Abstract
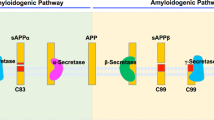
The abnormal deposition of amyloid β (Aβ), produced by proteolytic cleavage events of amyloid precursor protein involving the protease γ-secretase and subsequent polymerization into amyloid plaques, plays a key role in the neuropathology of Alzheimer’s disease (AD). Here we show that ErbB3 binding protein 1 (EBP1)/proliferation-associated 2G4 (PA2G4) interacts with presenilin, a catalytic subunit of γ-secretase, inhibiting Aβ production. Mice lacking forebrain Ebp1/Pa2g4 recapitulate the representative phenotypes of late-onset sporadic AD, displaying an age-dependent increase in Aβ deposition, amyloid plaques and cognitive dysfunction. In postmortem brains of patients with AD and 5x-FAD mice, we found that EBP1 is proteolytically cleaved by asparagine endopeptidase at N84 and N204 residues, compromising its inhibitory effect on γ-secretase, increasing Aβ aggregation and neurodegeneration. Accordingly, injection of AAV2-Ebp1 wild-type or an asparagine endopeptidase-uncleavable mutant into the brains of 5x-FAD mice decreased Aβ generation and alleviated the behavioral impairments. Thus, our study suggests that EBP1 acts as an inhibitor of γ-secretase on amyloid precursor protein cleavage and preservation of functional EBP1 could be a therapeutic strategy for AD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The RNA-seq raw data using this study from Ebp1-CKO are deposited in the Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra) with BioProjectID (PRJNA1187335); SRA (SRR31373365, SRR31373362, SRR31373364, SRR31373361, SRR31373363 and SRR31373360) and presented in supplementary data (Supplementary Table 3). The Gene Expression Omnibus Database with accession nos. GSE9770, GSE5281 and GSE36980 was obtained from the National Center for Biotechnology Information. All reagents including resources, antibodies, cell lines and animal models are available from commercial sources or the corresponding author. All data in the article can be obtained from the corresponding author upon reasonable request. Source data for all statistical data, unmodified gels and blots and original staining images are provided in this paper.
References
Bateman, R. J. et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 367, 795–804 (2012).
Duyckaerts, C., Delatour, B. & Potier, M. C. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 118, 5–36 (2009).
Martini, A. C., Forner, S., Trujillo-Estrada, L., Baglietto-Vargas, D. & LaFerla, F. M. Past to future: what animal models have taught us about Alzheimer’s disease. J. Alzheimers Dis. 64, S365–s378 (2018).
Götz, J., Bodea, L.-G. & Goedert, M. Rodent models for Alzheimer disease. Nat. Rev. Neurosci. 19, 583–598 (2018).
Park, H. H. et al. Characterization of age- and stage-dependent impaired adult subventricular neurogenesis in 5xFAD mouse model of Alzheimer’s disease. BMB Rep. 56, 520–525 (2023).
King, A. The search for better animal models of Alzheimer’s disease. Nature 559, S13–S15 (2018).
Kitazawa, M., Medeiros, R. & Laferla, F. M. Transgenic mouse models of Alzheimer disease: developing a better model as a tool for therapeutic interventions. Curr. Pharm. Des. 18, 1131–1147 (2012).
Chen, G.-F. et al. Amyloid β: structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 38, 1205–1235 (2017).
Im, D. & Choi, T. S. Distinctive contribution of two additional residues in protein aggregation of Aβ42 and Aβ40 isoforms. BMB Rep. 57, 263–272 (2024).
Selkoe, D. J. & Wolfe, M. S. Presenilin: running with scissors in the membrane. Cell 131, 215–221 (2007).
Haapasalo, A. & Kovacs, D. M. The many substrates of presenilin/γ-secretase. J. Alzheimers Dis. 25, 3–28 (2011).
De Strooper, B., Iwatsubo, T. & Wolfe, M. S. Presenilins and γ-secretase: structure, function, and role in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2, a006304 (2012).
Kimberly, W. T. et al. Notch and the amyloid precursor protein are cleaved by similar γ-secretase(s). Biochemistry 42, 137–144 (2003).
Hur, J. Y. γ-Secretase in Alzheimer’s disease. Exp. Mol. Med. 54, 433–446 (2022).
Thathiah, A. et al. The orphan G protein-coupled receptor 3 modulates amyloid-β peptide generation in neurons. Science 323, 946–951 (2009).
Liu, X. et al. β-Arrestin1 regulates γ-secretase complex assembly and modulates amyloid-β pathology. Cell Res. 23, 351–365 (2013).
Zhang, Z. et al. Cleavage of tau by asparagine endopeptidase mediates the neurofibrillary pathology in Alzheimer’s disease. Nat. Med. 20, 1254–1262 (2014).
Zhang, Z. et al. δ-Secretase cleaves amyloid precursor protein and regulates the pathogenesis in Alzheimer’s disease. Nat. Commun. 6, 8762 (2015).
Chen, G. et al. Netrin-1 receptor UNC5C cleavage by active δ-secretase enhances neurodegeneration, promoting Alzheimer’s disease pathologies. Sci. Adv. 7, eabe4499 (2021).
Dall, E. & Brandstetter, H. Structure and function of legumain in health and disease. Biochimie 122, 126–150 (2016).
Basurto-Islas, G., Grundke-Iqbal, I., Tung, Y. C., Liu, F. & Iqbal, K. Activation of asparaginyl endopeptidase leads to tau hyperphosphorylation in Alzheimer disease. J. Biol. Chem. 288, 17495–17507 (2013).
Hwang, I. & Ahn, J. Y. Dysregulation of epigenetic control contributes to schizophrenia-like behavior in Ebp1+/− mice. Int. J. Mol. Sci. 21, 2609 (2020).
Kim, Y., Ko, H. R., Hwang, I. & Ahn, J. Y. ErbB3 binding protein 1 contributes to adult hippocampal neurogenesis by modulating Bmp4 and Ascl1 signaling. BMB Rep. 57, 182–187 (2024).
Kraushar, M. L. et al. Protein synthesis in the developing neocortex at near-atomic resolution reveals Ebp1-mediated neuronal proteostasis at the 60S tunnel exit. Mol. Cell 81, 304–322.e316 (2021).
Ko, H. R. et al. Roles of ErbB3-binding protein 1 (EBP1) in embryonic development and gene-silencing control. Proc. Natl Acad. Sci. USA 116, 24852–24860 (2019).
Kim, B. S., Ko, H. R., Hwang, I., Cho, S. W. & Ahn, J. Y. EBP1 regulates Suv39H1 stability via the ubiquitin-proteasome system in neural development. BMB Rep. 54, 413–418 (2021).
Hwang, I. et al. Cerebellar dysfunction and schizophrenia-like behavior in Ebp1-deficient mice. Mol. Psychiatry 27, 2030–2041 (2022).
Hwang, I. et al. PA2G4/EBP1 ubiquitination by PRKN/PARKIN promotes mitophagy protecting neuron death in cerebral ischemia. Autophagy 20, 365–379 (2024).
Andrews, S. J., Fulton-Howard, B. & Goate, A. Interpretation of risk loci from genome-wide association studies of Alzheimer’s disease. Lancet Neurol. 19, 326–335 (2020).
Shigemizu, D. et al. Ethnic and trans-ethnic genome-wide association studies identify new loci influencing Japanese Alzheimer’s disease risk. Transl. Psychiatry 11, 151 (2021).
Bellenguez, C. et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 54, 412–436 (2022).
Readhead, B. et al. Multiscale analysis of independent Alzheimer’s cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron 99, 64–82.e67 (2018).
Liang, W. S. et al. Alzheimer’s disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proc. Natl Acad. Sci. USA 105, 4441–4446 (2008).
Liang, W. S. et al. Altered neuronal gene expression in brain regions differentially affected by Alzheimer’s disease: a reference data set. Physiol. Genomics 33, 240–256 (2008).
Hokama, M. et al. Altered expression of diabetes-related genes in Alzheimer’s disease brains: the Hisayama study. Cereb. Cortex 24, 2476–2488 (2014).
Liang, W. S. et al. Gene expression profiles in anatomically and functionally distinct regions of the normal aged human brain. Physiol. Genomics 28, 311–322 (2007).
Zhang, Y., Wu, K.-M., Yang, L., Dong, Q. & Yu, J.-T. Tauopathies: new perspectives and challenges. Mol. Neurodegeneration 17, 28 (2022).
Hardy, J. & Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 12, 383–388 (1991).
Wang, Y. P., Biernat, J., Pickhardt, M., Mandelkow, E. & Mandelkow, E. M. Stepwise proteolysis liberates tau fragments that nucleate the Alzheimer-like aggregation of full-length tau in a neuronal cell model. Proc. Natl Acad. Sci. USA 104, 10252–10257 (2007).
Ko, H. R. et al. Neuron-specific expression of p48 Ebp1 during murine brain development and its contribution to CNS axon regeneration. BMB Rep. 50, 126–131 (2017).
Multhaup, G., Huber, O., Buée, L. & Galas, M. C. Amyloid precursor protein (APP) metabolites APP intracellular fragment (AICD), Aβ42, and tau in nuclear roles. J. Biol. Chem. 290, 23515–23522 (2015).
Han, J. et al. Aberrant role of pyruvate kinase M2 in the regulation of γ-secretase and memory deficits in Alzheimer’s disease. Cell Rep. 37, 110102 (2021).
Steiner, H., Fukumori, A., Tagami, S. & Okochi, M. Making the final cut: pathogenic amyloid-β peptide generation by γ-secretase. Cell Stress 2, 292–310 (2018).
Wongtrakul, J. et al. Proteomic analysis of human glutathione transferase omega (hGSTO1) stable transfection in a 6-hydroxydopamine-induced neuronal cells. Gen. Physiol. Biophys. 37, 141–152 (2018).
Jia, Y. et al. Genetic dissection of glutathione S-transferase omega-1: identification of novel downstream targets and Alzheimer’s disease pathways. Neural Regen. Res. 17, 2452–2458 (2022).
Baglietto-Vargas, D. et al. Generation of a humanized Aβ expressing mouse demonstrating aspects of Alzheimer’s disease-like pathology. Nat. Commun. 12, 2421 (2021).
Umeda, T. et al. Neurofibrillary tangle formation by introducing wild-type human tau into APP transgenic mice. Acta Neuropathol. 127, 685–698 (2014).
Ahlemeyer, B., Halupczok, S., Rodenberg-Frank, E., Valerius, K. P. & Baumgart-Vogt, E. Endogenous murine amyloid-β peptide assembles into aggregates in the aged C57BL/6J mouse suggesting these animals as a model to study pathogenesis of amyloid-β plaque formation. J. Alzheimers Dis. 61, 1425–1450 (2018).
Xu, Z. et al. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Mol. Neurodegener. 10, 58 (2015).
Zhang, Z., Tian, Y. & Ye, K. δ-Secretase in neurodegenerative diseases: mechanisms, regulators and therapeutic opportunities. Transl. Neurodegeneration 9, 1 (2020).
Song, M. The asparaginyl endopeptidase legumain: an emerging therapeutic target and potential biomarker for Alzheimer’s disease. Int. J. Mol. Sci. 23, 10223 (2022).
Bahk, Y. Y. et al. Antigens secreted from Mycobacterium tuberculosis: identification by proteomics approach and test for diagnostic marker. Proteomics 4, 3299–3307 (2004).
Gobom, J., Nordhoff, E., Mirgorodskaya, E., Ekman, R. & Roepstorff, P. Sample purification and preparation technique based on nano-scale reversed-phase columns for the sensitive analysis of complex peptide mixtures by matrix-assisted laser desorption/ionization mass spectrometry. J. Mass Spectrom. 34, 105–116 (1999).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Acknowledgements
The authors thank Y.-K. Jung (Seoul National University, Korea) and J.H. Shin for providing Flag–PSEN plasmid and HT22 cell line, respectively. This research was supported by a grant from the Korea Dementia Research Project through the Korea Dementia Research Center, funded by the Ministry of Health and Welfare and Ministry of Science and ICT, Republic of Korea (grant no. HU21C0157) to J.-Y.A.
Author information
Authors and Affiliations
Contributions
B.-S.K., I.H., Young Kwan Kim and J.-Y.A. conceived the study and designed the experiments. B.-S.K., I.H., H.R.K., Young Kwan Kim, Y.C., S.L., S.N., J.-C.P. and S.E.L. performed experiments. B.-S.K., I.H., K.Y. and J.-Y.A. analyzed the data. H.J.K., S.W.S., D.-G.J. and Y.L. collected human brain samples. Yun Kyung Kim, J.-C.P., D.K., S.-W.C., K.Y. and J.-Y.A. provided supervision of the study and data interpretation. J.-Y.A. wrote the paper. K.Y. and J.-Y.A. read and edited the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Yadong Huang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 EBP1 is progressively decreased in Ebp1-CKO.
a, EBP1 RNA expression was analyzed using GEO datasets (GSE9770, GSE5281, and GSE36980) in human non-AD or AD brains (left, middle) and serial-aged brains (right). (left) Control: n = 71, AD: n = 34, (middle) Control: n = 47, AD: n = 32, (right) < 40: n = 12, 40-70: n = 9, 70-94: n = 16, 95-106: n = 4. b, Schematic strategy of CamkIIα-Cre; Ebp1flox/flox knockout mouse production. c, 2, and 6-month Ebp1-CKO and control mice brains were isolated, purified mRNA, and synthesized to cDNA. Comparative Ebp1 RNA expression in hippocampal and cortical tissues was analyzed with PCR. d, Ebp1-CKO and control mouse brains at serial ages (1, 4, 8, and 12 months) were applied to IHC with anti-EBP1 antibody (red) for detecting the decrease of progressive EBP1 protein expression. Nuclei were stained with DAPI. Quantification of EBP1 intensity in each region (bottom). Scale bar, 200 μm. n = 5. Data are presented as mean values ± SEM (a, d). *p < 0.05, **p < 0.005, ***p < 0.001, ****p < 0.0001 (a, d), ns; not significant. Student’s two-tailed unpaired t-test (a) and Two-way ANOVA followed by Sidak’s multiple comparison (d).
Extended Data Fig. 2 Aβ deposition is increased in the brains of Ebp1-CKO.
a, Black and white conversion images of Fig. 1b. Scale bar, 100 μm. b, Brain tissues of serial-aged Ebp1-CKO and 2, 4-month 5X-FAD mice were subjected to IHC with anti-Aβ (red) antibody. Neurons were marked with anti-Tuj1 antibody (green) and nuclei were counter-stained with DAPI. Scale bar, 20 μm. c-d, Brain tissues of Ebp1-CKO and control were immunostained with anti-Aβ antibody, and conversed to black and white images. c, Young-aged Ebp1-CKO at 1 and 3-month-old mice were sacrificed and isolated brain tissues were applied to IHC. d, 2, 4, 8, 10, and 15-month-old brains of control mice were isolated and subjected to IHC. Scale bar, 1 mm. e, Thioflavin-S staining was used to analyze thioflavin-S positive amyloid fibrils in 8 and 12-month Ebp1-CKO and control mice brains. White boxes in 8-month hippocampus images indicate the magnified CA1 region. White boxes in enlarged CA1 and cortical images show iAβ in each region. Scale bar, 500 μm.
Extended Data Fig. 3 Ebp1-CKO mice show AD-like phenotypes.
a-b, T2-weighted MR images (MRI) of the whole brain of Ebp1-CKO and control mice at 2 and 12 months. a, Measurement of hippocampus and cortex volume (right). #14 indicates the 14th order of the MRI series. b, #18 means the 18th order of the MRI series. (n = 3, each group). c-e, Hippocampus of 2, 8, 12-month Ebp1-CKO and control mice stained with anti-GFAP (green) and anti-Aβ (red) antibodies. Nuclei were stained with DAPI. c, Representative images of the whole hippocampus. Quantification of GFAP signals in the whole hippocampus (right). Scale bar, 500 μm. d-e, Magnified images of the CA1 and CA3 regions (d) and DG region (e) in (c). Quantification of astrocytes in DG (right). Scale bar, 20 μm. n = 5. f, RNA-seq analysis of AD-related genes in 10-month Ebp1-CKO brains. g, Experimental scheme of behavioral tests. h, Heatmaps indicating the track for searching the hidden platform in the Morris water maze test. i, Representative heatmaps in Y-maze showing alteration in arms. j, Heatmap images of novel object recognition test expressing the time spent performance in the experimental position. k, Training session in the novel object recognition test. n = 9. Data are presented as mean values ± SEM (a-e, k). *p < 0.05, **p < 0.005, ***p < 0.001, ****p < 0.0001, ns; not significant (a-e). Multiple unpaired t-tests (a, b) and Two-way ANOVA followed by Sidak’s multiple comparisons (c-e) were used to calculate the P values.
Extended Data Fig. 4 EBP1 proteolysis occurs in AD brains.
a, Serial-aged WT mouse brains were isolated and used for the comparison of EBP1 expression pattern between RNA and protein via PCR and immunoblots, respectively. n = 4 for IB and n = 6 for PCR. b, Brain lysates of human postmortem AD patients (n = 4) and age-matched controls (n = 4) were subjected to immunoblots with anti-EBP1 and anti-HSP70 antibodies. c, Brains of 8-month WT (n = 3) and 5X-FAD (n = 3) mice were applied to a cleavage assay in pH 7.4 cleavage buffer for 45 min. Two cleaved EBP1 fragments were only detected in 5X-FAD brain lysates with anti-EBP1 antibody. d, Hippocampal neurons at DIV7 applied Aβ peptide at 1 and 10 μM, and control peptide for 24 h were stained with indicated antibodies. White boxes on the left side indicate the soma of stained neurons. Scale bar, 20 μm and 5 μm, each. e, Brains of 8-month WT and 5X-FAD mice were isolated and subjected to IHC against anti-EBP1 (red), anti-EEA1 (blue), and anti-LAMP1 (green) antibodies. Analysis of the intensity of EBP1 with EEA1 and LAMP1 signals (right). Scale bar, 20 μm. f, Brains of 3 WT and Lgmn(-/-) mice were subjected to cleavage assay and cleaved EBP1 was detected by anti-EBP1 antibody. n = 3. g, Series of EBP1 N Mutants cleavage was quantified to the bar graphs. n = 4. Data are presented as mean values ± SEM (a, f, g). *p < 0.05, **p < 0.005, ***p < 0.001, ****p < 0.0001 (f, g), ns; not significant. Student’s two-tailed unpaired t-test (f) and Two-way ANOVA followed by Tukey’s multiple comparison (a, g).
Extended Data Fig. 5 AEP-mediated EBP1 cleavage is determined by either anti-N84 or N204 antibody.
a, A diagram illustration of N84- and N204-specific antibody production process. b-c, Antibody specificity test. b, In vitro cleavage assay with purified GST-EBP1 and rAEP proteins. Both the anti-N84 and anti-N204 antibodies detected the correct size of cleaved EBP1 with an anti-GST antibody. c, GST-tagged EBP1 fragments were transfected to HEK293T cells. Cells were lysed and conducted to IB with anti-GST antibody, anti-N84 and N204 antibodies. Red arrow: cleaved EBP1. d, Brains of 5X-FAD mice at serial ages by IHC with anti-Aβ (green) and anti-N84 or N204 antibodies (red). Nuclei were stained with DAPI. Scale bar, 100 μm. e, An indicated series of 5X-FAD brain lysates were subjected to IB for detecting the N84 and N204 fragments as aging. The intensity of N84 and N204 cleavage forms was quantified (right). n = 8. f, Hippocampus and cortex of 8-month 5X-FAD and WT mice immunostained with anti-EBP1 or N84 or N204 antibody (red) with anti-NeuN (green) antibody. Nuclei were stained with DAPI. Quantification of fluorescence (bottom). Scale bar, 50 μm. n = 5. g, Brains of 10-month control mice stained with anti-N84 or N204 antibodies. h, Peptide inhibition assay for exploring antibody specificity. 5-month 5X-FAD tissues were incubated with N84 or N204 peptides or control peptide as control. After incubation, tissue samples were stained with indicated antibodies. Scale bar, 100 μm. Data are presented as mean values ± SEM (e, f). *p < 0.05, **p < 0.005, ***p < 0.001, ****p < 0.0001 (e, f), ns; not significant. Multiple unpaired t-test (f) Two-way ANOVA followed by Tukey’s multiple comparison (e).
Extended Data Fig. 6 Localization and effects of cleaved EBP1 fragments in hippocampal neurons.
a, GFP-EBP1 fragments transfected hippocampal primary neurons at DIV9 were analyzed to show the nuclear translocation of 205–394 fragment. (left) After 24 h transfection, neurons were fixed with 4% PFA and stained with anti-NeuN antibody (purple). (right) Nuclei of GFP-plasmids overexpressed neurons were stained with DAPI. The expression pattern of each panel was depicted to the graphs. b, Hippocampal primary neurons at DIV9 were overexpressed the GFP-EBP1 fragments and stained with anti-Tuj1 antibody (red). White arrows indicate the axons of GFP-transfected neurons. Quantification of axonal length (μm) of GFP-positive neurons (right). The measured GFP-positive neurons were selected randomly and all data were normalized to GFP-Mock. Scale bar, 20 μm. n = 3. c, DIV9 5X-FAD primary hippocampal neurons were transfected with the indicated RFP-tagged plasmids and stained with anti-Tuj1 antibody (green). White arrows indicate the axons of GFP-transfected neurons. Axon length (μm) with Tuj1-positive neurons were quantified (right). The measured RFP-positive neurons were selected randomly and all data were normalized to RFP-Mock. Scale bar, 20 μm. n = 3. d, GFP-EBP1 plasmids were transfected to primary neurons at DIV9 and neurons were subjected to immunocytochemistry with anti-LAMP1 (red) and anti-EEA1 (blue) antibodies. White arrows mean the GFP-colocalization with EEA1-LAMP1 signals. Data are presented as mean values ± SEM (b, c). *p < 0.05, **p < 0.005, ***p < 0.001, ****p < 0.0001, ns; not significant (b, c). Student’s two-tailed unpaired t-test (b, c).
Extended Data Fig. 7 EBP1 effects on γ-secretase activity but not α/β-secretase activity or complex formation of γ-secretase.
a, Brains of 5-month WT and 5X-FAD, as well as 12-month Ebp1-CKO and control brains, were separated into membrane fractions. Membrane lysates were incubated with each secretase substrate at 37°C for 16 h. (left) α-secretase activity assay. (right) β-secretase activity assay. n = 7, each test. b, HT22 cells were co-transfected with the indicated plasmids, and cell lysates were subjected to luciferase reporter assay. n = 5. c, AAV-Mock, Ebp1, and N84A/N204A mutant virus were injected into 10-month Ebp1-CKO and control mice. After 2 months, brains were subjected to PLA assay using indicated antibodies. The whole CA1 region of mice were arranged. d, Membrane fraction of 9-month 5X-FAD and WT brains was subjected to γ-secretase cleavage assay with or without L-685,458. n = 3. e, 12-month Ebp1-CKO and control brains were isolated and lysates were applied to immunoprecipitation with anti-PSEN antibody. f, HEK293T cells were co-transfected with GST-EBP1 fragments and Flag-AICD plasmids and applied to GST pulldown assay. g, Representative confocal images of red dots PLA signals in the CA1 region. Brain tissues of 2 and 12-month Ebp1-CKO and control mice were applied to PLA staining. Scale bar, 100 μm. h, HT22 cells were co-transfected with GST-EBP1, pCAX-APP, and Flag-PSEN, and incubated for 24 h. Cells were lysed, and cell lysates were subjected to GST pulldown assay and immunoprecipitation with anti-Flag antibody. n = 3. Data are presented as mean values ± SEM (a, b, d). *p < 0.05, **p < 0.005, ***p < 0.001, ****p < 0.0001 (a, b, h), ns; not significant. Student’s two-tailed unpaired t-test (a, b, d) and Two-way ANOVA followed by Tukey’s multiple comparison (h).
Extended Data Fig. 8 Expression of AAV2-Mock, Ebp1-WT, and N84A/N204A mutant virus in the mice brain.
a, AAV2-Mock, Ebp1-WT, and N84A/N204A mutant virus-injected mice brains were lysed and subjected to IB with anti-EBP1 and anti-HSP70 antibodies for detecting the overexpressed EBP1 protein in AAV2-Ebp1 WT and N84,204A infected lanes. b, Indicated AAV2-virus transduced 1-month slice cultured whole brains were fixed and immunostained with anti-Aβ (green) and anti-NeuN antibodies (blue).
Extended Data Fig. 9 A schematic image of study.
a, EBP1 cleavage by AEP elicits neurodegeneration, leading to dismissal of γ-secretase control thereby accelerating i/eAβ generation.
Supplementary information
Supplementary Information
Headings of Supplementary Tables 1–6, Figs. 1–4 and Videos 1–3, and Supplementary Tables 1, 2, 5 and 6 and Figs. 1–4.
Supplementary Table 3
The raw data of RNA-seq analysis using 10-month-old Ebp1-CKO and control brains.
Supplementary Table 4
The raw data for MS.
Supplementary Video 1
Morris water maze raw data of AAV-injected 5x-FAD mice.
Supplementary Video 2
Y-maze raw data of AAV-injected 5x-FAD mice.
Supplementary Video 3
Novel object recognition test raw data of AAV-injected 5x-FAD mice.
Source data
Source Data Fig. 1
Statistical source data for Fig. 1.
Source Data Fig. 2
Statistical source data for Fig. 2.
Source Data Fig. 3
Statistical source data for Fig. 3.
Source Data Fig. 4
Statistical source data for Fig. 4.
Source Data Fig. 5
Statistical source data for Fig. 5.
Source Data Fig. 6
Statistical source data for Fig. 6.
Source Data Fig. 7
Statistical source data for Fig. 7.
Source Data Fig. 8
Statistical source data for Fig. 8.
Source Data Fig. 1
Unprocessed gels and blots of Fig. 1.
Source Data Fig. 3
Unprocessed gels and blots of Fig. 3.
Source Data Fig. 4
Unprocessed gels and blots of Fig. 4.
Source Data Fig. 6
Unprocessed gels and blots of Fig. 6.
Source Data Fig. 7
Unprocessed gels and blots of Fig. 7.
Source Data Extended Data Fig. 1
Statistical source data for Extended Data Fig. 1.
Source Data Extended Data Fig. 3
Statistical source data for Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Statistical source data for Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Statistical source data for Extended Data Fig. 5.
Source Data Extended Data Fig. 6
Statistical source data for Extended Data Fig. 6.
Source Data Extended Data Fig. 7
Statistical source data for Extended Data Fig. 7.
Source Data Extended Data Fig. 4
Unprocessed gels and blots of Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Unprocessed gels and blots of Extended Data Fig. 5.
Source Data Extended Data Fig. 7
Unprocessed gels and blots of Extended Data Fig. 7.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, BS., Hwang, I., Ko, H.R. et al. EBP1 potentiates amyloid β pathology by regulating γ-secretase. Nat Aging 5, 486–503 (2025). https://doi.org/10.1038/s43587-024-00790-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-024-00790-1