Abstract
Combating resistance to targeted therapy remains a major challenge to improving lung cancer care. Epithelial-mesenchymal transition (EMT) in tumour cells is an established non-genetic resistance mechanism to EGFR tyrosine kinase inhibitors (TKI) that is also associated with worse outcome in patients. Here we demonstrate that integrin-linked kinase (ILK) is an important driver of EMT-mediated TKI resistance in lung adenocarcinoma (LUAD) by promoting a drug-tolerant persister (DTP) cell phenotype. Our results indicate that high ILK expression is associated with EMT in LUAD patients and that genetic suppression of ILK can limit EMT progression and reduce the viability of DTP cells by impairing YAP activation, ultimately improving osimertinib (Osi) sensitivity in LUAD cells. Importantly, LUAD cells with high ILK expression are able to persist during EGFR-TKI treatment, acquiring additional genetic and phenotypic alterations to develop EGFR-TKI resistance. To improve clinical translatability of our findings, we showed that pharmacological inhibition of ILK can suppress EMT and improve Osi response in LUAD cells. Lastly, we found that strong immunohistochemistry staining of ILK in patient biopsies was significantly associated with and may be used to predict receptor tyrosine kinase-independent mechanisms of EGFR-TKI resistance. Overall, our results suggest that ILK is an important regulator of EGFR-TKI response and may be exploited as a predictor for acquired resistance, providing evidence for co-targeting ILK with EGFR to better control minimal residual disease and EGFR-TKI resistance in lung cancer.
Similar content being viewed by others
Introduction
Lung cancer remains the most prevalent cause of cancer-related deaths worldwide [1]. In ~20% of the most commonly diagnosed lung cancer subtype, lung adenocarcinoma (LUAD), activating mutations in epidermal growth factor receptor (EGFR) drive malignant cell proliferation [2]. The development of EGFR tyrosine kinase inhibitors (TKI) represented a significant improvement in lung cancer therapies. In particular, the 3rd generation EGFR tyrosine kinase inhibitor osimertinib (Osi) selectively inhibits mutant EGFR while sparing wild-type EGFR activity [2]—granting Osi exceptional potency and tolerance, and vastly improving patient outcomes [3]. However, as with other EGFR-TKIs, tumours inevitably resist Osi treatment [4], highlighting an urgent need to develop new approaches to combat TKI resistance and improve long-term survival of lung cancer patients.
Epithelial-mesenchymal transition (EMT) is an established non-genetic mechanism of EGFR-TKI resistance in LUAD [5,6,7,8] that is also associated with increased tumour invasion, metastasis, and lethality [9, 10]. While factors driving EMT in EGFR-TKI resistance remain unclear, there is evidence that a transitory drug-tolerant-persister (DTP) state may be involved [11]. DTP cells represent small fractions of cancer cells that are able to enter a reversible state of drug tolerance [12,13,14] and survive drug treatment without acquiring resistance-conferring genetic alterations. Compared to drug-sensitive cells, these DTP cells tend to have reduced proliferation, altered metabolism, and greater phenotypic plasticity that can promote attributes that enable survival and growth in the presence of EGFR-TKI [11, 15]. Clinically, these populations of DTP cells are best represented by minimal residual disease (MRD) in patients, in which the patients’ tumours no longer regress with continuous treatment; however, upon discontinuing treatment or when the cancer cells become stably resistant, their “residual” tumours readily relapse [11, 15]. Despite our limited understanding of the mechanisms underlying DTP cell survival, there is a growing appreciation for the interactions that exist between tumour cells and the extracellular matrix (ECM) in the tumour microenvironment. These cell-ECM interactions can promote drug persistence/resistance by altering signal transduction [16]. In particular, integrin signalling has been shown to stimulate EGFR activity and accelerate lung cancer progression [17,18,19,20]; conversely, EGFR activation has also been shown to attenuate the mechanical threshold for integrin tension and focal-adhesion formation [21]. Interestingly, inhibition of focal-adhesion kinase (FAK) improves Osi response in both sensitive and resistant EGFR-mutant LUAD cells [22], and both integrin β1 and FAK have been shown to promote persister cell survival and growth of residual disease during targeted therapy in melanoma [14] and LUAD [23].
Integrin-linked kinase (ILK) is an important regulator of integrin-mediated signal transduction, linking cellular ECM adhesion to proliferation and migration [24,25,26,27]. ILK has been implicated in the pathogenesis of various cancer types, mainly through the promotion of EMT [27, 28] via an integrin β1/FAK/ILK signalling axis that activates several EMT transcription factors – notably SNAIL, SLUG and TWIST [29,30,31,32,33]. ILK is considered an attractive option for targeted therapy development as it rarely mutates,is readily druggable [34, 35], and has recently been shown to be associated with worse outcomes in patients treated with EGFR-TKIs [36]. Based on these findings, we hypothesized that ILK may be involved in the development of EGFR-TKI resistance through the development of a DTP phenotype in lung cancer cells.
Herein, we show that high ILK expression is associated with EMT in EGFR-driven LUAD. Through genetic approaches, we have found that ILK drives EGFR-TKI resistance through regulation of DTP cell survival. Suppression of ILK sensitizes LUAD cells to TKI treatment by depleting DTP cells, which is potentiated when cells grow in ECM-rich microenvironments. Additionally, we found that the cytoprotective role of ILK is mediated by YAP activation during EGFR-TKI treatment, and that surviving DTP cells with high ILK expression may undergo EMT during the progression towards EGFR-TKI resistance. Lastly, the results of our study demonstrate the feasibility of combining EGFR and ILK inhibition to circumvent EMT-mediated TKI resistance.
Material and methods
Reagents
See Supplementary Table 1 for the list of reagents used.
Cell culture
Cells were cultured at 37 °C with 5% CO2 in a humidified atmosphere. HCC4006, NCI-H1975, PC9 and HCC4011 cells were cultured in RPMI 1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% or 5% fetal bovine serum (FBS; Thermo Fisher Scientific) and 1% Pen/Strep (Thermo Fisher Scientific). HEK293T cells were cultured in Dulbecco’s minimal essential medium (DMEM) supplemented with 10% FBS and 1% Pen/Strep. Cells were passaged with 0.25% Trypsin-EDTA (Thermo Fisher Scientific) once they reached ~80% confluency. Mycoplasma was tested monthly by PCR [37]. All cell lines used were authenticated in June 2022.
EMT Scoring, Gene set enrichment analysis, and heatmap
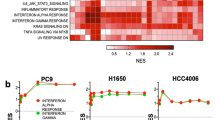
TCGA PanCan Atlas and GSE31210 datasets were filtered for LUAD patient samples with available gene expression data. TCGA PanCan Atlas and GSE31210 datasets were stratified and categorized into tertiles based on ILK expression level. EMT score for each sample was generated based on the gene list described in Tan et al. [38]. In brief, EMT Score was calculated by summing z-score of each gene in the EMT gene list multiplied by the weight of the EMT gene. Mesenchymal genes were given positive weights while epithelial genes were given negative weights. Gene Set Enrichment Analysis (GSEA) [39] was performed with the Hallmarks gene sets comparing high ILK-expressing tumour samples to low ILK-expressing tumour samples; genes were ranked on t-test P-value. GSEA comparing Osi-resistant and parental HCC4006 cells was performed with the Hallmark and Oncogenic Signatures gene sets with log2 ratio as the metric to rank genes. The DepMap cell line database was filtered for LUAD cell lines with activating EGFR mutations. Heatmaps were constructed and hierarchal clustering was performed using Morpheus (https://software.broadinstitute.org/morpheus). Genes used to construct the heatmap were the core enrichment genes from GSEA analysis of both TCGA PanCan Atlas and GSE31210 datasets in the Hallmark Epithelial-Mesenchymal Transition gene set.
Generation of Osi-resistant lines
H1975, PC9, HCC4011, and HCC4006 cells were cultured in escalating doses of Osi starting at 10 nM until they were capable of growing in 1 µM Osi (i.e. first concentration = 10 nM, then 30, 100, 300 nM, and finally 1 µM), at which point they were considered Osi-resistant. Media containing Osi was changed every 3–4 days and cells were passaged once they reached ~80% confluency with a higher dose of Osi supplemented in the media at each passage.
Fluorescent and confocal imaging
HCC4006 cells were cultured on coverslips prior to fixation with 10% phosphate-buffered formalin (Fisher Scientific, Hampton, NH, USA). The cells were permeabilized with 0.1% TRITON-X-100 (Fisher Scientific) in D-PBS (Thermo Fisher Scientific) and blocked with 4% FBS in D-PBS. The cells were subsequently stained with appropriate primary and secondary antibodies with 0.2 µg/mL DAPI. Coverslips were mounted on glass microscope slides with VECTASHIELD Antifade Mounting Medium (Newark, CA, USA). Confocal imaging was conducted using the Nikon A1-si Confocal & TIRF Microscope (objective lenses used: 20X/0.75NA and 40X/0.95NA).
RNA sequencing
RNA from parental and Osi-resistant HCC4006 cells was extracted using the QIAGEN RNeasy Mini kit (Hilden, Germany) following the manufacturer’s protocol. RNA sequencing was performed by Genewiz Azenta Life Sciences (Waltham, MA, USA). Salmon [40] was used to convert raw sequencing reads into quantifiable gene abundance. Resultant TPM values were used for GSEA. Differential gene expression analysis was performed with limma [41] and subsequent gene ontology analysis was performed with the PANTHER Overrepresentation Test [42] on the significantly upregulated and downregulated genes.
RT-qPCR
RNA was extracted using the QIAGEN RNeasy Mini kit (Hilden, Germany) following the manufacturer’s protocol. cDNA was synthesized using high-capacity RNA-to-cDNA kit (Applied Biosystems) for subsequent qPCR reactions with PowerUp SYBR Green Master Mix (Applied Biosystems) on the QuantStudio 5 real-time PCR system. See Supplementary Table 2 for the list of primers used.
MSK-IMPACT sequencing
Genomic DNA from parental and Osi-resistant HCC4006 cells were extracted using the QIAGEN DNeasy Blood and Tissue kit (Hilden, Germany). Genomic alterations were profiled using the MSK-IMPACT platform as previously described [43].
Western blot
Western blots were performed as described previously [44]. Briefly, cells were lysed in RIPA Lysis and Extraction Buffer (G-Biosciences, St. Louis, MO, USA) containing Halt protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific). Protein concentration was determined using a Pierce BCA protein assay kit (Thermo Fisher Scientific). Approximately 20 μg of lysates were denatured in NuPAGE LDS Sample Buffer (Thermo Fisher Scientific) and loaded on 4–12% Bis-Tris Gels (Thermo Fisher Scientific). After electrophoretic separation, the proteins were transferred onto PVDF membranes (Millipore Sigma, Billerica, MA, USA). The proteins of interest were detected using appropriate antibodies. Nuclear and cytoplasmic fractions were separated using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific) following the manufacturer’s protocol.
Plasmids and generation of stable cell lines
pLenti CMV ILK Blast was cloned from ILK cDNA from pcDNA3.1-ILK and pLenti CMV Empty Blast backbone plasmids by Gateway Cloning, using pCR8/GW/TOPO TA Cloning Kit and Gateway LR Clonase II Enzyme Mix (Thermo Fisher Scientific). pInducer20-SRC was cloned from SRC cDNA in pDONR223-SRC (a gift from William Hahn & David Root; Addgene plasmid # 23934 and pInducer20 (a gift from Stephen Elledge; Addgene plasmid # 44012) using pENTR/D-TOPO Cloning Kit and and Gateway LR Clonase II Enzyme Mix (Thermo Fisher Scientific). HEK293T cells co-transfected with pLenti CMV ILK Blast, pTRIPZ shNS (a gift from Sandra Demaria; Addgene plasmid # 127696), pTRIPz shILK B09, pLenti CMV GFP Blast (a gift from Eric Campeau & Paul Kaufman; Addgene plasmid # 17445), EF1a_mCherry_P2A_Hygro (a gift from Prashant Mali; Addgene plasmid # 135003), pInducer20-SRC, or pInducer20-GFP plasmids (as described previously [45]) together with pMD2.G, and psPAX2 plasmid constructs (pMD2.G and psPAX2 were gifts from Didier Trono; Addgene plasmid #12259 and #12260, respectively) using Lipofectamine 2000 (Thermo Fisher Scientific). After overnight incubation, the supernatant carrying the virus was filtered (0.45 µm) to remove cell debris and 8 µg/mL of Polybrene (Hexadimethrine bromide, Millipore Sigma) was subsequently added. The mixture was then added to the target cells. The plates and the media containing virus were spun down at 1200 × g for 60 min at 32 °C to aid with transduction efficiency. The cells were incubated overnight and then selected with 10 µg/mL of Blasticidin S (Thermo Fisher Scientific) for 5 days, 2 µg/mL of Puromycin (Millipore Sigma) for 5 days, or 800 µg/mL G418 (Thermo Fisher Scientific) for 14 days.
Matrigel-coated plates
Matrigel was diluted to 0.27 mg/mL in ice-cold RPMI 1640 media and added to cell culture plates, entirely covering the bottom of the plates. The plates were then incubated at 37 °C for 1 h to allow the Matrigel to coat the plate. The residual diluted Matrigel media were removed prior to seeding cells.
Cell viability assay and clonogenics
HCC4006 cells were pre-treated with 100 ng/mL of doxycycline (DOX) for 3 days prior to being seeded in 96-well plates and then incubated overnight. The cells were then treated with Osi (serial dilutions of 1/2 starting at 10 µM) for 3 days. Cell viability was determined by alamarBlue (Thermo Fisher Scientific) and fluorescent intensities (Ex/Em: 560/590 nm) were measured for each well using the Cytation 3 Multi Modal Reader with Gen5 software (BioTek Instruments, Inc, Winooski, VT, USA).
For clonogenic and persister cell assays, PC9, H1975 and HCC4006 cells were seeded with or without 100 ng/mL DOX at very low density in 6-well plates (100–150 cells for clonogenics and 200-300 cells for persister cell assay). After 3 days, the cells were treated with Osi for 7 days. For clonogenic assays, cells were fixed with 10% buffered formalin and 50% ice-cold methanol prior to staining with 0.5% crystal violet solution. For persister cell assays, the media supplemented with Osi was replaced with media without Osi and the persister cells were allowed to grow for 3–4 days. Subsequently, the cells were fixed and stained similarly to clonogenic assays. Media (with or without DOX/Osi) in both clonogenic and persister cell assays were changed every 3–4 days. Dried clonogenic plates were scanned using Epson Perfection V550 Photo Scanner. Colonies in the scanned images of the CV-stained plates were counted using FIJI/ImageJ [46]. Briefly, colonies on the plate were identified using the “Color Threshold” and “Watershed” commands. Identified particles were subsequently counted using the “Analyze Particles…” function.
IncuCyte growth assay
HCC4006 cells were seeded at 1000 cells per well in a 96-well plate and placed into the IncuCyte SX5 (Sartorius, Germany). Confluency was measured by phase-contrast microscopy at 4 different locations in each well every 4 hours until experimental endpoint.
Co-culture competition assay
HCC4006 pInducer20-GFP and pTRIPZ shNS/shILK cells were pre-treated with 100 ng/mL of DOX for 3 days to induce expression of their respective fluorescent proteins prior to being mixed together. A portion of the mixed cells was run on a BD LSR Fortessa Cell Analyzer to determine the baseline RFP:GFP proportional ratio. Meanwhile, another portion of the mixture was seeded and cultured overnight. These mixed cells were subsequently treated with either 100 nM Osi or DMSO control for 7 days before being run on the BD LSR Fortessa Cell Analyzer to determine RFP:GFP ratio. Similar methods were used for competition involving PC9 GFP cells and ILK overexpressing PC9 mCherry cells without the addition of DOX.
Image analysis and quantification of YAP staining
Confocal images were analyzed using FIJI/ImageJ. In each image, the DAPI signal was binarized to obtain the “nucleus mask”. Non-stringent threshold values were set to binarize the YAP signal to obtain the “cell mask”. The “Image Calculator” function was used to generate the “cytosol mask” by subtracting the “nucleus mask” from the “cell mask”. The “nucleus” and “cytosol” masks were then overlaid on top of the raw YAP signal to determine the nuclear/cytosolic YAP fluorescent intensity ratio. RFP signal was binarized to differentiate RFPhigh and RFPlow cells. Intensity profile plots were generated using the “Plot Profile” function in FIJI.
Mouse tumour xenografts
All animals used were bred and housed under specific pathogen-free conditions in the Animal Resource Centre at the BC Cancer Research Institute. 1 × 106 HCC4006 cells harbouring either DOX-inducible control shRNA or ILK-targeting shRNA constructs were re-suspended in serum-free RPMI 1640 medium and implanted into the dorsal flanks of immune incompetent male NRG mice 9–12 weeks of age. Upon palpable tumour formation 14 days following the implantation, half of the mice were transferred to a DOX diet to induce ILK knockdown. After the tumours grew to ~100-200 mm3, the mice were administered either 15 mg/kg Osi or vehicle control (6% DMSO and 30% PEG300 in water) by oral gavage three times a week. Upon reaching ethical tumour morbidity constraints, tumours were harvested for subsequent analyses.
Validation of knockdown by RT-qPCR
Tissue from isolated tumours was placed in RLT buffer (from the QIAGEN RNeasy kit) supplemented with 1% 2-mercaptoethanol (Thermo Fisher Scientific). A 1 mL syringe with 18 G needle was used to help dissociate the tissue. Subsequently, RNA was isolated using the RNeasy kit in accordance with the manufacturer’s protocol. cDNA was synthesized using high-capacity RNA-to-cDNA kit (Applied Biosystems) for subsequent qPCR reactions with PowerUp SYBR Green Master Mix (Applied Biosystems) on the ViiA 7 Real-Time PCR System (Applied Biosystems). Human specific primers were designed to only amplify human ILK and the housekeeping gene ACTB. Primer sequences used: ILK Forward—GGGGGAGAAGCCATGATCG; ILK Reverse—TCCTTCCCTGGATCACTCCAC; ACTB Forward—CCGCCGAGACCGCGT; ACTB Forward—TCATCATCCATGGTGAGCTGG.
ILK immunohistochemistry
Immunohistochemical (IHC) stains were performed on formalin-fixed paraffin-embedded (FFPE) tissues from clinical samples. Anti-ILK1 clone 4G9 (Cell Signaling Technology Inc., Danvers, MA, USA) was implemented using the Leica bond platform (antigen retrieval ER2 30′, primary antibody incubation 30’, detection: DAB retrieval kit). ILK immunoreactivity was validated using multi-tissue sections, with skeletal muscle as positive control and hepatocytes as negative control. ILK tumour expression was scored by a certified pathologist (CAFA) as positive or negative based on any level of cytoplasmic labelling. H-scores [47] were calculated by summing the product of multiplying the percentage of positive tumour cells (0–100%) by staining intensity (0 = no staining, 1+: weak, 2+: moderate, 3+: strong), yielding a range of H-scores from 0 to 300. Tissue samples were collected under MSKCC IRB-approved biospecimen collection protocols, and informed consent was obtained. Genomic alterations were profiled using the MSK-IMPACT platform as previously described [43].
Statistical analysis
All statistical analyses were performed using GraphPad Prism, version 9 (GraphPad Software, San Diego, CA, USA) and R, version 4.1.0. The specific statistical tests used and the number of independent replicates that were performed are described in the figure legends. P-values less than 0.05 were considered statistically significant. The number of experimental replicates were based on the observed effect sizes and variances in pilot experiments in order to achieve a reasonable statistical power.
Results
High ILK expression is associated with an EMT gene signature in EGFR-mutant LUAD
We first examined ILK expression in tumour samples from patients diagnosed with EGFR-driven LUAD. Performing immunohistochemistry (IHC) staining on pre-therapy LUAD tumours (18 samples from 10 patients) revealed that all EGFR-mutant tumours stained positively for ILK (Fig. 1a), with varying expression among samples (median H-score of 150; ranging from 100-300) (Fig. 1a, b; see Supplementary Fig. S1a for negative staining control). As ILK has been demonstrated to drive EMT in many cancer types [29, 31, 48,49,50], we aimed to determine whether ILK expression is associated with EMT in EGFR-driven LUAD. Using a comprehensive EMT Scoring system gene list [38], we derived an EMT Score for LUAD patient samples in two publicly available databases: TCGA PanCan Atlas LUAD and GSE31210. We binned the patient samples into tertiles based on their ILK expression and found that high ILK-expressing tumour samples had significantly higher EMT Scores compared to low ILK-expressing samples in both datasets (Fig. 1c, d). As an orthogonal approach, we also performed Gene Set Enrichment Analysis (GSEA) on these two clinical datasets and demonstrated that there is a significant positive correlation between high ILK expression and an EMT gene signature enrichment (Fig. 1e, f), with EMT being the top result in both datasets. However, bulk patient tumour samples also contain non-cancer mesenchymal cell populations [51]. To confirm that this correlation was, at least in part, intrinsic to the cancer cells, the DepMap cell line database was used to perform hierarchal clustering based on the expression of EMT core enrichment genes found in GSEA. The resultant heatmap (Fig. 1g) demonstrated that LUAD cell lines harbouring activating EGFR mutations with high ILK expression clustered separately from cell lines with low ILK expression, indicating that the association between ILK and EMT can be observed in tumour cell lines independent from stromal cells found in lung tumours.
a IHC staining of ILK in EGFR-mutant LUAD patient tumours. Representative images of staining scores 1, 2, or 3 are shown. Black scale bar = 100 µm. b Summary of ILK staining in pre-treated EGFR-mutant LUAD tumours (n = 18 from 10 patients). c, d Comparison of EMT Score between high-expressing vs low-expressing ILK EGFR-mutant LUAD patient tumour samples in c TCGA PanCan Atlas LUAD (n = 24 each) and d Japanese LUAD microarray cohort (n = 42 each; GSE31210). Wilcoxon rank sum Test were used to statistically compare high vs low ILK samples. e, f Enrichment plots of the top Hallmark gene set from GSEA comparing high to low ILK-expressing EGFR Mut LUAD tumours from indicated datasets. g Heatmap of DepMap EGFR Mut LUAD cell lines indicating expression of core enrichment genes from the EMT gene set of patient tumours in (e, f).
ILK is associated with EMT-mediated Osi resistance
As EMT can mediate EGFR-TKI resistance in LUAD [5,6,7,8], we asked if ILK has a role in EMT-mediated Osi resistance. We first generated multiple osimertinib-resistant (OsiR) EGFR-mutant LUAD cell lines (PC9, H1975, HCC4011, and HCC4006) (Supplementary Fig. S1b). MSK-IMPACT sequencing of these parental-resistant pairs revealed that H1975, PC9, and HCC4011 had acquired genetic alterations that reactivated the RTK signalling pathways (i.e. bypass signalling). Only HCC4006 was found to have not acquired any genetic alterations that reactivated the RTK signalling pathways (Supplementary Fig. S1c). HCC4006 cells demonstrated a more mesenchymal-like phenotype in the drug-resistant state relative to its parental (Par) epithelial-like counterpart. These cells notably lost expression of the epithelial marker ECAD while upregulating expression of mesenchymal markers NCAD and vimentin (Fig. 2a). We also found upregulation of the pro-EMT transcription factor SNAIL with decreased expression of SLUG (Supplementary Fig. S1e). Confocal imaging of HCC4006 OsiR cells supported the western blot results and demonstrated a loss of membrane ECAD and substantially higher levels of vimentin staining (Fig. 2b). These findings suggest EMT as a potential mechanism by which HCC4006 cells became EGFR-TKI resistant. Interestingly, the HCC4006 cell line also had the highest basal protein levels of ILK (Fig. 2a), potentially linking ILK with EMT-associated Osi resistance.
a Western blot of ILK and EMT marker levels in parental (Par) and osimertinib (Osi) resistant EGFR-mutant cell lines. b Immunofluorescence images of HCC4006 Par and OsiR cells. c Enrichment plot of the top enriched Hallmark gene set from GSEA comparing HCC4006 OsiR (n = 2 experimental replicates) to Par (n = 2 experimental replicates) cells. d Volcano plot of significant differentially expressed genes. Genes were considered differentially expressed if adjusted P-value (by the Benjamini-Hochberg method) was less than 0.05, which corresponds to a raw P-value of ~0.008. e RT-qPCR validation of representative epithelial, mesenchymal and EMT transcription factor genes (n = 3 each). Student’s t-test were used to statistically compare Par vs OsiR (*p < 0.05; **p < 0.01; ***p < 0.001). Bar graphs with error bars represent the mean ± SEM. f Top 6 downregulated and upregulated biological processes from Gene Ontology analysis (PANTHER Over Representation test) of differentially expressed genes in HCC4006 OsiR versus Par cells. Bolded are known biological processes that ILK regulates. Osi was maintained at 1 µM in OsiR cells in these experiments.
Next, we performed RNA-sequencing on HCC4006 OsiR and HCC4006 Par cells. In agreement with the findings above, GSEA showed that an EMT gene signature was significantly enriched in HCC4006 OsiR cells (Fig. 2c) and several EMT-associated genes were differentially expressed in HCC4006 OsiR cells compared to parental cells (Fig. 2d). Upon closer inspection of the canonical markers of EMT, we saw an overall decrease in genes associated with epithelial differentiation (KRT19, MUC1, CDH1, and EPCAM), an overall increase in genes associated with a mesenchymal-like state (VIM, MMP3, and FAP), and a marked increase in canonical EMT transcription factor genes (SNAI1, TWIST1, ZEB1) (Fig. 2e). Using Gene Ontology analysis on significantly differentially expressed genes, we found that genes involved with cell-cell adhesion were downregulated while genes involved with cell-matrix adhesion and EMT were upregulated in OsiR cells (Fig. 2f).
HCC4006 OsiR cells did not exhibit re-activation of EGFR—a potential TKI resistance mechanism - as shown by a lack of phosphorylated-EGFR (P-EGFR) in the OsiR cells (Supplementary Fig. S1d). HCC4006 OsiR cells also did not have altered levels of MAPK signalling downstream of EGFR as indicated by phosphorylated-ERK1/2 (P-ERK1/2) (Supplementary Fig. S1d) and increased PI3K signalling – namely phosphorylated-mTOR (P-mTOR), phosphorylated-RICTOR (P-RICTOR), and phosphorylated-AKT (P-AKT) (Supplementary Fig. S1d). We also found OsiR cells had increased focal-adhesion signalling indicated by phosphorylated-FAK (P-FAK) (Supplementary Fig. S1f). Taken together, these results suggest that the mechanism underlying Osi resistance in HCC4006 cells involves changes in two biological processes that ILK is known to regulate, namely EMT and focal-adhesion signalling.
ILK knockdown in treatment-naïve HCC4006 increases Osi sensitivity
To establish whether the associations between ILK, EMT, and Osi resistance are causative, we employed pTRIPZ doxycycline (DOX) inducible shILK and shNS control constructs (Fig. 3a) that enable conditional knockdown of ILK in HCC4006 cells. The addition of DOX significantly lowered the protein level of ILK and also reduced expression of the ILK binding partnerα-Parvin in cells treated with DMSO or Osi (Fig. 3b). PINCH was not observed to change with ILK knockdown in HCC4006 cells treated with DMSO. However, upon treatment with Osi (10 and 50 nM) PINCH was elevated. This elevation in PINCH was suppressed by DOX, suggesting that the ILK-PINCH-Parvin (IPP) complex necessary for ILK’s downstream functions [25, 27] became more stable following Osi treatment and that ILK knockdown destabilized the formation of IPP complex.
a Schematic of pTRIPZ shRNA constructs. b Validation of knockdown in HCC4006 shILK cells; western blot of proteins in the IPP complex in HCC4006. c Three day Osi dose-response in HCC4006 shILK (n = 3) and shNS (n = 3) ± DOX. Multiple t-tests with Holm-Sidak correction were used to statistically compare DOX vs No DOX at each Osi concentration (*p < 0.05). d Clonogenic assay of HCC4006 shILK (n = 10) and shNS (n = 9) cells treated with Osi for 7 days ± DOX. Quantification of colony count is below the representative plates. Multiple paired t-tests with Holm-Sidak correction were used to statistically compare DOX vs No DOX at each Osi concentration (*p < 0.05; **p < 0.01). Error bars represent the mean ± SEM. DOX concentration = 100 ng/mL.
ILK knockdown increased HCC4006 cell sensitivity to acute (3 day) Osi treatment across concentrations from 50 nM to 1 µM (Fig. 3c). Next, we assessed the role of ILK during a longer-term (7 day) Osi treatment (10 nM and 100 nM) by quantifying the effect of ILK knockdown on the colony-forming potential in HCC4006 cells. We found that both DOX (i.e. ILK knockdown) and Osi treatment alone suppressed the number of colonies formed (Fig. 3d), but greater suppression was observed with ILK knockdown combined with Osi treatment. Moreover, acute treatment of Osi increased expression of EMT markers NCAD, vimentin, SNAIL, and SLUG in HCC4006 cells while ILK knockdown suppressed NCAD and SNAIL during Osi treatment (Supplementary Fig. S2a), suggesting that ILK may have a role in Osi-mediated EMT in HCC4006 cells. Together, these results suggest that ILK expression plays a role in promoting Osi insensitivity in LUAD cells.
ILK-driven Osi insensitivity is independent of EGFR/ERK/AKT re-activation
In an effort to understand the mechanisms by which ILK affects Osi resistance, we first examined whether ILK reactivates EGFR or the MAPK signalling pathway, as these are common mechanisms of Osi insensitivity/resistance [4]. As expected, we found that acute treatment (3 days) with 10 nM and 50 nM Osi lowered P-EGFR levels in a dose-dependent manner and this translated into a decrease in phosphorylated ERK1/2 (P-ERK1/2), particularly in cells treated with a higher Osi concentration (50 nM Osi) (Supplementary Fig. S3). However, ILK knockdown did not affect P-EGFR or P-ERK1/2 after Osi treatment (Supplementary Fig. S3), indicating that the mechanism by which ILK drives Osi insensitivity is independent of EGFR or MAPK signalling. Next, we examined the PI3K signalling pathway, which is regulated by EGFR and ILK, and is also increased in HCC4006 OsiR cells (Supplementary Fig. S1d). At 10 nM Osi, but not 50 nM Osi, P-AKT was induced in a manner that was suppressed by ILK knockdown (Supplementary Fig. S3), perhaps due to compensatory increase in ILK activity that leads to increased AKT signalling following 10 nM Osi treatment. However, with 50 nM Osi treatment, mutant EGFR and downstream AKT signalling was suppressed beyond rescuable effect by ILK. Phosphorylated mTOR (P-mTOR) was not affected by Osi treatment, while P-RICTOR was upregulated in response to Osi. RICTOR cooperates with ILK to signal downstream to AKT [30], although P-RICTOR was only slightly reduced by ILK knockdown (Supplementary Fig. S3). We, therefore, conclude that the influence of ILK on Osi response cannot be explained by the re-activation of PI3K signalling via mTOR or RICTOR.
ILK knockdown in HCC4006 OsiR cells does not re-sensitize cells to Osi
To determine if ILK suppression can be used to directly combat EGFR-TKI resistance in OsiR cells, we first tested whether HCC4006 OsiR cells depend on ILK as a survival mechanism. However, we found that HCC4006 OsiR cells were significantly more resistant to ILK inhibition (QLT0267) than parental HCC4006 cells (Supplementary Fig. S4a). Next, we asked if ILK may be important to maintain Osi resistance in HCC4006 OsiR cells. DOX-inducible ILK knockdown could not re-sensitize HCC4006 OsiR cells to acute/longer-term Osi treatment (Supplementary Fig. S4b, c). Taken together, our results suggest that ILK is important for survival of treatment naïve HC4006 parental cells, but HCC4006 cells that have further evolved to become Osi-resistant do not require ILK to maintain resistance.
To better understand the ILK-independent survival of HCC4006 OsiR cells, we re-examined MSK-IMPACT sequencing results to determine if OsiR cells had acquired mutations that may maintain Osi resistance. We found that HCC4006 OsiR cells had acquired several mutations of particular interest in comparison to the parental HCC4006 cells (Supplementary Fig. S1c). HCC4006 OsiR cells had acquired point mutations in ERG, SMARCA4, DNMT3B, and KMT2D, which are all genes that have been previously implicated in EMT [52,53,54,55]. Since the OsiR cells show evidence of undergoing EMT (Fig. 2), these mutation data may help to explain why HCC4006 OsiR cells are independent of ILK for survival after the development of Osi resistance.
ILK drives EMT-mediated Osi resistance by promoting DTP survival in HCC4006 cells
ILK protects HCC4006 cells against Osi treatment, but is dispensable for the survival of Osi-resistant cells. ILK has been shown to drive EMT [25, 27, 30, 31, 33, 48, 56], and OsiR cells displayed a more mesenchymal phenotype than parental cells. We, therefore, hypothesized that ILK is important for the transition of HCC4006 cells towards EMT-mediated Osi resistance. To test this hypothesis, we generated Osi-resistant cells from HCC4006 shNS/shILK in the presence or absence of DOX-inducible ILK knockdown. In HCC4006 cells with intact ILK expression (i.e., shNS control cells or shILK cells without DOX), Osi resistance was associated with increased mesenchymal marker expression (NCAD, vimentin, and SNAIL) along with a loss of ECAD expression (Fig. 4a). Importantly, we also observed up-regulation of ILK in these OsiR lines compared to parental HCC4006 shNS/shILK cells. HCC4006 cells that became Osi resistant in the presence of ILK knockdown had reduced expression of NCAD and SNAIL, although the levels of ECAD and vimentin were unchanged with ILK knockdown (Fig. 4a). These data indicate that ILK mediates mesenchymal transition during the progression of HCC4006 cells towards EMT-driven Osi resistance.
a Western blot of EMT markers in HCC4006 shNS, and shILK cells ± DOX Par and OsiR. b Western blot of ILK and EMT markers following treatment with DMSO or 100 nM Osi for 7 days and subsequent removal of Osi. c Schematic of persister cell assay. d Persister clonogenic assay of HCC4006 shILK (n = 6) and shNS (n = 6) cells treated with Osi for 7 days ± DOX. Quantification of colony count is below the representative plates. Multiple paired t-tests with Holm-Sidak correction were used to statistically compare DOX vs No DOX at each Osi concentration (*p < 0.05; **p < 0.01). Error bars represent the mean ± SEM. DOX concentration = 100 ng/mL.
Non-genetic mechanisms of drug resistance, such as EMT, often arise from a transitory DTP state prior to acquiring the genetic/phenotypic changes necessary to support growth in the presence of the drug [11, 15]. Therefore, we rationalized that ILK may be promoting the survival of DTP cells during Osi treatment. In order to test this, we treated HCC4006 cells with 100 nM Osi for 7 days, leaving only the DTP cells. In comparison with HCC4006 cells treated with DMSO, Osi DTP cells had elevated levels of ILK. This increase in ILK expression is correlated with an upregulation of EMT markers, namely NCAD, vimentin, and SNAIL (Fig. 4b). As the DTP state is transitory and reversible, we then tested if the increase in ILK and EMT marker expression would regress following Osi removal. In accordance with our hypothesis, ILK and EMT markers (NCAD and SNAIL) decreased in expression following Osi removal (Fig. 4b).
To study the role of ILK on DTP cell survival, we performed persister cell assays (Fig. 4c), a modified clonogenic survival assay, by first treating HCC4006 cells with Osi at IC75-95 concentrations (100 nM, 300 nM, and 1 µM) for 7 days. Due to the low abundance of DTP cells and their inability to proliferate sufficiently to form visible colonies in the presence of drug, Osi was removed from media at the end of the treatment period (i.e. 7 days), allowing these DTP colonies to proliferate to a quantifiable size. The resultant colonies were stained with crystal violet and counted. We observed that DOX pre-treatment in HCC4006 shILK, but not shNS, cells significantly decreased the DTP colony count (Fig. 4c), suggesting that ILK promotes the survival of these Osi DTP cells.
We then asked if ILK suppression can be exploited to counter Osi tolerance once the DTP state has already been established. Acute treatment (3 days) of Osi revealed that HCC4006 DTP cells were less sensitive than their parental counterpart (Fig. S5a). DOX-inducible knockdown of ILK in HCC4006 DTP cells was unable to re-sensitize these cells to Osi (Fig. S5a), suggesting that ILK no longer affects survival after the formation of the DTP state. Next, we also asked whether ILK knockdown during Osi treatment may affect the recurrence rate of HCC4006 DTP cells. To answer this, we analyzed the growth kinetics of HCC4006 Osi DTP cells made in the presence or absence of ILK knockdown following Osi removal. ILK knockdown throughout the Osi treatment period did not affect the growth rate of HCC4006 DTP cells following Osi removal (Fig. S5b), suggesting ILK knockdown does not affect rate of DTP regrowth in HCC4006 cells. Taken all together, our findings suggest that ILK is primarily important for the depth of response to Osi treatment in LUAD cells.
YAP activation underlies the cytoprotective effects of ILK
YAP activation has recently been shown to be important for DTP survival following Osi treatment in EGFR-mutant LUAD [57,58,59] and YAP has been previously demonstrated to subsequently drive EMT transcriptional programming [59]. ILK has also been shown to be an important integrin signalling mediator for YAP activation [60, 61]. Therefore, we next asked whether ILK activates YAP in DTP cells during Osi treatment. To first explore this possibility, we compared YAP expression levels and transcriptional activity in HCC4006 OsiR cells to its parental counterpart from our RNA-seq results. We found that HCC4006 OsiR cells had higher expression of YAP1 (Fig. 5a) and were significantly enriched for YAP transcriptional signatures (Fig. 5b). We validated these results at the protein level by Western blot and found elevated levels of YAP protein in OsiR cells (Fig. 5c). To demonstrate ILK’s role in YAP activation, we performed immunofluorescent confocal imaging for YAP subcellular localization (Supplementary Fig. S6a); as YAP becomes activated, it forms the YAP/TAZ-TEAD complex that translocates into the nucleus and drives YAP-associated transcriptional activity [62]. Our results showed that the basal, untreated HCC4006 cells, with or without ILK knockdown, did not have strong YAP activation, as indicated by the overall weak nuclear/cytoplasmic YAP staining (Fig. 5d–f). Treatment of these HCC4006 cells with 100 nM Osi for 3 days with or without ILK knockdown significantly increased nuclear/cytoplasmic YAP intensity (Fig. 5d–f), suggesting elevated YAP activity was important for survival against EGFR inhibition. ILK knockdown in both DMSO and Osi-treated HCC4006 cells, significantly decreased nuclear/cytoplasmic YAP intensity, indicating the existence of an ILK-dependent mechanism for YAP activation (Fig. 5d–f). Importantly, we noticed that HCC4006 shILK cells treated with DOX had variable levels of RFP expression (proportional to the shRNA levels as represented in Fig. 3a), indicating potential heterogeneity in the level of ILK knockdown across the assayed cellular population. To better delineate the effects of ILK knockdown on YAP activity, we quantified YAP fluorescent intensity specifically in RFPlow (relatively little to no knockdown) and RFPhigh (relatively greater knockdown) HCC4006 shILK cells after DOX treatment (Supplementary Fig. S6b). We found that nuclear to cytoplasmic YAP intensity ratio was significantly elevated in RFPlow cells upon Osi treatment (Fig. 5g, h); however, Osi treatment did not increase YAP activity in RFPhigh cells. Moreover, YAP activity in Osi treated cells was significantly decreased in RFPhigh cells in comparison to RFPlow cells (Fig. 5g, h), further demonstrating the role of ILK in YAP activation in response to Osi treatment. As an orthogonal approach, we also examined levels of phosphorylated YAP (P-YAP), a marker of inactivated YAP, in the nuclear and cytoplasmic fractions of HCC4006 shILK/NS cells by Western blot. We found that cytoplasmic P-YAP was decreased and nuclear YAP was elevated in Osi-treated (100 nM) cells (Fig. S7a). ILK knockdown elevated cytoplasmic P-YAP in both DMSO and Osi-treated cells (Fig. S7a). Lastly, to determine if ILK’s effects on YAP activity translated to a change in the transcriptional activity of YAP-TEAD, we performed RT-qPCR for CCN2 (also known as CTGF), an established target of YAP-TEAD transcription [63], on Osi-treated (100 nM) HCC4006 shILK cells. We found that Osi massively increased the expression of CCN2, while ILK knockdown suppressed CCN2 expression in both DMSO and Osi-treated cells (Fig. S7c).
a YAP1 expression in HCC4006 Par and OsiR cells (n = 2 experimental replicates each). b Enrichment plot of the YAP expression signature from the Oncogenic Signature gene sets from GSEA comparing HCC4006 OsiR to Par cells. c Western blot of YAP, P-SFK and SRC in HCC4006 Par and OsiR cells. d Representative immunofluorescent confocal imaging of Osi/DMSO-treated HCC4006 shILK cells ± DOX. e Insets of example HCC4006 cells treated with DMSO or Osi. YAP intensity profile across the white line is plotted on the right. f Quantification of nuclear/cytoplasmic YAP intensity in Osi (100 nM) treated HCC4006 shILK (n = 12–14 images in each condition across 3 experimental replicates) and shNS (n = 12 images in each condition across 3 experimental replicates) cells. g Insets of 2 example Osi treated HCC4006 shILK+DOX cells with high and low RFP expression neighbouring each other. YAP intensity profile across the white line is plotted on the right. h Quantification of nuclear/cytoplasmic YAP intensity in Osi/DMSO-treated HCC4006 shILK cells +DOX (n = 12–14 images in each condition across 3 experimental replicates). i Representative western blot of P-SFK and SRC in Osi-treated HCC4006 shILK cells ± DOX. j Quantification of nuclear/cytoplasmic YAP intensity in Osi (50 nM) treated HCC4006 cells ± dasatinib (30 nM DASA) (n = 12 images in each condition across 3 experimental replicates). Statistical analyses were performed with One-way ANOVA with Sidak post-hoc test (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001). k Validation of DOX-inducible SRC overexpression HCC4006 shILK p20-GFP/SRC cells ± DOX. l Quantification of GFP or SRC rescue in Osi-treated HCC4006 shILK DTP cells (n = 3 each). Colony counts were normalized to the No DOX condition for each Osi concentration. Multiple paired t-tests with Holm-Sidak correction were used to statistically compare DOX vs No DOX at each Osi concentration (*p < 0.05; **p < 0.01). Error bars represent the mean ± SEM. DOX concentration = 100 ng/mL.
Lastly, we further assessed the importance of YAP activation on DTP survival through pharmacological inhibition of YAP/TAZ-TEAD (1µM K-975). K-975 (24 h treatment) was sufficiently able to significantly decrease the expression of YAP/TAZ-TEAD target genes CCN2 and IGFBP3 [64] (Fig. S7c). K-975, similar to ILK knockdown, significantly reduced colony formation (10 nM and 100 nM Osi) (Supplementary Fig. S8a) and DTP cell viability (100 nM, 300 nM, and 1 µM Osi) (Supplementary Fig. S8c) in HCC4006 cells treated with Osi. Taken together, our results demonstrate that ILK regulates YAP activation in HCC4006 cells after EGFR inhibition, providing a potential mechanism for DTP survival in cells with high ILK expression.
ILK signals through SFKs, but not FAK, to drive DTP survival
To determine how ILK may lead to YAP activation and DTP survival, we examined two known intermediates of integrin signalling, SRC-family kinases (SFKs) and FAK. These kinases are known to be associated with ILK and have been shown to stimulate YAP activation [65,66,67,68,69,70]. In addition, they have been associated with Osi insensitivity in LUAD [22, 71, 72]. HCC4006 OsiR cells had elevated levels of P-FAK and P-SFK when compared to its parental counterpart (Fig. 5c and Supplementary Fig. S1e). Moreover, ILK knockdown in HCC4006 cells reduced the level of P-SFK and P-FAK in Osi treated cells (Fig. 5i and Supplementary Fig. S9a).
To directly assess whether FAK or SFK signalling underlies the phenotype observed with ILK knockdown, we first employed the use of a FAK-specific inhibitor, PF573228, which has previously been shown to sensitize other EGFR-mutant LUAD cells to Osi [22]. However, treatment with a sub-lethal concentration of PF573228 (300 nM) in HCC4006 cells did not phenocopy the effects of ILK knockdown, despite diminishing P-FAK levels induced by 50 nM Osi (Supplementary Fig. S9b). PF573228 treatment did not improve sensitivity in HCC4006 cells treated with 10 nM and 100 nM Osi for 7 days (Supplementary Fig. S9c). On the other hand, sub-lethal inhibition of SFKs by dasatinib (30 nM) phenocopied the effects of ILK knockdown, not only reducing YAP activation following 50 nM Osi treatment (Fig. 5j), but also colony formation (10 nM and 100 nM Osi) (Supplementary Fig. S8b) and DTP survival (100 nM, 300 nM, and 1 µM Osi) (Supplementary Fig. S8d) in HCC4006 cells treated with Osi, in agreement with previous findings [22, 67]. Moreover, overexpression of SRC, but not GFP control, in HCC4006 shILK cells (Fig. 5k) rescued the effects of ILK knockdown in Osi-treated (100 nM, 300 nM, and 1 µM Osi) HCC4006 DTP cells (Fig. 5l and Supplementary Fig. S10a-c), further demonstrating the importance of SFKs in mediating the cytoprotective effects of ILK. Taken together, these results suggest that while FAK activation may require the presence of ILK, it is independent from the mechanism by which ILK mediates Osi insensitivity in HCC4006 cells. In contrast, SFK activation is relevant for the cytoprotective effects of ILK, bridging ILK to YAP activation in this model system.
Extracellular Matrix increases dependences on ILK-mediated cytoprotective effects during Osi treatment
The binding of integrins to ECM is important for ILK’s physiological functions [25, 27, 73]. While ILK provides a survival advantage during Osi treatment, we next asked whether the presence of ECM that mimics an in situ setting amplifies this effect. To do this, we performed a flow cytometry-based competition assay (Fig. 6a) between GFP-expressing HCC4006 cells with intact ILK expression and RFP-expressing HCC4006 shILK cells with ILK knockdown (or shNS control) cultured on either regular or Matrigel-coated plates, as a form of ECM (heavily enriched with laminin – the canonical integrin ligand) [74]. To account for any intrinsic competitive disadvantage associated with ILK knockdown, we normalized our results to the DMSO-treated control cells cultured on regular plates. As anticipated, ILK knockdown yielded a competitive disadvantage to HCC4006 cells in the context of EGFR inhibition, as observed by the decrease in RFP/GFP ratio compared to the vehicle control when these cells were cultured in 100 nM Osi for 7 days (Fig. 6b). Interestingly, ILK knockdown in the presence of Matrigel coating alone also decreased the competitive viability of DMSO-treated HCC4006 cells (Fig. 6b), further demonstrating the importance of ILK when cells adhere to ECM. The effects of ILK knockdown were further amplified when cells cultured on Matrigel were treated with 100 nM Osi (Fig. 6b), demonstrating that ECM is required for the survival effects of ILK in HCC4006 cells. Next, we asked how ECM can affect the viability of Osi DTP cells (see Fig. S12 for DMSO control). ILK knockdown in Osi-treated (100 nM, 300 nM, and 1 µM) HCC4006 cells cultured on Matrigel-coated plates significantly decreased the survival of DTP cells (Fig. 6c). The reduction in viability was more prominent (~70% reduction in DTP viability) for cells cultured on Matrigel-coated plates than cells on regular plates (~50% reduction in DTP viability) (Fig. 4c), suggesting the contribution of ILK in promoting Osi-resistant DTP survival is prevalent in ECM-rich microenvironment contexts.
a Schematic of competition assay. b Quantification of normalized RFP/GFP ratio in Osi (100 nM) treated HCC4006 shILK/GFP or HCC4006 shNS/GFP cells plated on either regular or Matrigel-coated plates (n = 3 experimental replicates in each condition). One-way ANOVA was used to statistically compare across these various conditions (*p < 0.05; **p < 0.01; ***p < 0.001). c Persister clonogenic assay of HCC4006 shILK (n = 9) and shNS (n = 9) cells plated on Matrigel-coated plates and treated with Osi for 7 days ± DOX. Quantification of colony count is below the representative plates. d Validation of ILK knockdown in H1975 shILK cells. e Persister clonogenic assays of H1975 shILK cells cultured on Matrigel-coated plates treated with Osi for 7 days ± DOX. Multiple paired t-tests with Holm-Sidak correction were used to statistically compare DOX vs No DOX at each Osi concentration (*p < 0.05; ***p < 0.001). DOX concentration = 100 ng/mL. f Validation of ILK overexpression in PC9 ILK cells. g Persister clonogenic assays of PC9 and PC9 ILK cells on Matrigel-coated plates treated with Osi for 7 days. Quantifications of colony count are below the representative plates. Multiple t-tests were used to statistically compare PC9 vs PC9 ILK at each Osi concentration (*p < 0.05). h Validation of ILK overexpression in PC9 ILK mCherry cells vs GFP cells. i Quantification of mCherry/GFP ratio in Osi/DMSO-treated PC9 mCherry/GFP or PC9 ILK mCherry/GFP cells plated on Matrigel-coated plates (n = 3 experimental replicates in each condition). One-way ANOVA was used to statistically compare across these various conditions (****p < 0.0001). Error bars represent the mean ± SEM.
To confirm our findings in other model systems, we next aimed to assess the effect of ILK in DTP survival in additional EGFR-mutant LUAD cell lines, one with moderate levels of ILK expression (H1975) and one with low basal ILK (PC9) (Fig. 1g). Using the same inducible knockdown construct described above (Fig. 3a), the addition of DOX sufficiently reduced ILK protein level in H1975 cells (Fig. 6d). We found that ILK knockdown in H1975 cells cultured on regular plates did not have significant effects on colony count following 10 nM or 100 nM Osi treatment (or DMSO control) for 7 days (Supplementary Fig. S13a); however, ILK knockdown in H1975 cells cultured on Matrigel significantly reduced the colony count in DMSO and 100 nM Osi (Supplementary Fig. S13b), suggesting that ECM is required for ILK’s function in H1975 cells. We found that ILK knockdown significantly reduced DTP viability (100 nM and 300 nM Osi; 1 µM did not yield a single viable colony), on both regular and Matrigel-coated plates (Fig. 6e and Supplementary Fig. S13c), with more profound effects when these cells were plated on Matrigel. In parallel, we further examined the DTP protective role of ILK in PC9s, a cell line with low basal ILK expression. We observed that constitutive ILK overexpression in PC9 cells (Fig. 6f) did not significantly affect clonogenic survival in response to 10 nM or 100 nM Osi treatment (or DMSO control), both on regular or Matrigel-coated plates (Supplementary Fig. S14a, b). However, we found that ILK overexpression significantly increased DTP colony counts in PC9 cells treated with 100 nM, 300 nM, and 1 µM Osi, but only when cultured on Matrigel (Fig. 6g and Supplementary Fig. S9c). These results were complemented by a competition assay in PC9 cells cultured on Matrigel. In PC9 cells without ILK overexpression, mCherry-labelled cells competed evenly with GFP-labelled cells treated with 100 nM Osi/DMSO for 7 days (Fig. 6i). Introducing ILK overexpression to these mCherry-labelled cells (Fig. 6h) significantly enriched for the PC9 cells with ILK overexpression, but only when these cells were treated with Osi (Fig. 6i). In summary, our results in H1975 and PC9 cells further confirmed our findings in HCC4006, highlighting that ILK promotes Osi DTP survival in the context of EGFR-mutant LUAD in ECM-rich conditions.
ILK knockdown reduces persistent tumours in vivo
Lastly, two-dimensional cell culture does not recapitulate the complex ECM structure in the tumour microenvironment. We therefore sought to translate the effects of ILK knockdown on Osi response in vivo (Fig. 7a). NRG mice were subcutaneously implanted with both HCC4006 shNS and shILK cells and subsequently fed a chow or DOX diet, providing mouse ECM for the formation of tumours. These mice were subsequently gavaged with either Osi or vehicle (Veh) control. We found that the DOX diet reduced ILK expression levels (Fig. 7b) and that ILK knockdown alone did not significantly affect subcutaneous HCC4006 tumour formation. When these mice were dosed with Osi, both HCC4006 shNS and shILK tumours regressed >70% in size and remained small for the duration of the treatment (Vehicle tumours = 0.53 ± 0.17 g; Osi tumours = 0.15 ± 0.05 g; weights reported here are mean±95%CI), suggesting that these residual tumours were “persisting” during Osi treatment. In accordance with findings in vitro, we observed that only in mice fed with a DOX diet, Osi persistent HCC4006 shILK tumours were ~50% smaller than the persistent HCC4006 shNS tumours (Fig. 7c). Importantly, the reduction in persistent tumour size corresponded to a significant decrease in tumour YAP levels as detected by western blot (Fig. 7d). Lastly, as these HCC4006 tumour xenografts contain non-tumour cells that may also express activated YAP, we performed confocal imaging on tumour sections to confirm YAP was reduced in tumour cells following ILK knockdown (Fig. 7e). Using RFP expression as a marker of the implanted tumour cells, we observed that HCC4006 shILK persistent tumours had notably lower nuclear YAP intensity and percent YAP positive area in comparison to the control shNS persistent tumours (Fig. 7f), suggesting that these tumours required YAP to persist during Osi treatment. Taken together, our results indicate that the cytoprotective role of ILK and its activation of YAP are also applicable in vivo.
a Schematic of HCC4006 shNS/shILK tumour xenograft experiment. b Validation of ILK knockdown by RT-qPCR. Ratio paired t-test was used to statistically compare between HCC4006 shNS vs shILK tumours (n = 7) in the same mouse (***p < 0.001). c Tumour weights of implanted HCC4006 shNS and shILK tumours extracted from mice gavaged with Vehicle (Veh; n = 4) or Osi (n = 3) at endpoint. Ratio paired t-test was used to statistically compare between HCC4006 shNS vs shILK tumours (n = 3) in the same mouse (**p < 0.01). d Representative western blot of HCC4006 shNS and shILK tumours treated with Veh or Osi. Quantification of YAP band density are plotted on the right. Ratio paired t-test was used to statistically compare between HCC4006 shNS vs shILK tumours (n = 3) in the same mouse (*p < 0.05). e Representative images of YAP staining in HCC4006 shNS and shILK tumours. White arrows indicate regions of YAP staining. f Quantification of nuclear YAP intensity and %YAP positive area between shNS vs shILK tumours (n = 4 images/section × 2 section/tumour × 3 tumours). Student’s t-tests were used to statistically compare HCC4006 shNS vs shILK persistent tumours (*p < 0.05, ***p < 0.001). Error bars represent the mean ± SEM.
Pharmacological inhibition of ILK can be used in combination with Osi to reduce viability of DTP cells
To improve the translatability of our findings, we examined the feasibility of combining pharmacological inhibition of ILK with Osi. Sub-lethal inhibition of ILK by 5 µM QLT0267 in HCC4006 cells improved Osi response compared to cells treated with DMSO (Fig. 8a). Moreover, while ILK inhibition did not significantly reduce colony-forming potential (DMSO, 10 nM, or 100 nM Osi) in HCC4006 cells cultured on Matrigel (Fig. 8c), QLT0267 significantly reduced Osi DTP survival (100 nM, 300 nM, and 1 µM Osi) (Fig. 8c, d), further demonstrating that the cytoprotective role of ILK is specific to DTPs. Furthermore, ILK inhibition suppressed EMT in Osi-treated (10 nM and 50 nM) HCC4006 cells, as indicated by the reduced levels of NCAD, vimentin, and SNAIL in comparison to cells treated with DMSO control (Fig. 8b). Lastly, we found that ILK inhibition delays the onset of Osi resistance in HCC4006 cells treated with escalating concentrations of Osi (Fig. 9e). Overall, our results demonstrate that co-targeting ILK is a viable strategy in combating DTP survival and EMT-mediated Osi resistance in EGFR-mutant LUAD.
a Three days Osi dose-response in HCC4006 cells (n = 3 experimental replicates) ± ILKi (5 µM QLT0267). Multiple t-tests with Holm-Sidak correction were used to statistically compare DOX vs No DOX at each Osi concentration (*p < 0.05). b Representative western blot of mesenchymal markers in Osi-treated HCC4006 cells ± ILKi (5 µM QLT0267). c Clonogenic and d persister clonogenic assays of HCC4006 cells cultured on Matrigel-coated plates treated with Osi for 7 days ± ILKi (5 µM QLT0267). Quantification of colony count is below the representative plates. Multiple paired t-tests with Holm–Sidak correction were used to statistically compare ILKi vs DMSO at each Osi concentration (*p < 0.05; **p < 0.01). e Onset of resistance of HCC4006 cells dose-escalated with either Osi alone or Osi + ILKi.
a ILK expression (by H-Score) in EGFR-TKI resistant patient tumour samples with RTK pathway-independent (RTK-) mechanisms (n = 12) vs RTK pathway-dependent (RTK+) mechanisms (n = 48). Mann–Whitney Test was used to statistically compare RTK- vs RTK+ resistant samples. b Coincidence of high vs low ILK expression and RTK+ vs RTK- resistance mechanisms in 18 LUAD patients post-treatment with EGFR-TKI. P values were calculated by a two-tailed 2 × 2 Fisher’s Exact Test to assess contingency across the two variables.
ILK expression is associated with RTK-independent mechanisms of EGFR-TKI resistance in clinical biopsies
Lastly, to assess whether ILK expression can be used to predict adaptive EGFR-TKI resistance in patients, we performed IHC for ILK on 60 EGFR-mutant LUAD tumours from 18 patients resistant to EGFR-TKIs (post-therapy with EGFR-TKIs) and correlated the ILK expression levels with potential genetic mechanisms of resistance as determined by MSK-IMPACT profiling. We observed that ILK expression (by H-Score) was significantly elevated in resistant samples lacking acquired genetic alterations in EGFR and/or other RTKs and their associated downstream signalling pathways (i.e. RAS-MAPK and PI3K-AKT pathways) (termed RTK-) when compared to resistant sample with acquired alterations in RTK signalling (RTK+) (Fig. 9a). In accordance, patients with higher ILK levels are more associated with RTK- resistance mechanisms while patients with lower ILK are associated with RTK+ resistance mechanisms (Fig. 9b). Of note, the only patient with high ILK tumour expression (3 samples with H-Score of 300) and concatenate RTK+ alteration (specifically EGFR/MET amplifications) was rationally treated with both osimertinib and savolitinib, but the patient unfortunately developed resistance to both TKIs.
Taken together with our in vitro findings, these clinical results in patients suggest that ILK may regulate the survival of residual tumours, providing the opportunity for these tumours to acquire “off-pathway” mutations that mediate EGFR-TKI resistance. In further support of our observations and our in vitro model system, one patient with high tumour ILK expression developed Osi-resistant LUAD with an acquired SMARCA4 alteration, mirroring that observed in HCC4006 OsiR cells (Supplementary Fig. S1c). In summary, our results indicate that ILK expression is associated with RTK-independent mechanisms of EGFR-TKI resistance and can potentially be exploited as a predictive marker for acquired resistance.
Discussion
Resistance to EGFR-TKIs remains a major hurdle to curing LUAD patients. Non-genetic mechanisms of resistance, like treatment-induced EMT, are historically less well-characterized than genetic mechanisms of resistance. While high ILK expression has recently been shown to correlate with worse outcomes in patients treated with EGFR-TKIs [36], the mechanism by which this occurs was not elucidated. Even though ILK has repeatedly been demonstrated to drive EMT in other cancer types [27, 30, 31, 48, 50, 56, 75], its role in mediating EMT in LUAD is much less established. This could be due to the fact that high expression of ILK is not directly associated with EMT in LUAD. In our analysis, we found that HCC4006, despite having the highest ILK expression of all EGFR-driven LUAD cell lines remains epithelial-like in its basal state (see Figs. 1e and 2a). However, HCC4006 and LUAD tumours expressing high ILK are enriched for EMT-related gene expression signatures, suggesting that the cells/primary tumours may be “primed” to execute EMT reprogramming in response to specific stimuli. Indeed, our data suggest that ILK can mediate EMT in EGFR-driven LUAD in the context of EGFR-TKI treatment.
In vitro models of EGFR-TKI resistance have demonstrated that individual cell lines can develop resistance through different mechanisms, with variable contributions of EMT-driven resistance varying across cell lines [76]. Interestingly, the frequency of EMT as a resistance mechanism is correlated with ILK expression. EGFR-mutant LUAD cell lines with high ILK expression (i.e. HCC4006 and HCC2279) undergo EMT in nearly all reported EGFR-TKI resistance studies [7, 77, 78], while cell lines with low ILK expression (i.e. PC9) rarely ever undergo EMT. Consistent with this observation, cell lines with moderate ILK expression (i.e. H1975, HCC827) undergo EMT in some of the reported studies [5, 76, 77]. As mentioned above, this suggests that ILK expression levels can potentially “prime” EGFR-mutant LUAD cells to undergo EMT or other non-genetic mechanisms during EGFR-TKI treatment. Our study found that lowering ILK expression in LUAD during EGFR-TKI treatment decreased the activation of EMT programs in the progression towards a resistant phenotype, complementing this hypothesis.
Adaptive, non-genetic mechanism of drug resistance arises from a transitory DTP state and there is increasing evidence that integrin signalling and cytoskeleton reorganization play an important role in the ability of a tumour cell to tolerate EGFR-TKIs [16, 57, 79, 80]. Our results contribute to this growing body of evidence, where we also observed that LUAD cells increased integrin/focal-adhesion signalling following EGFR inhibition, increasing their dependency on these signals for survival. We found that ILK, a mediator of integrin signalling and an effector for cytoskeleton remodelling [25, 27, 73, 81], can as a result, drive DTP survival and regulate the depth of response to Osi treatment. This observation was especially apparent when the LUAD cells were grown in an ECM-rich microenvironment, providing the ECM substrates for integrins to bind, both in vitro and in vivo, suggesting that ILK activity and its association with integrins are especially important for its cytoprotective functions. Future work involving specific ECM components and genetic manipulations of integrin subunits may help better delineate the precise interactions between ECM-integrin-ILK that ILK requires to promote DTP survival.
During the progression from tolerance to resistance, DTP cells often acquire additional genetic/phenotypic changes to support growth in the presence of the drug. In our model of EMT-mediated Osi resistance, we found that ILK was not required to maintain Osi resistance nor was ILK suppression able to reverse the mesenchymal-like cell state. MSK-IMPACT sequencing identified several mutations that may have contributed to this phenotype. However, in the scope of our study, we have not identified the precise combinations of mutations that permit LUAD cells to become ILK independent, nor have we characterized whether particular mutations are necessary in the progression towards EMT-mediated Osi resistance. One limitation of our study is that we have not clonally traced DTP cells as they become Osi-resistant cells. Future work with single-cell barcoding technology may address some of these questions.
The signalling mechanisms by which ILK promotes EMT and drug resistance have been described in other cancer types [31, 81, 82]. However, the mechanism by which ILK acts in LUAD has only been investigated in a few studies [75, 83]. Here we describe the role of ILK in promoting the activation of SFKs, which can subsequently activate YAP, allowing LUAD cells to persist during Osi treatment. Of note, our results indicate that ILK-dependent increase in YAP activity follows EGFR inhibition, suggesting an increased dependency on integrin-ILK-mediated signalling for survival during EGFR-TKI treatment. Our results agree with previous findings where ILK was found to be important for YAP activation under the context of metastatic colonization in LUAD [60] and breast cancer [61, 81]. Contrary to previous findings [23, 66, 68, 70], we identified SFKs, and not FAK, as the important downstream kinases for DTP survival in our model. According to the biochemistry work by Kim et al. [84], the interaction between ILK and SRC/SFK to induce cytoskeleton remodelling occurs predominantly in the absence of EGFR-ERK activation, and their results suggest that EGFR-ERK recruits active SRC away from ILK. Provided that forementioned study applies to LUAD cells, EGFR inhibition would interrupt the interaction between EGFR and SRC, and instead, SRC interacts with ILK to mediate downstream effects (i.e. activate YAP). Complementing our results, SFKs have been shown to mediate resistance to EGFR-TKIs [22, 71, 72] and the suppression of ILK has been shown to sensitize tumour cells to SFK inhibition in chronic myeloid leukaemia [85] and breast cancer [86]. However, the specific signalling cascade by which Osi induces YAP activation through ILK remains to be elucidated.
The knowledge gained from DTP cells in our model may be translatable to minimal residual disease (MRD) in patients. Currently, MRD remains a major hurdle in improving patient outcomes. While circulating tumour DNA assays are being developed to detect MRD [87, 88], there is a lack of a robust predictor for residual disease following targeted therapy. In terms of translation to the clinic, our results indicate that patient tumour ILK expression should be considered prior to and during EGFR-TKI therapy as it may serve as a valuable predictor of MRD and RTK-independent mechanisms of resistance. Based on our observations, EGFR-TKIs may even induce EMT in tumours with high ILK expression. Therefore, patient tumour ILK expression in pathology samples, assessed by RNA or protein-based assays (i.e. IHC), may dictate the necessity for combination therapies to specifically combat MRD and circumvent EGFR-TKI resistance.
Finally, we found that ILK inhibition can be combined with Osi to reduce DTP survival during treatment. However, the current use of ILK inhibitors in animal models have been severely limited by their potency and these inhibitor primarily target the kinase function of ILK; unlike genetic knockdown, pharmacological ILK inhibition may still leave its scaffolding functions intact [89], exemplifying a need for more effective inhibitors to be designed. An alternative approach would be to target other proteins in the ILK-SFK-YAP axis. Currently, YAP/TAZ-TEAD inhibitors are actively being designed for preclinical applications. Previous studies have also shown that SFK inhibition (dasatinib) is effective in combination with Osi in LUAD [22], which led to the Phase I clinical trial of combining Osi with dasatinib. However, while effective, dasatinib was found to be toxic to the patients [90]. Based on our findings, targeting the ILK-SFK-YAP pathway may be more viable in select patients with high tumour ILK expression or tumours with high levels of ECM deposition to better limit DTP survival and EMT-mediated resistance.
Overall, the results of our study demonstrate that ILK expression is an important determinant of Osi response in EGFR-mutant LUAD. We found that tumour cells with high ILK expression are primed to respond to Osi treatment by activating YAP through SFKs, which provides the ability to tolerate drug treatment and survive. The hallmarks of these ILK-high DTP cells are their phenotypical plasticity (i.e. activation of EMT transcriptional programs) and their ability to persist in response to Osi treatment, which generates a window of opportunity for clonal evolution and the acquisition of additional mutations/properties that confer stable resistance to Osi. In contrast, cells with low ILK expression cannot activate YAP nor undergo mesenchymal reprogramming during Osi treatment, ultimately reducing their ability to persist during Osi treatment and their potential to become resistant (Fig. 10). Our results suggest that ILK may be exploited as a predictor for acquired resistance in lung cancer patients. We provide evidence that co-targeting ILK with EGFR may be a viable strategy to better control minimal residual disease and EGFR-TKI resistance in lung cancer.
Data availability
The data that support the findings of this study are available on request. Patient data will require approval by ethics committee.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49.
Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–54.
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–25.
Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J cancer. 2019;121:725–37.
Fukuda K, Takeuchi S, Arai S, Kita K, Tanimoto A, Nishiyama A, et al. Glycogen synthase kinase-3 inhibition overcomes epithelial-mesenchymal transition-associated resistance to osimertinib in EGFR-mutant lung cancer. Cancer Sci. 2020;111:2374–84.
Jiang XM, Xu YL, Yuan LW, Zhang LL, Huang MY, Ye ZH, et al. TGFbeta2-mediated epithelial-mesenchymal transition and NF-kappaB pathway activation contribute to osimertinib resistance. Acta Pharmacol Sin. 2021;42:451–9.
Suda K, Tomizawa K, Fujii M, Murakami H, Osada H, Maehara Y, et al. Epithelial to mesenchymal transition in an epidermal growth factor receptor-mutant lung cancer cell line with acquired resistance to erlotinib. J Thorac Oncol. 2011;6:1152–61.
Weng CH, Chen LY, Lin YC, Shih JY, Lin YC, Tseng RY, et al. Epithelial-mesenchymal transition (EMT) beyond EGFR mutations per se is a common mechanism for acquired resistance to EGFR TKI. Oncogene. 2019;38:455–68.
Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol cell Biol. 2019;20:69–84.
Mittal V. EpitheliaL mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13:395–412.
Cabanos HF, Hata AN. Emerging insights into targeted therapy-tolerant persister cells in cancer. Cancers. 2021;13:2666.
Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80.
Shen S, Faouzi S, Bastide A, Martineau S, Malka-Mahieu H, Fu Y, et al. An epitranscriptomic mechanism underlies selective mRNA translation remodelling in melanoma persister cells. Nat Commun. 2019;10:5713.
Hirata E, Girotti MR, Viros A, Hooper S, Spencer-Dene B, Matsuda M, et al. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin beta1/FAK signaling. Cancer cell. 2015;27:574–88.
De Conti G, Dias MH, Bernards R. Fighting drug resistance through the targeting of drug-tolerant persister cells. Cancers. 2021;13:1118.
Wu P, Gao W, Su M, Nice EC, Zhang W, Lin J, et al. Adaptive Mechanisms of Tumor Therapy Resistance Driven by Tumor Microenvironment. Front cell developmental Biol. 2021;9:641469.
Dingemans AM, van den Boogaart V, Vosse BA, van Suylen RJ, Griffioen AW, Thijssen VL. Integrin expression profiling identifies integrin alpha5 and beta1 as prognostic factors in early stage non-small cell lung cancer. Mol Cancer. 2010;9:152.
Haake SM, Plosa EJ, Kropski JA, Venton LA, Reddy A, Bock F, et al. Ligand-independent integrin beta1 signaling supports lung adenocarcinoma development. JCI insight. 2022;7:e154098.
Morello V, Cabodi S, Sigismund S, Camacho-Leal MP, Repetto D, Volante M, et al. beta1 integrin controls EGFR signaling and tumorigenic properties of lung cancer cells. Oncogene. 2011;30:4087–96.
Wang Y, Hou K, Jin Y, Bao B, Tang S, Qi J, et al. Lung adenocarcinoma-specific three-integrin signature contributes to poor outcomes by metastasis and immune escape pathways. J Transl Intern Med. 2021;9:249–63.
Rao TC, Ma VP, Blanchard A, Urner TM, Grandhi S, Salaita K, et al. EGFR activation attenuates the mechanical threshold for integrin tension and focal adhesion formation. J Cell Sci. 2020;133:jcs238840.
Ichihara E, Westover D, Meador CB, Yan Y, Bauer JA, Lu P, et al. SFK/FAK signaling attenuates osimertinib efficacy in both drug-sensitive and drug-resistant models of EGFR-mutant lung cancer. Cancer Res. 2017;77:2990–3000.
Haderk F, Chou YT, Cech L, Fernandez-Mendez C, Yu J, Olivas V, et al. Focal adhesion kinase-YAP signaling axis drives drug-tolerant persister cells and residual disease in lung cancer. Nat Commun. 2024;15:3741.
Elad N, Volberg T, Patla I, Hirschfeld-Warneken V, Grashoff C, Spatz JP, et al. The role of integrin-linked kinase in the molecular architecture of focal adhesions. J cell Sci. 2013;126:4099–107.
Gorska A, Mazur AJ. Integrin-linked kinase (ILK): the known vs. the unknown and perspectives. Cell Mol Life Sci. 2022;79:100.
Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat Rev Cancer. 2005;5:51–63.
McDonald PC, Dedhar S. New Perspectives on the Role of Integrin-Linked Kinase (ILK) Signaling in Cancer Metastasis. Cancers. 2022;14:3209.
Tsirtsaki K, Gkretsi V. The focal adhesion protein Integrin-Linked Kinase (ILK) as an important player in breast cancer pathogenesis. Cell Adhes Migr. 2020;14:204–13.
Gil D, Zarzycka M, Ciolczyk-Wierzbicka D, Laidler P. Integrin linked kinase regulates endosomal recycling of N-cadherin in melanoma cells. Cell Signal. 2020;72:109642.
Serrano I, McDonald PC, Lock FE, Dedhar S. Role of the integrin-linked kinase (ILK)/Rictor complex in TGFbeta-1-induced epithelial-mesenchymal transition (EMT). Oncogene. 2013;32:50–60.
Tsoumas D, Nikou S, Giannopoulou E, Champeris Tsaniras S, Sirinian C, Maroulis I, et al. ILK expression in colorectal cancer is associated with EMT, cancer stem cell markers and chemoresistance. Cancer Genomics Proteom. 2018;15:127–41.
Zhuang X, Lv M, Zhong Z, Zhang L, Jiang R, Chen J. Interplay between intergrin-linked kinase and ribonuclease inhibitor affects growth and metastasis of bladder cancer through signaling ILK pathways. J Exp Clin Cancer Res. 2016;35:130.
Yang J, Hou Y, Zhou M, Wen S, Zhou J, Xu L, et al. Twist induces epithelial-mesenchymal transition and cell motility in breast cancer via ITGB1-FAK/ILK signaling axis and its associated downstream network. Int J Biochem Cell Biol. 2016;71:62–71.
Kalra J, Warburton C, Fang K, Edwards L, Daynard T, Waterhouse D, et al. QLT0267, a small molecule inhibitor targeting integrin-linked kinase (ILK), and docetaxel can combine to produce synergistic interactions linked to enhanced cytotoxicity, reductions in P-AKT levels, altered F-actin architecture and improved treatment outcomes in an orthotopic breast cancer model. Breast Cancer Res. 2009;11:R25.
Lee SL, Hsu EC, Chou CC, Chuang HC, Bai LY, Kulp SK, et al. Identification and characterization of a novel integrin-linked kinase inhibitor. J Med Chem. 2011;54:6364–74.
Karachaliou N, Cardona AF, Bracht JWP, Aldeguer E, Drozdowskyj A, Fernandez-Bruno M, et al. Integrin-linked kinase (ILK) and src homology 2 ___domain-containing phosphatase 2 (SHP2): Novel targets in EGFR-mutation positive non-small cell lung cancer (NSCLC). EBioMedicine. 2019;39:207–14.
Uphoff CC, Drexler HG. Detecting mycoplasma contamination in cell cultures by polymerase chain reaction. Methods Mol Biol. 2011;731:93–103.
Tan TZ, Miow QH, Miki Y, Noda T, Mori S, Huang RY, et al. Epithelial-mesenchymal transition spectrum quantification and its efficacy in deciphering survival and drug responses of cancer patients. EMBO Mol Med. 2014;6:1279–93.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods. 2017;14:417–9.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47.
Mi H, Muruganujan A, Casagrande JT, Thomas PD. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc. 2013;8:1551–66.
Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703–13.
Inoue Y, Nikolic A, Farnsworth D, Shi R, Johnson FD, Liu A, et al. Extracellular signal-regulated kinase mediates chromatin rewiring and lineage transformation in lung cancer. eLife. 2021;10:e66524.
Unni AM, Lockwood WW, Zejnullahu K, Lee-Lin SQ, Varmus H. Evidence that synthetic lethality underlies the mutual exclusivity of oncogenic KRAS and EGFR mutations in lung adenocarcinoma. eLife. 2015;4:e06907.
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat methods. 2012;9:676–82.
Fedchenko N, Reifenrath J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue - a review. Diagn Pathol. 2014;9:221.
Feng Y, Le F, Tian P, Zhong Y, Zhan F, Huang G, et al. GTW inhibits the epithelial to mesenchymal transition of epithelial ovarian cancer via ILK/AKT/GSK3beta/Slug signalling pathway. J Cancer. 2021;12:1386–97.
Gil D, Ciolczyk-Wierzbicka D, Dulinska-Litewka J, Laidler P. Integrin-linked kinase regulates cadherin switch in bladder cancer. Tumour Biol. 2016;37:15185–91.
Lin L, Luo X, Wang L, Xu F, He Y, Wang Q, et al. BML-111 inhibits EMT, migration and metastasis of TAMs-stimulated triple-negative breast cancer cells via ILK pathway. Int Immunopharmacol. 2020;85:106625.
Szabo PM, Vajdi A, Kumar N, Tolstorukov MY, Chen BJ, Edwards R, et al. Cancer-associated fibroblasts are the main contributors to epithelial-to-mesenchymal signatures in the tumor microenvironment. Sci Rep. 2023;13:3051.
Gupta S, Iljin K, Sara H, Mpindi JP, Mirtti T, Vainio P, et al. FZD4 as a mediator of ERG oncogene-induced WNT signaling and epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2010;70:6735–45.
Kim J, Jang G, Sim SH, Park IH, Kim K, Park C. SMARCA4 depletion induces cisplatin resistance by activating YAP1-mediated epithelial-to-mesenchymal transition in triple-negative breast cancer. Cancers. 2021;13:5474.
Park JH, Shin JM, Yang HW, Park IH. DNMTs Are Involved in TGF-beta1-induced epithelial-mesenchymal transitions in airway epithelial cells. Int J Mol Sci. 2022;23:3003.
Zhang Y, Donaher JL, Das S, Li X, Reinhardt F, Krall JA, et al. Genome-wide CRISPR screen identifies PRC2 and KMT2D-COMPASS as regulators of distinct EMT trajectories that contribute differentially to metastasis. Nat Cell Biol. 2022;24:554–64.
Li CL, Li J, Gong SY, Huang M, Li R, Xiong GX, et al. Targeting the ILK/YAP axis by LFG-500 blocks epithelial-mesenchymal transition and metastasis. Acta Pharmacologica Sin. 2021;42:1847–59.
Criscione SW, Martin MJ, Oien DB, Gorthi A, Miragaia RJ, Zhang J, et al. The landscape of therapeutic vulnerabilities in EGFR inhibitor osimertinib drug tolerant persister cells. NPJ Precis Oncol. 2022;6:95.
Pfeifer M, Brammeld JS, Price S, Pilling J, Bhavsar D, Farcas A, et al. Genome-wide CRISPR screens identify the YAP/TEAD axis as a driver of persister cells in EGFR mutant lung cancer. Commun Biol. 2024;7:497.
Kurppa KJ, Liu Y, To C, Zhang T, Fan M, Vajdi A, et al. Treatment-induced tumor dormancy through YAP-mediated transcriptional reprogramming of the apoptotic pathway. Cancer Cell. 2020;37:104–22.e112.
Er EE, Valiente M, Ganesh K, Zou Y, Agrawal S, Hu J, et al. Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization. Nat Cell Biol. 2018;20:966–78.
Serrano I, McDonald PC, Lock F, Muller WJ, Dedhar S. Inactivation of the Hippo tumour suppressor pathway by integrin-linked kinase. Nat Commun. 2013;4:2976.
Shreberk-Shaked M, Oren M. New insights into YAP/TAZ nucleo-cytoplasmic shuttling: new cancer therapeutic opportunities?. Mol Oncol. 2019;13:1335–41.
Totaro A, Panciera T, Piccolo S. YAP/TAZ upstream signals and downstream responses. Nat cell Biol. 2018;20:888–99.
Kaneda A, Seike T, Danjo T, Nakajima T, Otsubo N, Yamaguchi D, et al. The novel potent TEAD inhibitor, K-975, inhibits YAP1/TAZ-TEAD protein-protein interactions and exerts an anti-tumor effect on malignant pleural mesothelioma. Am J Cancer Res. 2020;10:4399–415.
Hsu PC, Yang CT, Jablons DM, You L. The crosstalk between Src and Hippo/YAP signaling pathways in non-small cell lung cancer (NSCLC). Cancers. 2020;12:1361.
Kim NG, Gumbiner BM. Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway. J Cell Biol. 2015;210:503–15.
Lamar JM, Xiao Y, Norton E, Jiang ZG, Gerhard GM, Kooner S, et al. SRC tyrosine kinase activates the YAP/TAZ axis and thereby drives tumor growth and metastasis. J Biol Chem. 2019;294:2302–17.
Li J, Zhang X, Hou Z, Cai S, Guo Y, Sun L, et al. P130cas-FAK interaction is essential for YAP-mediated radioresistance of non-small cell lung cancer. Cell Death Dis. 2022;13:783.
Rigiracciolo DC, Nohata N, Lappano R, Cirillo F, Talia M, Adame-Garcia SR, et al. Focal Adhesion Kinase (FAK)-Hippo/YAP transduction signaling mediates the stimulatory effects exerted by S100A8/A9-RAGE system in triple-negative breast cancer (TNBC). J Exp Clin cancer Res. 2022;41:193.
Zhang B, Zhang Y, Zhang J, Liu P, Jiao B, Wang Z, et al. Focal Adhesion Kinase (FAK) Inhibition Synergizes with KRAS G12C Inhibitors in Treating Cancer through the Regulation of the FAK-YAP Signaling. Adv Sci. 2021;8:e2100250.
Fan PD, Narzisi G, Jayaprakash AD, Venturini E, Robine N, Smibert P, et al. YES1 amplification is a mechanism of acquired resistance to EGFR inhibitors identified by transposon mutagenesis and clinical genomics. Proc Natl Acad Sci USA. 2018;115:E6030–E6038.
Sato H, Kubota D, Qiao H, Jungbluth A, Rekhtman N, Schoenfeld AJ, et al. SRC Family Kinase inhibition targets YES1 and YAP1 as primary drivers of lung cancer and as mediators of acquired resistance to ALK and epidermal growth factor receptor inhibitors. JCO Precis Oncol. 2022;6:e2200088.
Li F, Zhang Y, Wu C. Integrin-linked kinase is localized to cell-matrix focal adhesions but not cell-cell adhesion sites and the focal adhesion localization of integrin-linked kinase is regulated by the PINCH-binding ANK repeats. J Cell Sci. 1999;112:4589–99.
Aisenbrey EA, Murphy WL. Synthetic alternatives to Matrigel. Nat Rev Mater. 2020;5:539–51.
Chen D, Zhang Y, Zhang X, Li J, Han B, Liu S, et al. Overexpression of integrin-linked kinase correlates with malignant phenotype in non-small cell lung cancer and promotes lung cancer cell invasion and migration via regulating epithelial-mesenchymal transition (EMT)-related genes. Acta Histochem. 2013;115:128–36.
Ohara S, Suda K, Mitsudomi T. Cell line models for acquired resistance to first-line osimertinib in lung cancers-applications and limitations. Cells. 2021;10:354.
Suda K, Murakami I, Yu H, Kim J, Tan AC, Mizuuchi H, et al. CD44 Facilitates Epithelial-to-Mesenchymal Transition Phenotypic Change at Acquisition of Resistance to EGFR Kinase Inhibitors in Lung Cancer. Mol Cancer Ther. 2018;17:2257–65.
Ware KE, Hinz TK, Kleczko E, Singleton KR, Marek LA, Helfrich BA, et al. A mechanism of resistance to gefitinib mediated by cellular reprogramming and the acquisition of an FGF2-FGFR1 autocrine growth loop. Oncogenesis. 2013;2:e39.
Adua SJ, Arnal-Estape A, Zhao M, Qi B, Liu ZZ, Kravitz C, et al. Brain metastatic outgrowth and osimertinib resistance are potentiated by RhoA in EGFR-mutant lung cancer. Nat Commun. 2022;13:7690.
Haderk F, Fernández-Méndez C, Čech L, Yu J, Meraz IM, Olivas V, et al. A focal adhesion kinase-YAP signaling axis drives drug tolerant persister cells and residual disease in lung cancer. bioRxiv. 2021:2021.2010.2023.465573.
Qin X, Lv X, Li P, Yang R, Xia Q, Chen Y, et al. Matrix stiffness modulates ILK-mediated YAP activation to control the drug resistance of breast cancer cells. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165625.
Jia Z. Role of integrin-linked kinase in drug resistance of lung cancer. OncoTargets Ther. 2015;8:1561–5.
Nikou S, Arbi M, Dimitrakopoulos FD, Sirinian C, Chadla P, Pappa I, et al. Integrin-linked kinase (ILK) regulates KRAS, IPP complex and Ras suppressor-1 (RSU1) promoting lung adenocarcinoma progression and poor survival. J Mol Histol. 2020;51:385–400.
Kim YB, Choi S, Choi MC, Oh MA, Lee SA, Cho M, et al. Cell adhesion-dependent cofilin serine 3 phosphorylation by the integrin-linked kinase.c-Src complex. J Biol Chem. 2008;283:10089–96.
Rothe K, Babaian A, Nakamichi N, Chen M, Chafe SC, Watanabe A, et al. Integrin-linked kinase mediates therapeutic resistance of quiescent CML stem cells to tyrosine kinase inhibitors. Cell Stem Cell. 2020;27:110–24.e119.
Beetham H, Griffith BGC, Murina O, Loftus AEP, Parry DA, Temps C, et al. Loss of integrin-linked kinase sensitizes breast cancer to SRC inhibitors. Cancer Res. 2022;82:632–47.
Frisone D, Friedlaender A, Addeo A. The role and impact of minimal residual disease in NSCLC. Curr Oncol Rep. 2021;23:136.
Pellini B, Chaudhuri AA. Circulating tumor DNA minimal residual disease detection of non-small-cell lung cancer treated with curative intent. J Clin Oncol. 2022;40:567–75.
Li Y, Tan X, Dai C, Stolz DB, Wang D, Liu Y. Inhibition of integrin-linked kinase attenuates renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:1907–18.
Kim C, Liu SV, Crawford J, Torres T, Chen V, Thompson J, et al. A phase i trial of dasatinib and osimertinib in TKI naive patients with advanced egfr-mutant non-small-cell lung cancer. Front Oncol. 2021;11:728155.
Author information
Authors and Affiliations
Contributions
Rocky Shi: Conceptualization, methodology, investigation, validation, formal analysis, writing – original draft, visualization. Dylan A Farnsworth: Methodology, investigation, formal analysis. Christopher A Febres-Aldana: Methodology, investigation, formal analysis, writing – review & editing. Justine LM Chow: Investigation. Ravinder Sheena: Investigation. Tejveer Atwal: Investigation. Juan Luis Gomez Marti: Investigation. Samantha Li: Investigation. Kiersten N Thomas: Investigation. Che-Min Lee: Investigation. Shannon J Awrey: Investigation. Paul C McDonald: Project administration, supervision. Romel Somwar: Project administration, writing – review & editing, supervision. Shoukat Dedhar: Conceptualization, supervision, resources, writing – review & editing. Marc Ladanyi: Supervision, resources, writing – review & editing. Kevin L Bennewith: Conceptualization, supervision, resources, project administration, funding acquisition, writing – review & editing. William W Lockwood: conceptualization, supervision, resources, project administration, funding acquisition, writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approvals
Patient tissue samples were collected under MSKCC IRB-approved biospecimen collection protocols, and informed consent was obtained. All housing, care, and experimental conditions and protocols for mice were followed in accordance with the guidelines of the University of British Columbia Animal Care Committee and the Canadian Council for Animal Care.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shi, R., Farnsworth, D.A., Febres-Aldana, C.A. et al. Drug tolerance and persistence to EGFR inhibitor treatment are mediated by an ILK-SFK-YAP signaling axis in lung adenocarcinoma. Oncogene (2025). https://doi.org/10.1038/s41388-025-03461-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41388-025-03461-6