009).
Bhatt, K. K., Patelia, E. M. & Mori, A. Simultaneous Estimation of Pregabalin and Methylcobalamine in pharmaceutical formulation by RP-HPLC method. J. Anal. Bioanal Tech. 4 (1), 1–4. https://doi.org/10.4172/2155-9872.1000159 (2013).
Gade, S. T. et al. ;7(14):205–212. (2018). https://doi.org/10.20959/wjpr201814-12463
Chandak, D. & Sharma, P. Development and validation for simultaneous Estimation of gabapentin, mecobalamin and alpha lipoic acid in tablet formulation. Res. J. Sci. Technol. 12 (1), 74–78. https://doi.org/10.5958/2349-2988.2020.00010.8 (2020).
Bakshi, A., Monika, K., Bhutada, S. & Raju, M. B. Simultaneous Estimation of Gabapentin and methylcobalamin in bulk and pharmaceutical dosage form by RP-HPLC. J. Drug Delivery Ther. 9 (1), 170–174. https://doi.org/10.22270/jddt.v9i1.2204 (2019).
Mahgoub, S. M. et al. Analytical assessment of a novel RP-HPLC method for the concurrent quantification of selected pharmaceutical drugs Levodopa and carbidopa using eight greenness metrics comparing to the lean six Sigma approach. Sustain. Chem. Pharm. 36, 101291. https://doi.org/10.1016/j.scp.2023.101291 (2023).
Pena-Pereira, F., Tobiszewski, M., Wojnowski, W. & Psillakis, E. A tutorial on agreeprep an analytical greenness metric for sample Preparation. Adv. Sample Preparation. 3, 100025. https://doi.org/10.1016/j.sampre.2022.100025 (2022).
Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: green analytical procedure index. Talanta 181, 204–209. https://doi.org/10.1016/j.talanta.2018.01.013 (2018).
Ahmed, H. A. et al. Eco-friendly chromatographic techniques for appraisal of amlodipine, hydrochlorothiazide, telmisartan, and their related substances in dosage form: application to six Sigma and content uniformity approaches. Sustain. Chem. Pharm. 38, 101469. https://doi.org/10.1016/j.scp.2024.101469 (2024).
Manousi, N., Wojnowski, W., Płotka-Wasylka, J. & Samanidou, V. Blue applicability grade index (BAGI) and software: a new tool for the evaluation of method practicality. Green. Chem. 25 (19), 7598–7604. https://doi.org/10.1039/D3GC02347H (2023).
Nowak, P. M., Wietecha-Posłuszny, R. & Pawliszyn, J. White analytical chemistry: an approach to reconcile the principles of green analytical chemistry and functionality. TrAC Trends Anal. Chem. 138, 116223. https://doi.org/10.1016/j.trac.2021.116223 (2021).
Gałuszka, A., Migaszewski, Z. M., Konieczka, P. & Namieśnik, J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC Trends Anal. Chem. 37, 61–72. https://doi.org/10.1016/j.trac.2012.03.013 (2012).
Hartman, R., Helmy, R., Al-Sayah, M. & Welch, C. J. Analytical method volume intensity (AMVI): A green chemistry metric for HPLC methodology in the pharmaceutical industry. Green. Chem. 13 (4), 934–939. https://doi.org/10.1039/C0GC00524J (2011).
Hicks, M. B. et al. Making the move towards modernized greener separations: introduction of the analytical method greenness score (AMGS) calculator. Green Chem. 21 (7), 1816–1826. https://doi.org/10.1039/C8GC03875A (2019).
Gaber, Y., Törnvall, U., Kumar, M. A., Amin, M. A. & Hatti-Kaul, R. HPLC-EAT (Environmental assessment Tool): a tool for profiling safety, health and environmental impacts of liquid chromatography methods. Green. Chem. 13 (8), 2021–2025. https://doi.org/10.1039/C0GC00667J (2011).
ICH Harmonized Tripartite Guideline. Curr. Step ; 4: 1–17. (2005).
Swartz, M. E. & Krull, I. S. Analytical Method Development and Validation (CRC, 2018). https://doi.org/10.1201/9781315275161
Acknowledgements
The current work was supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R419), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Samar M. Mahgoub and Mahmoud A. Mohamed. wrote the main manuscript text, Project Administration, Formal analysis, and methodology; Ammena Y. Binsaleh and Bandar R. Alsehli. Visualization, Investigation, Conceptualization, resources; Fahad M. Alminderej and Sayed M. Saleh. Review & editing, software, supervision, and investigation; All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mahgoub, S.M., Alminderej, F.M., Binsaleh, A.Y. et al. Green and white analytical approach for parallel quantification of gabapentin and methylcobalamin in medicinal products using inventive RP-HPLC technique. Sci Rep 15, 20263 (2025). https://doi.org/10.1038/s41598-025-07056-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-07056-9