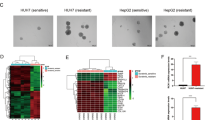
Abstract
Resistance to tyrosine kinase inhibitors (TKIs) dampens their clinical efficacy in hepatocellular carcinoma (HCC). Stress granules formed by phase separation are essential to stress response and can be involved in therapy resistance, but their mechanisms in HCC are unclear. Here our screen shows that the atypical serine/threonine kinase RIOK1 is highly expressed in HCC, linked to poor prognosis, and transcriptionally activated by NRF2 under various stress conditions. RIOK1 undergoes liquid–liquid phase separation by incorporating IGF2BP1 and G3BP1 into stress granules that sequestrate PTEN messenger RNA reducing its translation. This process activates the pentose phosphate pathway, facilitating stress resolution and cytoprotection against TKI. We further show that the small-molecule inhibitor chidamide downregulates RIOK1 and enhances TKI efficacy. RIOK1-positive stress granules are found in donafenib-resistant tumors from patients with HCC. These findings reveal a link between stress granule dynamics, metabolic reprogramming and HCC progression, offering the potential means to improve TKI efficacy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
RNA-seq data supporting the findings of this study have been deposited in the National Genomics Data Center (HRA005434, CRA021525). Publicly available metabolomics data can be accessed via MetaboLights (MTBLS11963). The scRNA-seq data to explore relationships between RIOK1 and glutamine metabolism genes in publicly available hepatocellular carcinoma studies are from the China National GeneBank Database (CNP0000650). Gene expression and clinical information were obtained from TCGA, ICGC and GEO databases (GSE22058, GSE25097, GSE36376, GSE76427, GSE54236, GSE63898 and GSE64041). Source data are provided with this paper. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
Code availability
No unique code was developed for this study.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA 71, 209–249 (2021).
Yang, C. et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 20, 203–222 (2023).
Huang, A., Yang, X. R., Chung, W. Y., Dennison, A. R. & Zhou, J. Targeted therapy for hepatocellular carcinoma. Signal Transduct. Target. Ther. 5, 146 (2020).
Galluzzi, L., Yamazaki, T. & Kroemer, G. Linking cellular stress responses to systemic homeostasis. Nat. Rev. Mol. Cell Biol. 19, 731–745 (2018).
Dai, W. et al. OGDHL silencing promotes hepatocellular carcinoma by reprogramming glutamine metabolism. J. Hepatol. 72, 909–923 (2020).
Wei, L. et al. Genome-wide CRISPR/Cas9 library screening identified PHGDH as a critical driver for sorafenib resistance in HCC. Nat. Commun. 10, 4681 (2019).
Liu, Q. et al. Glycogen accumulation and phase separation drives liver tumor initiation. Cell 184, 5559–5576.e5519 (2021).
Xie, J. et al. Targeting androgen receptor phase separation to overcome antiandrogen resistance. Nat. Chem. Biol. 18, 1341–1350 (2022).
Lu, B. et al. Pharmacological inhibition of core regulatory circuitry liquid-liquid phase separation suppresses metastasis and chemoresistance in osteosarcoma. Adv. Sci. 8, e2101895 (2021).
Jain, S. et al. ATPase-modulated stress granules contain a diverse proteome and substructure. Cell 164, 487–498 (2016).
Yang, P. et al. G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. Cell 181, 325–345.e328 (2020).
Protter, D. S. W. & Parker, R. Principles and properties of stress granules. Trends Cell Biol. 26, 668–679 (2016).
McCormick, C. & Khaperskyy, D. A. Translation inhibition and stress granules in the antiviral immune response. Nat. Rev. Immunol. 17, 647–660 (2017).
Namkoong, S., Ho, A., Woo, Y. M., Kwak, H. & Lee, J. H. Systematic characterization of stress-induced RNA granulation. Mol. Cell 70, 175–187.e178 (2018).
Khong, A. et al. The stress granule transcriptome reveals principles of mRNA accumulation in stress granules. Mol. Cell 68, 808–820.e805 (2017).
Somasekharan, S. P. et al. G3BP1-linked mRNA partitioning supports selective protein synthesis in response to oxidative stress. Nucleic Acids Res. 48, 6855–6873 (2020).
Grabocka, E. & Bar-Sagi, D. Mutant KRAS enhances tumor cell fitness by upregulating stress granules. Cell 167, 1803–1813.e1812 (2016).
Shi, Q. et al. Prostate cancer-associated SPOP mutations enhance cancer cell survival and docetaxel resistance by upregulating Caprin1-dependent stress granule assembly. Mol. Cancer 18, 170 (2019).
Li, M. et al. Sestrin2-mediated disassembly of stress granules dampens aerobic glycolysis to overcome glucose starvation. Cell Death Discov. 9, 127 (2023).
Ameismeier, M. et al. Structural basis for the final steps of human 40S ribosome maturation. Nature 587, 683–687 (2020).
Widmann, B. et al. The kinase activity of human Rio1 is required for final steps of cytoplasmic maturation of 40S subunits. Mol. Biol. Cell 23, 22–35 (2012).
Hu, Q. et al. MTAP deficiency-induced metabolic reprogramming creates a vulnerability to cotargeting de novo purine synthesis and glycolysis in pancreatic cancer. Cancer Res. 81, 4964–4980 (2021).
Hong, X. et al. Targeting posttranslational modifications of RIOK1 inhibits the progression of colorectal and gastric cancers. eLife 7, e29511 (2018).
Tarumoto, Y. et al. LKB1, salt-inducible kinases, and MEF2C are linked dependencies in acute myeloid leukemia. Mol. Cell 69, 1017–1027.e1016 (2018).
Angermayr, M., Roidl, A. & Bandlow, W. Yeast Rio1p is the founding member of a novel subfamily of protein serine kinases involved in the control of cell cycle progression. Mol. Microbiol. 44, 309–324 (2002).
Ferreira-Cerca, S., Kiburu, I., Thomson, E., LaRonde, N. & Hurt, E. Dominant Rio1 kinase/ATPase catalytic mutant induces trapping of late pre-40S biogenesis factors in 80S-like ribosomes. Nucleic Acids Res. 42, 8635–8647 (2014).
Hüttelmaier, S. et al. Spatial regulation of β-actin translation by Src-dependent phosphorylation of ZBP1. Nature 438, 512–515 (2005).
Lambrianidou, A. et al. mTORC2 deploys the mRNA binding protein IGF2BP1 to regulate c-MYC expression and promote cell survival. Cell Signal. 80, 109912 (2021).
Niewidok, B. et al. Single-molecule imaging reveals dynamic biphasic partition of RNA-binding proteins in stress granules. J.Cell Biol. 217, 1303–1318 (2018).
Protter, D. S. W. et al. Intrinsically disordered regions can contribute promiscuous interactions to RNP granule assembly. Cell Rep. 22, 1401–1412 (2018).
Bugaj, L. J., Choksi, A. T., Mesuda, C. K., Kane, R. S. & Schaffer, D. V. Optogenetic protein clustering and signaling activation in mammalian cells. Nat. Methods 10, 249–252 (2013).
Stöhr, N. et al. IGF2BP1 promotes cell migration by regulating MK5 and PTEN signaling. Genes Dev. 26, 176–189 (2012).
Zhou, Y. et al. RNA damage compartmentalization by DHX9 stress granules. Cell 187, 1701–1718.e1728 (2024).
Fonteneau, G. et al. Stress granules determine the development of obesity-associated pancreatic cancer. Cancer Discov. 12, 1984–2005 (2022).
Cheng, J. et al. TRIM21 and PHLDA3 negatively regulate the crosstalk between the PI3K/AKT pathway and PPP metabolism. Nat. Commun. 11, 1880 (2020).
Hong, X. et al. PTEN antagonises Tcl1/hnRNPK-mediated G6PD pre-mRNA splicing which contributes to hepatocarcinogenesis. Gut 63, 1635–1647 (2014).
Polat, I. H. et al. Glutamine modulates expression and function of glucose 6-phosphate dehydrogenase via NRF2 in colon cancer cells. Antioxidants 10, 1349 (2021).
Kudo, Y. et al. PKCλ/ι loss induces autophagy, oxidative phosphorylation, and NRF2 to promote liver cancer progression. Cancer Cell 38, 247–262.e211 (2020).
Leung, H. W. et al. NRF2/SHH signaling cascade promotes tumor-initiating cell lineage and drug resistance in hepatocellular carcinoma. Cancer Lett. 476, 48–56 (2020).
Su, H. et al. Cancer cells escape autophagy inhibition via NRF2-induced macropinocytosis. Cancer Cell 39, 678–693.e611 (2021).
Cui, Q. et al. Diverse CMT2 neuropathies are linked to aberrant G3BP interactions in stress granules. Cell 186, 803–820.e825 (2023).
Gwon, Y. et al. Ubiquitination of G3BP1 mediates stress granule disassembly in a context-specific manner. Science 372, eabf6548 (2021).
Paget, M. et al. Stress granules are shock absorbers that prevent excessive innate immune responses to dsRNA. Mol. Cell 83, 1180–1196.e1188 (2023).
Lee, A. K. et al. Translational repression of G3BP in cancer and germ cells suppresses stress granules and enhances stress tolerance. Mol. Cell 79, 645–659.e649 (2020).
Bley, N. et al. Stress granules are dispensable for mRNA stabilization during cellular stress. Nucleic Acids Res. 43, e26 (2015).
Somasekharan, S. P. et al. Regulation of AR mRNA translation in response to acute AR pathway inhibition. Nucleic Acids Res. 50, 1069–1091 (2022).
Zhao, Z. et al. QKI shuttles internal m(7)G-modified transcripts into stress granules and modulates mRNA metabolism. Cell 186, 3208–3226.e3227 (2023).
Sun, X. et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 63, 173–184 (2016).
Chen, J. et al. CRISPR-Cas9-based genome-wide screening identified novel targets for treating sorafenib-resistant hepatocellular carcinoma: a cross-talk between FGF21 and the NRF2 pathway. Sci. China Life Sci. 65, 1998–2016 (2022).
Qin, S. et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallel-controlled phase II-III trial. J. Clin. Oncol. 39, 3002–3011 (2021).
Yuan, Y. et al. Chidamide augment sorafenib-derived anti-tumor activities in human osteosarcoma cells lines and xenograft mouse model. Med. Oncol. 39, 87 (2022).
Liu, Y. et al. Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J. Hepatol. 78, 770–782 (2023).
Ries, R. J. et al. m(6)A enhances the phase separation potential of mRNA. Nature 571, 424–428 (2019).
Jin, H. et al. EGFR activation limits the response of liver cancer to lenvatinib. Nature 595, 730–734 (2021).
Acknowledgements
We thank Zelgen Biopharmaceuticals Co. for providing donafenib. We sincerely thank M. Chen from the Analytical Applications Center, Shimadzu (China) Co. for technical support on MS imaging. This work was funded by the National Natural Science Foundation of China (no. 82103219 to F.Z.M.; U19A2008 and 81972307 to L.X.L.; 82101480 to C.T.Z.; 82272700 to R.P.S.; and 82170618 to J.Z.W.; 82272787 to J.B.W.); National Key R&D Program of China (grant no. 2019YFA0709300 to L.X.L.); Joint Fund for Medical Artificial Intelligence of the First Affiliated Hospital of USTC (MAI2023Q034 to F.Z.M.); Provincial Natural Science Foundation of Anhui (no. 2108085QH343 to F.Z.M.; 2108085QH325 to C.T.Z.; 2108085MH288 to R.P.S.; 2208085J4 to J.B.W.; and 2208085MH245 to S.G.Z.;); China Postdoctoral Science Foundation (2022T150623 to F.Z.M.; 2020M682023 to F.Z.M.; and 2021TQ0324 to C.T.Z.); Scientific Research Project of Anhui Provincial Department of Education (2022AH020077 to S.G.Z.); Youth Innovation Key Fund Project of USTC (WK9110000153 to F.Z.M., WK9110000164 to C.T.Z., YD9100002003 to R.P.S. and YD9110002048 to J.B.W.) and the Beijing CSCO Clinical Oncology Research Foundation (Y-JS2019-014 to J.B.W.)
Author information
Authors and Affiliations
Contributions
L.X.L. conceived the project; L.X.L., J.Z.W., R.P.S. and S.G.Z. designed the research, J.B.W. provided guidance, supervision and overall design of the supplementary experiments. F.Z.M., H.R.L. and C.X.W. performed the experiments. Y.B.H., Y.F.L. and C.T.Z. analyzed results and made the figures. F.Z.M., D.L.C. and R.FT. wrote the paper. T.F.Z., S.Y.Z., Y.Y.L., B.Z. and C.D.L. provided clinical samples. J.X., W.X.X. and S.X.P. contributed to scientific discussion. X.D.S., Y.L. and J.B.W. revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks Jorge Moscat, Huiyong Yin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 RIOK1 is commonly upregulated in HCC and is associated with poor patient survival.
a. Hierarchical clustering of TCGA data analysis showed highly expressed kinase-related expression matrix of HCC tumors in public TCGA 50 paired-RNA-seq datasets (n = 50 patients). b–e, To visually illustrate the distribution of glutamine in liver cancer and adjacent normal tissues, representative mass spectrometry imaging result are shown for Patient 1 (b) and Patient 2 (c), along with the statistical analysis (d) of the tumor–normal tissue boundary in human samples (n = 3 patients; b-d) and mouse samples of SgP53/Myc spontaneous liver tumor (e, n = 1 mouse). The black circle represents liver tumor tissues. f, Pairwise comparisons of RIOK1 immunostaining scores in matched HCC and adjacent normal tissues in a cohort of 112 patients (n = 112 patients). g-h, Spatial transcriptomics expression in log-normalized (logNorm) counts for RIOK1 and glutamine metabolism genes in tumor tissues from patients with HCC. i, Comparisons of RIOK1 mRNA expression in HCC and adjacent tissues in nine datasets from the ICGC, TCGA and GEO databases. Data source and statistical analysis (left) and box plot depicting the range of RIOK1 expression values in defined tissues (right). Box plots show 25th, 50th (median) and 75th percentiles, with whiskers extending to 1.5× interquartile range of the hinge. j-k, Kaplan–Meier plots of OS and DFS of patients with HCC from the TCGA HCC cohort stratified according to high or low RIOK1 mRNA levels based on a median cutoff (n = 181 patients/group). l-m, RIOK1 protein (l) and mRNA (m) expression levels in a panel of HCC cell lines (n = 3 independent experiments, 2 technical replicates). Both blots are representative of n = 3 independent experiments. Data were analyzed by unpaired, two-tailed Student’s t-tests (d and f). All quantitative data are represented as mean ± SD.
Extended Data Fig. 2 RIOK1 is associated with the PI3K/AKT pathway in HCC cells.
a-c, Heatmap of markedly differentially expressed genes based on RNA-seq analysis of HCCLM3 cells without or with knockdown of RIOK1 (a, n = 1 independent culture per cell line). KEGG pathway enrichment analysis of differentially expressed genes after RIOK1 knockdown (b). Volcano plot depicting differentially expressed genes from (a), highlighting those associated with the PI3K/AKT pathway (c). d–f, Expression of RIOK1, PTEN, p53, p27, p21, p16 and γ-H2AX in HCC cell lines after manipulating RIOK1. Western blot analysis after overexpressing HA–RIOK1 in SNU449 or PLC/PRF/5 cells (left) or after shRNA knockdown of RIOK1 in HCCLM3 and MHCC-97H cells (right) (d). QPCR analysis of mRNA levels of RIOK1, PTEN, p21 and p27 in SNU449 cells (e) or HCCLM3 cells (f) after RIOK1 overexpression (Ctrl and HA–RIOK1) and knockdown (sh-ctrl, sh-RIOK-1 and sh-RIOK-2), respectively (n = 3 independent experiments, 2 technical replicates). Error bars indicate mean ± SD. Data were analyzed by two-way ANOVA with Tukey’s multiple comparisons test. g, Western blot analysis of AKT-mTORC1 signaling pathway proteins levels in SNU449 and PLC cells transduced with control or RIOK1 overexpression constructs or HCCLM3 transduced with shRNA-ctrl, shRIOK1-1 and shRIOK1-2 constructs for 48 h, respectively. All blots are representative of n = 3 independent experiments.
Extended Data Fig. 3 RIOK1 silencing inhibits the assembly of stress granules in HCC cells.
a, LC-MS (left) and MS-MS spectra to identify RIOK1 (left), G3BP1 (middle) and IGF2BP1 (right) in RIOK1 immunoprecipitates (related to Fig. 2a). b-c, Illustration depicting full-length RIOK1 (b) and IGF2BP1 (c) along with the design of truncation constructs used for mapping analyses (related to Fig. 2i and j). d, Western blotting analysis of SNU449 cells with indicated constructs. e-h, Representative confocal images of RIOK1/IGF2BP1(e) and G3BP1/ IGF2BP1(f) in indicated PLC/RNF/5 cells. The average number of SGs/SG-positive cell (g) and SG index (h) based on G3BP1 immunofluorescence were shown. n = 3 independent experiments/4 images each. i-k, Representative confocal images of indicated HCCLM3 cells after treating with As2O3 (0.5 mM) to observe the dynamics of SG assembly (i). The average number of SGs/SG-positive cell (j) and SG index (k) were shown based on G3BP1 immunofluorescence. n = 3 independent experiments, 2 biological replicates. l, Representative confocal images of indicated SNU449 cells. m, Confocal image of LLPS droplet formed in vitro incorporating RIOK1–GFP, IGF2BP1–BFP and G3BP1–mCherry (n = 3 independent experiments). n, Representative smFISH images and protein expression of indicated SNU449 cells (n = 3 independent experiments). o, Quantification of SG index in the indicated SNU449 cells subjected to glutamine deprivation or donafenib as detected by eIF4G, respectively (n = 4 images, 3 biological replicates). p, WB of lysates of SNU449 cells expressing the indicated shRNAs of G3BP2 and ‘synthetic’ constructs. q-r, Representative immunostaining (q) and quantification of SG index (r) as detected by of G3BP2, eIF4G and eIF3η in SNU449 cells expressing the indicated constructs under oxidative stress (sodium arsenate, 500 μM, 1 hr). n = 4 images, 3 biological replicates. s, Representative immunostaining of SNU449 cells transfected with indicated constructs. Error bars indicate mean ± SD. Data were analyzed by unpaired, two-tailed Student’s t-tests (g, h and o), one-way ANOVA with Tukey’s multiple comparisons test (r) and two-way ANOVA with Sidak’s multiple comparisons test (j and k). All microscopy images and blots are representative of n = 3 independent experiments.
Extended Data Fig. 4 Silencing RIOK1 inhibits pentose phosphate pathway and tumor growth.
a, KEGG pathway enrichment analysis of different metabolites in HCCLM3 cells with RIOK1 knockdown and ctrl group. b, Heatmap representing markedly changed metabolites associated with nucleotides and pentose phosphate pathway in HCCLM3 cells with RIOK1 knockdown and ctrl group, as determined using untargeted metabolomics (n = 5 independent cultures per cell line). c-d, Images depict the scheme to selectively inactivate the RIOK1 gene in murine livers by targeting exon 2 using Cre-lox technology (c). Representative RT–PCR-based genotyping assays to identify mice bearing the floxed RIOK1 allele (fl/fl) (top panel) in combination with albumin (Alb) driven expression of Cre recombinase (Alb-CreERT2 transgene) (bottom panel) (d). e-f, Construct design (e) and experimental scheme (f) for the spontaneous liver tumor model induced by hydrodynamic tail vein injection with the C-myc proto-oncogene transposon system in combination with CRISPR–Cas9 targeting of Trp53 (SgP53/Myc). g, Experimental scheme for the spontaneous liver tumor model induced by DEN/CCl4 treatment. h, Representative Ki67 stained liver sections were shown refer to Fig. 5g. n = 3 mice. i, Western blotting showing the expression of RIOK1, PTEN and G6PD in liver tumors from Fig. 5g; n = 6 mice/group). j, GO functional analysis diagram of markedly differentially expressed genes in RIOK1 knockout tumors (n = 5 mice/group). k-l, Hepatic spontaneous neoplasms were challenged by hydrodynamic tail vein (HTV) injection of Sleeping Beauty (SB) transposase vector encoding MycOE, Trp53KO in both RIOK1fl/fl and RIOK1ΔAlb group mice. In a separate cohort of mice, we administered a combination of SB/MycOE, IGF2BP1 Y396A, and Trp53KO plasmids. Representative freshly excised livers (k) and Liver/body weight ratios (l) indicative of tumor burden in the different genotypes (n = 3 mice/group). Data are represented as mean ± SD and analyzed by one-way ANOVA with Tukey’s multiple comparisons test.
Extended Data Fig. 5 RIOK1 ablation enhances the sensitivity of HCC cells to stress.
a, Analysis of the proximal promoter region of RIOK1 using JASPAR reveals two high-scoring binding motifs for NRF2. b, QPCR analyses of RIOK1 mRNA expression in SNU449 cells cultured under normal or glutamine starvation (-GLN) conditions for up to 48 h. n = 3 independent experiments, 2 biological replicates/experiments. c, Immunoblotting of SNU449 and PLC/RNF/5 cells stimulated by glucose deficiency and hypoxia. d, Western blotting analysis of HCCLM3 cells stimulated by glutamine deficiency, sorafenib and donafenib. e-h, Confocal images of HCCLM3 cells cultured without GLN for 48 h (e) or transduced with sh-Ctrl or shRIOK1 and treated for 48 h with 10 μM donafenib (f). And the corresponding SG index of GLN deprivation(g)and donafenib (h) based on G3BP1 immunofluorescence were shown. n = 3 independent experiments. i-j, Immunoprecipitations performed using control IgG (IgG-R) or anti-IGF2BP1 in indicated HCCLM3 cells cultured with or without glutamine (i) or donafenib (j) for 48 h. k, Cell viability measurements of indicated SNU449 cells over 1-7 days in the presence or absence of GLN (n = 3 biological replicates/group). l, Cell viability of indicated HCCLM3 cells with or without GLN (n = 3 biological replicates/group). m, Confocal images of indicated HCCLM3 cells cultured for 48 h in the absence of GLN and stained as per panel (Fig. 6n). n = 3 independent experiments. n, Representative gating strategy for analyzing propidium iodide stained HCCLM3 cells death using flow cytometry. The gating strategy was as follows: cells were first gated based on SSC-A/FSC-A to exclude debris, followed by FSC-H/FSC-A to select single cells, and finally SSC-A/PI+ to identify apoptotic cells. o-p, Representative IHC staining against Ki67 (o) in HCCLM3 xenograft tissues treated with vehicle (−) or donafenib (+) (related to Fig. 5s). The fraction Ki67 positive cell was quantified (p, n = 3 independent experiments). Data were analyzed by unpaired, two-tailed Student’s t-tests (g and h), one-way ANOVA with Tukey’s multiple comparisons test (b and p) and two-way ANOVA with Tukey’s multiple comparisons test (k and l). All quantitative data are represented as mean ± SD. All blots are representative of n = 3 independent experiments.
Extended Data Fig. 6 Chidamide suppress SGs formation.
a, 3D representation of seven high-scoring candidate small molecules docking with RIOK1 identified using in silico analyses (related to Fig. 7a). b, Schematic representing the development of the HCC PDX models in NSG-nude mice and experimental treatment schemes using chidamide. c-d, Images (n = 2 images, 3 biological replicates) were analyzed to assess the average number of SGs/SG-positive cell based on G3BP1 immunofluorescence (c) and the fraction of PTEN mRNA in SGs (d) (related to Fig. 7l). e-g, Representative confocal images of staining against RIOK1 (green), G3BP1 (red) and PTEN mRNA (purple) in HCCLM3 cells cultured without GLN for 48 h in the absence and presence of 10 μM chidamide (e). Images (n = 2 images, 3 biological replicates) were analyzed to assess the average number of SGs/SG-positive cell based on G3BP1 immunofluorescence (f) and the fraction of PTEN smFISH in SGs (g). In c, d, f and g, error bars indicate mean ± SD for 3 independent experiments in which 2 fields of view. Data were analyzed by unpaired, two-tailed Student’s t-tests.
Extended Data Fig. 7 Chidamide potentiates the actions of TKIs against HCC cells.
a-b, Cell viability measurements in HCCLM3 (a) and MHCC-97H (b) cells over 24–72 h after treatment with the indicated concentrations of donafenib and chidamide. n = 4 biological replicates. c, Colony formation assays conducted in MHCC-97H cells treated with donafenib, chidamide and chidamide plus donafenib. Images are representative of n = 3 independent experiments. d-f, Cell viability of MHCC-97H cells over 24–72 h after treatment with chidamide alone or in combination with lenvatinib (d), sorafenib (e) and regorafenib (f). n = 4 biological replicates. g–i, Cell viability of HCCLM3 cells over 24–72 h after treatment with chidamide alone or in combination with lenvatinib (g), sorafenib (h) and regorafenib (i). n = 4 biological replicates. j–l, Colony formation assays conducted in HCCLM3 cells treated with vehicle control (DMSO) or 2.5 μM chidamide in combination with the indicated concentrations of lenvatinib (j), sorafenib (k), and regorafenib (l). Images are representative of n = 3 independent experiments. m-o, The drug combination index analysis of Chidamide and donafenib (m), Chidamide and sorafenib (n), Chidamide and Lenvatinib (o). p, Representative HE images of patient-derived organoids derived from HCC tissues(n = 3 independent experiments). q. Schematic representing the development of the HCC PDX models in NSG-nude mice and experimental treatment schemes using chidamide or combining chidamide and donafenib. r-s, The ki67 IHC staining (r) and quantification (s) of HCC tumors treated with donafenib, chidamide and the combination treatment. in various liver tissues (n = 3 mice/group). t, Schematic depicting the autologous Hep1-6 HCC tumor model in C57/BL mice. u. Experimental scheme for implementing the SgP53/Myc spontaneous liver tumor model with the primary measure of survival data. v-w, Quantification of animal body weights during treatment in the PDX/NSG (n = 5 mice/group; v) and Hep1-6/C57/BL (n = 6 mice/group; w) murine tumor models (related to Fig. 7q and s, respectively). Data were analyzed by one-way ANOVA with Tukey’s multiple comparisons test (s) and two-way ANOVA with Tukey’s multiple comparisons test (v and w). All quantitative data are represented as mean ± SD. All microscopy images are representative of n = 3 independent experiments.
Supplementary information
Supplementary Video 1
Overexpression of RIOK1 resulted in the sustained formation of stress granules.
Supplementary Table 1
Supplementary Tables 1–6.
Source data
Source Data All Figures
Unprocessed western blots.
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meng, F., Li, H., Huang, Y. et al. RIOK1 phase separation restricts PTEN translation via stress granules activating tumor growth in hepatocellular carcinoma. Nat Cancer (2025). https://doi.org/10.1038/s43018-025-00984-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43018-025-00984-5