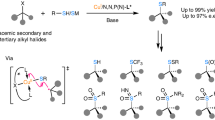
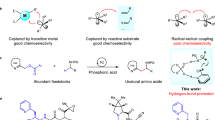
Abstract
Methods for direct enantioselective oxidation of C(sp3)–H bonds will revolutionize the preparation of chiral alcohols and their derivatives. Enzymatic catalysis, which uses key metal-oxo species to facilitate efficient hydrogen atom abstraction, has evolved as a highly selective approach for C–H oxidation in biological systems. Despite its effectiveness, reproducing this function and achieving high stereoselectivity in biomimetic catalysts has proven to be a daunting task. Here we present a copper-based biomimetic catalytic system that achieves highly efficient asymmetric sp3 C–H oxidation with C–H substrates as the limiting reagent. A Cu(II)-bound tert-butoxy radical is responsible for the site-selective C–H bond cleavage, which resembles the active site of copper-based enzymes for C–H oxidation. The developed method has been successfully accomplished with good functional group compatibility and exceptionally high site- and enantioselectivity, which is applicable for the late-stage oxidation of bioactive compounds.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this Article are available from the Cambridge Crystallographic Data Centre with the deposition number CCDC 2391309. All other data supporting the findings of this study are available within the Article and its Supplementary Information, or from the corresponding author upon reasonable request.
References
Santaniello, E., Ferraboschi, P., Grisenti, P. & Manzocchi, A. The biocatalytic approach to the preparation of enantiomerically pure chiral building blocks. Chem. Rev. 92, 1071–1140 (1992).
Noyori, R. & Ohkuma, T. Asymmetric catalysis by architectural and functional molecular engineering: practical chemo- and stereoselective hydrogenation of ketones. Angew. Chem. Int. Ed. 40, 40–73 (2001).
Labinger, J. A. & Bercaw, J. E. Understanding and exploiting C–H bond activation. Nature 417, 507–514 (2002).
Chakrabarty, S., Wang, Y., Perkins, J. C. & Narayan, A. R. H. Scalable biocatalytic C–H oxyfunctionalization reactions. Chem. Soc. Rev. 49, 8137–8155 (2020).
Golden, D. L., Suh, S.-E. & Stahl, S. S. Radical C(sp3)–H functionalization and cross-coupling reactions. Nat. Rev. Chem. 6, 405–427 (2022).
White, M. C. & Zhao, J. Aliphatic C–H oxidations for late-stage functionalization. J. Am. Chem. Soc. 140, 13988–14009 (2018).
Che, C.-M., Lo, V. K.-Y., Zhou, C.-Y. & Huang, J.-S. Selective functionalisation of saturated C–H bonds with metalloporphyrin catalysts. Chem. Soc. Rev. 40, 1950–1975 (2011).
Milan, M., Salamone, M., Costas, M. & Bietti, M. The quest for selectivity in hydrogen atom transfer based aliphatic C–H bond oxygenation. Acc. Chem. Res. 51, 1984–1995 (2018).
Chen, M. S. & White, M. C. Combined effects on selectivity in Fe-catalyzed methylene oxidation. Science 327, 566–571 (2010).
Horn, E. J. et al. Scalable and sustainable electrochemical allylic C–H oxidation. Nature 533, 77–81 (2016).
Saint-Denis, G., Zhu, R.-Y., Chen, G., Wu, Q.-F. & Yu, J.-Q. Enantioselective C(sp3)‒H bond activation by chiral transition metal catalysts. Science 359, eaao4798 (2018).
Covell, D. J. & White, M. C. A chiral Lewis acid strategy for enantioselective allylic C–H oxidation. Angew. Chem. Int. Ed. 47, 6448–6451 (2008).
Wang, P.-S. et al. Asymmetric allylic C–H oxidation for the synthesis of chromans. J. Am. Chem. Soc. 137, 12732–12735 (2015).
Cianfanelli, M. et al. Enantioselective C–H lactonization of unactivated methylenes directed by carboxylic acids. J. Am. Chem. Soc. 142, 1584–1593 (2020).
Nie, X., Ye, C.-X., Ivlev, S.-I., & Meggers, E. Nitrene-mediated C–H oxygenation: catalytic enantioselective formation of five-membered cyclic organic carbonates. Angew. Chem. Int. Ed. 61, e202211971 (2022).
Ortiz de Montellano, P. R. Hydrocarbon hydroxylation by cytochrome P450 enzymes. Chem. Rev. 110, 932–948 (2010).
Ciano, L., Davies, G. J., Tolman, W. B. & Walton, P. H. Bracing copper for the catalytic oxidation of C–H bonds. Nat. Catal. 1, 571–577 (2018).
Wang, V. C. C. et al. Alkane oxidation: methane monooxygenases, related enzymes, and their biomimetics. Chem. Rev. 117, 8574–8621 (2017).
Lieberman, R. L. & Rosenzweig, A. C. Crystal structure of a membrane-bound metalloenzyme that catalyses the biological oxidation of methane. Nature 434, 177–182 (2005).
Balasubramanian, R. et al. Oxidation of methane by a biological dicopper centre. Nature 465, 115–119 (2010).
Elwell, C. E. et al. Copper–oxygen complexes revisited: structures, spectroscopy, and reactivity. Chem. Rev. 117, 2059–2107 (2017).
Kharasch, M. S. & Sosnovsky, G. The reactions of t-butyl perbenzoate and olefins—a stereospecific reaction. J. Am. Chem. Soc. 80, 756 (1958).
Kropf, H., Schröer, R. & Fösing, R. Kharasch-Sosnovsky-Reaktionen von Alkynen und Tetramethylallen. Synthesis 12, 894–896 (1977).
Tran, B. L., Driess, M. & Hartwig, J. F. Copper-catalyzed oxidative dehydrogenative carboxylation of unactivated alkanes to allylic esters via alkenes. J. Am. Chem. Soc. 136, 17292–17301 (2014).
Golden, D. L. et al. Benzylic C–H esterification with limiting C–H substrate enabled by photochemical redox buffering of the Cu catalyst. J. Am. Chem. Soc. 145, 9434–9440 (2023).
Eames, J. & Watkinson, M. Catalytic allylic oxidation of alkenes using an asymmetric Kharasch-Sosnovsky reaction. Angew. Chem. Int. Ed. 40, 3567–3571 (2001).
Zhang, B., Zhu, S.-F. & Zhou, Q.-L. Copper-catalyzed enantioselective allylic oxidation of acyclic olefins. Tetrahedron Lett. 54, 2665–2668 (2013).
Clark, J. S., Tolhurst, K. F., Taylor, M. & Swallow, S. Enantioselective propargylic oxidation. Tetrahedron Lett. 39, 4913–4916 (1998).
Andrus, M. B. & Lashley, J. C. Copper catalyzed allylic oxidation with peresters. Tetrahedron 58, 845–866 (2002).
Andrus, M. B. & Zhou, Z. Highly enantioselective copper-bisoxazoline-catalyzed allylic oxidation of cyclic olefins with tert-butyl p-nitroperbenzoate. J. Am. Chem. Soc. 124, 8806–8807 (2002).
Wang, F., Chen, P. & Liu, G. Copper-catalyzed radical relay for asymmetric radical transformations. Acc. Chem. Res. 51, 2036–2046 (2018).
Li, J. et al. Site-specific allylic C–H bond functionalization with a copper-bound N-centred radical. Nature 574, 516–521 (2019).
Hu, H. et al. Copper-catalysed benzylic C–H coupling with alcohols via radical relay enabled by redox buffering. Nat. Catal. 3, 358–367 (2020).
Walling, C. & Zavitsas, A. A. The copper-catalyzed reaction of peresters with hydrocarbons. J. Am. Chem. Soc. 85, 2084–2090 (1963).
Kochi, J. K. & Mains, H. E. Studies on the mechanism of the reaction of peroxides and alkenes with copper salts. J. Org. Chem. 30, 1862–1872 (1965).
Beckwith, A. L. J. & Zavitsas, A. A. Allylic oxidations by peroxy esters catalyzed by copper salts. The potential for stereoselective syntheses. J. Am. Chem. Soc. 108, 8230–8234 (1986).
Smith, K., Hupp, C. D., Allen, K. L. & Slough, G. A. Catalytic allylic amination versus allylic oxidation: a mechanistic dichotomy. Organometallics 24, 1747–1755 (2005).
Quinn, R. K. et al. Site-selective aliphatic C–H chlorination using N-chloroamides enables a synthesis of chlorolissoclimide. J. Am. Chem. Soc. 138, 696–702 (2016).
Janzen, E. G., Coulter, G. A., Oehler, U. M. & Bergsma, J. P. Solvent effects on the nitrogen and β-hydrogen hyperfine splitting constants of aminoxyl radicals obtained in spin trapping experiments. Can. J. Chem. 60, 2725–2733 (1982).
Bors, W., Michel, C. & Stettmaier, K. Radical species produced from the photolytic and pulse-radiolytic degradation of tert-butyl hydroperoxide. An EPR spin trapping investigation. J. Chem. Soc., Perkin Trans. 2, 1513–1517 (1992).
Gephart, R. T. III et al. Reaction of CuI with dialkyl peroxides: CuII-alkoxides, alkoxy radicals, and catalytic C–H etherification. J. Am. Chem. Soc. 134, 17350–17353 (2012).
An, Q. et al. Identification of alkoxy radicals as hydrogen atom transfer agents in Ce-catalyzed C–H functionalization. J. Am. Chem. Soc. 145, 359–376 (2023).
Fan, L.-F., Liu, R., Ruan, X.-Y., Wang, P.-S. & Gong, L.-Z. Asymmetric 1,2-oxidative alkylation of conjugated dienes via aliphatic C–H bond activation. Nat. Synth. 1, 946–955 (2022).
Chen, J. et al. Photoinduced copper-catalyzed asymmetric C–O cross-coupling. J. Am. Chem. Soc. 143, 13382–13392 (2021).
Wang, P.-Z. et al. Photoinduced copper-catalyzed asymmetric three-component coupling of 1,3-dienes: an alternative to Kharasch–Sosnovsky reaction. Angew. Chem. Int. Ed. 60, 22956–22962 (2021).
Zhu, X. et al. Asymmetric radical carboesterification of dienes. Nat. Commun. 12, 6670 (2021).
Nie, Z. et al. Copper-catalyzed radical enantioselective carbo-esterification of styrenes enabled by a perfluoroalkylated-PyBox ligand. Angew. Chem. Int. Ed. 61, e202202077 (2022).
Sowden, R. J., Yasmin, S., Rees, N. H., Bell, S. G. & Wong, L.-L. Biotransformation of the sesquiterpene (+)-valencene by cytochrome P450cam and P450BM-3. Org. Biomol. Chem. 3, 57–64 (2005).
Yan, X.-T. et al. Hyperhubeins A–I, bioactive sesquiterpenes with diverse skeletons from hypericum hubeiense. J. Nat. Prod. 86, 119–130 (2023).
Zhang, F. et al. Concise, scalable and enantioselective total synthesis of prostaglandins. Nat. Chem. 13, 692–697 (2021).
Baars, H., Classen, M. J. & Aggarwal, V. K. Synthesis of alfaprostol and PGF2α through 1,4-addition of an alkyne to an enal intermediate as the key step. Org. Lett. 19, 6008–6011 (2017).
Chen, X., Li, H.-H. & Kramer, S. Photoinduced copper-catalyzed enantioselective allylic C(sp3)–H oxidation of acyclic 1-aryl-2-alkyl alkenes as limiting substrates. Angew. Chem. Int. Ed. 63, e202413190 (2024).
Liu, X.-M. et al. Catalytic asymmetric oxidative coupling between C(sp3)–H bonds and carboxylic acids. Preprint at ChemRxiv https://doi.org/10.26434/chemrxiv-2024-hqqb0 (2024).
Tang, S., Xu, H., Dang, Y. & Yu, S. Photoexcited copper-catalyzed enantioselective allylic C(sp3)–H acyloxylation of acyclic internal alkenes. J. Am. Chem. Soc. 146, 27196–27203 (2024).
Acknowledgements
Financial support was provided by the National Key R&D Program of China (grant 2021YFA1500100), the National Natural Science Foundation of China (grants 22331012, 91956202, 92256301 and 21821002), the Science and Technology Commission of Shanghai Municipality (grants 20JC1417000 and 21520780100) and the International Partnership Program (grant 121731KYS-B20190016) of the Chinese Academy of Sciences and the Research Grants Council of Hong Kong (HKUST 16300620 and 16302222). H.Z. thanks Y. Zhang for the EPR manipulation and analysis. G.L. acknowledges support from the Tencent Foundation through the New Cornerstone Science Foundation.
Author information
Authors and Affiliations
Contributions
H.Z., Y.Z., J.W. and G.L. conceived the work and designed the experiments. H.Z. and Y.Z. performed the predominated laboratory experiments, and J.W. contributed partly. T.Y. and Z.L. conducted DFT calculation. H.Z., Y.Z., P.C., Z.L. and G.L. analysed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Albert Poater and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–14, Tables 1–5, methods and references.
Supplementary Data 1
DFT-calculated Cartesian coordinates.
Supplementary Data 2
X-ray structure of (S)-9.
Supplementary Data 3
checkCIF report.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, H., Zhou, Y., Yang, T. et al. Site- and enantioselective allylic and propargylic C–H oxidation enabled by copper-based biomimetic catalysis. Nat Catal 8, 58–66 (2025). https://doi.org/10.1038/s41929-024-01276-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-024-01276-4