Abstract
Glycoprotein nonmetastatic melanoma protein B (GPNMB) is a type I transmembrane protein initially identified in nonmetastatic melanomas and has been associated with human heart failure; however, its role in cardiac injury and function remains unclear. Here we show that GPNMB expression is elevated in failing human and mouse hearts after myocardial infarction (MI). Lineage tracing and bone-marrow transplantation reveal that bone-marrow-derived macrophages are the main source of GPNMB in injured hearts. Using genetic loss-of-function models, we demonstrate that GPNMB deficiency leads to increased mortality, cardiac rupture and rapid post-MI left ventricular dysfunction. Conversely, increasing circulating GPNMB levels through viral delivery improves heart function after MI. Single-cell transcriptomics show that GPNMB enhances myocyte contraction and reduces fibroblast activation. Additionally, we identified GPR39 as a receptor for circulating GPNMB, with its absence negating the beneficial effects. These findings highlight a pivotal role of macrophage-derived GPNMBs in post-MI cardiac repair through GPR39 signaling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Frangogiannis, N. G. Pathophysiology of myocardial infarction. Compr. Physiol. 5, 1841–1875 (2015).
Frangogiannis, N. G. Emerging roles for macrophages in cardiac injury: cytoprotection, repair, and regeneration. J. Clin. Invest. 125, 2927–2930 (2015).
Tzahor, E. & Dimmeler, S. A coalition to heal-the impact of the cardiac microenvironment. Science 377, eabm4443 (2022).
Gnecchi, M., Zhang, Z., Ni, A. & Dzau, V. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 103, 1204–1219 (2008).
Weterman, M. A. et al. nmb, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts. Int. J. Cancer 60, 73–81 (1995).
Tsou, P. S. & Sawalha, A. H. Glycoprotein nonmetastatic melanoma protein B: a key mediator and an emerging therapeutic target in autoimmune diseases. FASEB J. 34, 8810–8823 (2020).
Furochi, H. et al. Osteoactivin fragments produced by ectodomain shedding induce MMP-3 expression via ERK pathway in mouse NIH-3T3 fibroblasts. FEBS Lett. 581, 5743–5750 (2007).
Lazaratos, A. M., Annis, M. G. & Siegel, P. M. GPNMB: a potent inducer of immunosuppression in cancer. Oncogene 41, 4573–4590 (2022).
Zhou, L. et al. Glycoprotein non-metastatic melanoma protein b (Gpnmb) is highly expressed in macrophages of acute injured kidney and promotes M2 macrophages polarization. Cell Immunol. 316, 53–60 (2017).
Kumagai, K. et al. Glycoprotein nonmetastatic melanoma B (Gpnmb)-positive macrophages contribute to the balance between fibrosis and fibrolysis during the repair of acute liver injury in mice. PLoS ONE 10, e0143413 (2015).
Yu, B., Alboslemy, T., Safadi, F. & Kim, M. H. Glycoprotein nonmelanoma Clone B regulates the crosstalk between macrophages and mesenchymal stem cells toward wound repair. J. Invest. Dermatol. 138, 219–227 (2018).
Frara, N. et al. Transgenic expression of Osteoactivin/gpnmb enhances bone formation in vivo and osteoprogenitor differentiation ex vivo. J. Cell. Physiol. 231, 72–83 (2016).
Jarve, A. et al. Adverse left ventricular remodeling by glycoprotein nonmetastatic melanoma protein B in myocardial infarction. FASEB J. 31, 556–568 (2017).
Lin, L. Y. et al. Systems genetics approach to biomarker discovery: GPNMB and heart failure in mice and humans. G3 8, 3499–3506 (2018).
Cordero, P. et al. Pathologic gene network rewiring implicates PPP1R3A as a central regulator in pressure overload heart failure. Nat. Commun. 10, 2760 (2019).
Liu, Y. et al. RNA-seq identifies novel myocardial gene expression signatures of heart failure. Genomics 105, 83–89 (2015).
Li, S. et al. Cardiomyocytes disrupt pyrimidine biosynthesis in nonmyocytes to regulate heart repair. J. Clin. Invest. 132, e149711 (2022).
Yokota, T. et al. Type V collagen in scar tissue regulates the size of scar after heart injury. Cell 182, 545–562.e23 (2020).
Dieckmann, B. W., Paguaga, M. E., McCollum, G. W., Penn, J. S. & Uddin, M. I. Role of NLRP3 inflammasomes in monocyte and microglial recruitments in choroidal neovascularization. ImmunoHorizons 8, 363–370 (2024).
Chung, J.-S., Sato, K., Dougherty, I. I., Cruz, P. D. Jr. & Ariizumi, K. DC-HIL is a negative regulator of T lymphocyte activation. Blood 109, 4320–4327 (2007).
Mulder, K. et al. Cross-tissue single-cell landscape of human monocytes and macrophages in health and disease. Immunity 54, 1883–1900.e1885 (2021).
Kim, S. H., Lee, K. Y. & Chang, K. The protective role of TREM2 in the heterogenous population of macrophages during post-myocardial infarction inflammation. Int. J. Mol. Sci. 24, 5556 (2023).
Cuzon Carlson, V. C. et al. Modulation of Gpr39, a G-protein coupled receptor associated with alcohol use in non-human primates, curbs ethanol intake in mice. Neuropsychopharmacology 44, 1103–1113 (2019).
Dong, X., Tang, S., Zhang, W., Gao, W. & Chen, Y. GPR39 activates proliferation and differentiation of porcine intramuscular preadipocytes through targeting the PI3K/AKT cell signaling pathway. J. Recept. Signal. Transduct. Res. 36, 130–138 (2016).
Młyniec, K., Budziszewska, B., Holst, B., Ostachowicz, B. & Nowak, G. GPR39 (zinc receptor) knockout mice exhibit depression-like behavior and CREB/BDNF down-regulation in the hippocampus. Int. J. Neuropsychopharmacol. 18, pyu002 (2014).
Ono, Y., Tsuruma, K., Takata, M., Shimazawa, M. & Hara, H. Glycoprotein nonmetastatic melanoma protein B extracellular fragment shows neuroprotective effects and activates the PI3K/Akt and MEK/ERK pathways via the Na+/K+-ATPase. Sci. Rep. 6, 23241 (2016).
Fujio, Y., Nguyen, T., Wencker, D., Kitsis, R. N. & Walsh, K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 101, 660–667 (2000).
Nagoshi, T. et al. PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J. Clin. Invest. 115, 2128–2138 (2005).
Qin, W., Cao, L. & Massey, I. Y. Role of PI3K/Akt signaling pathway in cardiac fibrosis. Mol. Cell. Biochem. 476, 4045–4059 (2021).
Saade, M., Araujo de Souza, G., Scavone, C. & Kinoshita, P. F. The role of GPNMB in Inflammation. Front. Immunol. 12, 674739 (2021).
Taya, M. & Hammes, S. R. Glycoprotein non-metastatic melanoma protein B (GPNMB) and cancer: a novel potential therapeutic target. Steroids 133, 102–107 (2018).
Kuleshov, M. V. et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44, W90–W97 (2016).
Wasala, L. P., Hakim, C. H., Yue, Y., Yang, N. N. & Duan, D. Systemic delivery of adeno-associated viral vectors in mice and dogs. Methods Mol. Biol. 1937, 281–294 (2019).
Li, S. et al. Metabolic reprogramming and epigenetic changes of vital organs in SARS-CoV-2-induced systemic toxicity. JCI Insight 6, e145027 (2021).
Pereira, A. H. M., Cardoso, A. C. & Franchini, K. G. Isolation, culture, and immunostaining of neonatal rat ventricular myocytes. STAR Protoc. 2, 100950 (2021).
Acknowledgements
This study was supported by grants from the National Institutes of Health (HL149658, HL152176, HL149687, AR075867, DK132735 (A.D.) and T32 DK007044-41 (D.R.S.)). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
S.R. and J.Q. performed majority of the experiments. B.T. and B.S. performed animal surgeries. F.M. and M.P. performed single-nuclear gene expression analysis. R.W. assisted in generation of the GPNMB KO animals and S.L. assisted in viral expression experiments. E.C. assisted with immunostaining. N.E.T., D.R.S., A.P., K.V.G. and P.L.S.M.G. performed experiments related to the identification of binding partners of GPNMB and A.J.L. performed analysis on the strength of association of GPNMB in human heart failure. Y.Z. and J.W. constructed the plasmids. B.H. generated GPR39 KO mice. A.D. conceptualized the project, designed experiments, supervised data collection and analysis and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks Kory Lavine and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 GPNMB expression in the infarcted heart using immunofluorescent staining and flow cytometry.
a, Immunofluorescent staining, and confocal microscopy of GPNMB expression in uninjured (remote) and injured regions of heart demonstrating that GPNMB (green, arrows) is not coexpressed with CD90.2, CD31, CD146 and NG2 markers (red, arrows) at Day 7 post infarction (Representative images, n=3 animals). b, Flow cytometry of nonmyocytes in the heart 7 days after MI demonstrating the expression of GPNMB in macrophages (CD45, Ly6C, CD68 and CD11b, n=5 animals). Data presented as mean±SEM.
Extended Data Fig. 2 Flow cytometry to demonstrate expression of RFP+ and GFP+ cells in infarcted hearts of Ccr2RFPCx3cr1GFP mice.
a, Flow cytometry gating controls on nonmyocytes isolated from wild-type mice (using unstained controls for GPNMB and gates for RFP and GFP). b, GPNMB expression in infarcted hearts of Ccr2RFPCx3cr1GFP mice on day 7 following MI demonstrating GPNMB is predominantly expressed in the GFP and RFP positive cells (62.7± 10% (GFP) and 23.5±6% (RFP), n=3 animals/group).
Extended Data Fig. 3 Global depletion of GPNMB doesn’t affect the electrical activation pattern.
a, Surface ECG of age and sex matched WT and GPNMB KO animals demonstrating similar electrical activation patterns. b, ECG intervals showing PR, QRS, QT intervals and heart rate in WT and GPNMB KO animals (mean±SEM, n=3, Two-tailed Student’s t-test, NS=Not significant).
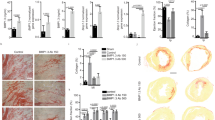
Extended Data Fig. 4 GPNMB deficiency increases infarct size but did not affect the area at risk after MI.
a, Images showing the transverse slices of Triphenyltetrazolium chloride (TTC) stained heart of wild-type and GPNMB KO mice on day 3 of myocardial injury. The red zone indicates viable heart tissue, and the white zone (arrows) represents non-viable infarct area. b, Relative % of infarct area in the hearts of WT and GPNMB KO mice on day 3 after myocardial injury (mean±SEM, n=4 animals, **p<0.0044 by two-tailed Student’s t-test). c, Representative image showing the transverse slices of hearts of WT and GPNMB KO mice stained with Evans blue at 24hr after myocardial injury. The red zone (arrow) indicates area of risk and blue zone indicates non-risk area. d, Relative % of area at risk in the hearts of WT and GPNMB KO mice. (mean±SEM, n=3 animals, Two-tailed Student’s t-test, NS=Not significant).
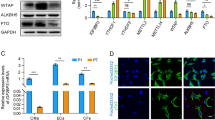
Extended Data Fig. 5 Effects of GPNMB depletion on bone marrow macrophage and peripheral immune cell composition.
a, Flow cytometry of bone marrow macrophages isolated from WT and GPNMB KO mice demonstrating the percentage of CD68, CD206, CD11b and Ly6A/E expressing cell population. b, Flow cytometry of peripheral immune cells isolated from WT and GPNMB KO animals demonstrating the percentage of CD11b, CD68, CD3 and CD19 expressing cells (mean±SEM, n=3 animals/group, * p<0.016 by Two-tailed Student's t-test, NS=Not significant).
Extended Data Fig. 6 Resident macrophage composition in hearts of WT and GPNMB KO animals.
Flow cytometry of macrophages isolated from WT and GPNMB KO hearts demonstrating the percentage of CD11b, CD68, CD206, and Ly6A/E expressing cell population. (mean±SEM, n=3 animals/group, Two-tailed Student’s t test, NS=Not significant).
Extended Data Fig. 7 Canonical genes used to identify cell population in the heart following single nuclear seq.
Dot plot demonstrating clusters of genes used to identify cardiomyocyte and nonmyocyte cell populations in snRNA-seq of the heart at 7 days after MI (n=4 animals).
Extended Data Fig. 8 GPNMB is predominantly expressed in specific subclusters of macrophages in the infarcted heart.
a, UMAP of myeloid subtype clusters in the injured murine heart on day 7 after MI. b, Cluster markers of myeloid subtype populations in the injured heart on day 7 after MI. c, UMAP showing GPNMB expression in specific subclusters (1&2). d, UMAP showing FABP5 expression in specific subclusters (1&2). e, f, UMAP showing the robust expression of GPNMB in TREM2 macrophage cluster of macrophage/monocyte atlas of healthy and pathologic tissues in humans (Mudler et al., 2021).
Extended Data Fig. 9 Genetic deletion of GPR39 did not influence cardiac function in normal conditions.
(a) Ejection fraction, (b) Fractional shortening and LV chamber size (LVID) in systole (c), and diastole (d) (Data presented as mean±SEM, n=10 animals/group, Two-tailed Student’s t-test, NS=Not significant).
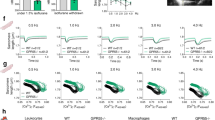
Extended Data Fig. 10 Genetic deletion of GPR39 did not affect post-MI cardiac function.
a, B-mode and M-mode echocardiogram demonstrating diminished contractile function with chamber dilatation at 4 weeks of cardiac injury in WT and GPR39 KO animals (orange arrows, diastole; blue arrows, systole). b, EF and FS and LV chamber size (LVID) in systole and diastole over 4 weeks after cardiac injury in WT and GPR39 KO animals (Baseline n=10, WT week 1 n=9, week2 n=7, Week4 n=7; GPR39 KO week 1 n=9, week2 n=9, Week4 n=7. Two-way ANOVA with Tukey’s multiple comparison). Note cardiac function at baseline is not different between WT and GPR39 KO animals. c, Masson trichrome staining demonstrating the scar in the apex and mid-ventricle (arrows). d, Quantitation of fibrotic scar area in the apex and mid-ventricle. (Data presented as mean±SEM. n=7 animals, Two-tailed Student’s t test, NS=Not significant).
Supplementary information
Supplementary Data 1
Proteomic data 1 related to Fig. 6.
Supplementary Data 2
Proteomic data 2 related to Fig. 6.
Supplementary Data 3
Proteomic data 3 related to Fig. 6.
Supplementary Table 1
Differential blood counts showing no significant difference between WT and GPNMB KO animals. Each value represents mean ± s.d., n = 5 animals.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Source Data Figs. 1c, 5a, 6h,g,h,i and 7a.
Unprocessed western blots.
Source Data Fig. 8a,f,g
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramadoss, S., Qin, J., Tao, B. et al. Bone-marrow macrophage-derived GPNMB protein binds to orphan receptor GPR39 and plays a critical role in cardiac repair. Nat Cardiovasc Res 3, 1356–1373 (2024). https://doi.org/10.1038/s44161-024-00555-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44161-024-00555-4
This article is cited by
-
Macrophage GPNMB-mediated cardiac repair
Nature Cardiovascular Research (2024)