Abstract
To assess the association between cerebral arterial stiffness measured using the carotid–cerebral pulse wave velocity (ccPWV) and the functional outcome after acute ischemic stroke (AIS). We prospectively studied 336 consecutive patients (mean age, 60.58 ± 9.89 years; 73.81% male) with first-ever AIS who underwent examination by multimodal brain magnetic resonance, ccPWV, echocardiography, and carotid ultrasonography during the admission period. Ischemic stroke subtypes were classified using the Trial of Org 10172 in Acute Stroke Treatment classification system. A poor functional outcome was defined as a modified Rankin Scale score >2 at 3 months after stroke onset. We observed that 110 (32.74%) patients had a poor functional outcome. Multivariate logistic regression analysis revealed that ccPWV ≥ 6.66 m/s, calculated from the receiver operating characteristic (ROC) curve, was an independent predictor of a poor functional outcome [adjusted odds ratio, 6.27; 95% confidence interval (CI), 2.07–18.99]. ROC curve analysis showed that the area under the curve of ccPWV for predicting the 3-month functional outcome after AIS was 85.27% (95% CI, 80.95–89.58; P < 0.001). The optimal ccPWV cutoff point was 6.66 m/s, with a sensitivity of 82.73% and specificity of 77.88%. Furthermore, the addition of ccPWV to the predictive model significantly improved the discrimination ability for a poor functional outcome. Cerebral arterial stiffness, measured using the ccPWV on admission, has value as an independent prognostic factor for predicting the long-term functional outcome in patients with AIS.
Similar content being viewed by others
Introduction
Stroke currently remains the leading cause of premature mortality and long-term disability worldwide [1, 2]. Due to differences in clinical parameters, such as age, initial severity, infarct size, risk factors, and serum markers, the outcome of acute stroke may vary widely [3]. However, the predictive ability of these parameters appears to be limited, highlighting the need to develop novel prognostic markers.
In the last decade, several other possible prognostic factors have been identified, the clinical significance of which still needs to be fully determined, especially in terms of early neurological outcomes [3,4,5,6,7,8,9,10,11]. Among those factors, arterial stiffness, especially as evaluated by the pulse wave velocity (PWV), is of particular interest [3, 5,6,7,8,9]. The PWV reflects arterial stiffness, and it is one of the earliest detectable signs of functional and structural changes in the vascular wall [12,13,14,15]. In clinical practice, the carotid–femoral PWV (cfPWV) mainly reflects the stiffness of central arteries, and the brachial–ankle PWV (baPWV) focuses on the stiffness of central and peripheral arteries [12, 13, 16,17,18]. Some studies showed that an increased PWV, including the cfPWV and baPWV, was independently associated with a poor functional outcome after acute ischemic stroke (AIS), but other studies presented different conclusions [3, 5,6,7,8,9]. Recently, the carotid–cerebral PWV (ccPWV) was shown to have a good correlation with the baPWV, and it can be determined noninvasively and easily; thus, it is becoming available as a means of measuring cerebral arterial stiffness [19, 20]. It mainly measures the stiffness of the segment (C-M segment) between the common carotid artery (CCA) and the ipsilateral middle cerebral artery (MCA). Therefore, it is necessary to evaluate the applicability of the ccPWV as a prognostic marker of early functional outcomes after AIS.
The aim of the present study was to evaluate the association between cerebral arterial stiffness confirmed by the ccPWV and the 3-month functional outcome after AIS.
Materials and methods
Patients
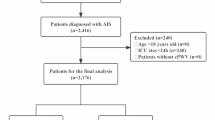
This study was a hospital-based, prospective observational study. Between June 2012 and May 2018, 1040 consecutive patients with first-ever acute ischemic stroke (AIS, within 7 days of symptom onset) were recorded in our AIS database. Among them, we consecutively enrolled anterior circulation infarction patients who underwent examination by multimodal brain magnetic resonance (MR), ccPWV, echocardiography and carotid ultrasonography during the admission period. We excluded patients who had [1] a history of previous stroke; [2] arrhythmia that could influence accurate assessment of the PWV; [3] a history of radiation therapy due to a head and neck cancer, which may promote cerebral artery atherosclerosis; [4] a high or medium risk of potential cardiac sources of embolism based on the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification system; [5, 21] a history of endovascular therapy due to anterior circulation disease, which may change the natural stiffness of the corresponding vascular segment; and [6] unsuitable temporal windows for conducting ccPWV measurements. Written informed consent for this study was obtained from either the patient or a member of his or her family. The ethics committee of the Second Affiliated Hospital of Guangzhou Medical University approved the study protocol. Good Clinical Practice guidelines in accordance with the Declaration of Helsinki were used, and the privacy of the patients was strictly protected.
Data acquisition
We collected data on sex, age, National Institute of Health Stroke Scale (NIHSS) score on admission, use of thrombolytics on admission, presence of risk factors [including hypertension, diabetes mellitus, coronary heart disease, hyperlipidemia, current smoking, systolic blood pressure (BP), and body mass index (BMI)], laboratory data, stroke subtype, use of medications during admission, and functional outcome for each patient (Table 1). Hypertension was defined as present if the subject had been previously diagnosed by a cardiology physician and were routinely receiving antihypertensive therapy. Patients were defined as having type 2 diabetes if they had known diabetes treated by diet, oral hypoglycemic drugs, or insulin before the stroke. Hypercholesterolemia was defined as the presence of a total cholesterol blood level ≥200 mg/dl. Coronary artery disease included any history of heart attack/myocardial infarction, angina, or coronary heart disease. Stroke subtypes were classified using the TOAST classification system. Cerebral angiographic findings for classification were obtained by MR angiography, computed tomography angiography, or digital subtraction angiography, which were performed on admission. The long-term functional outcome was assessed using the modified Rankin Scale (mRS) via a direct interview performed by a clinician or through a telephone interview conducted by a well-trained research nurse at 3 months after stroke onset. A poor functional outcome was defined as an mRS score >2 in this study.
Measurement of cerebral arterial stiffness
We evaluated the cerebral arterial stiffness of each patient by measuring the ccPWV. As previously described [19, 20], the ccPWV was measured by experienced operators using a special two-channel TCD (TCD-2000M; Beijing Chioy Medical Technology Co., Ltd., Beijing, China) with 2-MHz and 4-MHz ultrasound transducers with the patient in the supine position after at least 5 min of rest. The 2-MHz probe was held in a temporal window for detecting the proximal part of the MCA, and the 4-MHz transducer, in an angle fixator of thirty degrees, was placed on the ipsilateral pulsation point of the CCA beside the thyroid notch in the neck of the patient to detect the CCA. The transit time (Δt, ms) of the pulse wave traveling between the two insonation sites was automatically measured by the arterial pulse wave analysis system. The mean transit time (Δmt) was then determined from 10 consecutive cardiac cycles. The distance (D, m) traveled by the pulse wave was defined as the body surface distance (D1, m) between the two probes measured using a tape measure plus cosine 30° of the detecting depth (D2, m) for CCA, namely, D = D1 + D2 × cosine 30° [19, 20]. Thus, the ccPWV on each side was calculated as ccPWV = D/Δmt (m/s). Cerebral arterial stiffness was evaluated on the side with the greater value. The validity and reproducibility of the ccPWV measurements were previously reported elsewhere [19].
Statistical analyses
All baseline variables were analyzed. Data are expressed as the mean ± standard deviation (SD) or median (25th and 75th percentiles) for continuous variables and as the frequency and percentage for discrete variables. Comparisons between patients with favorable and more severe outcomes were performed by unpaired Student’s t-test or the Mann–Whitney U test where appropriate for continuous variables and the chi-squared test for categorical variables. To evaluate the discrimination ability of the ccPWV in predicting functional outcomes, receiver operating characteristic (ROC) curve analysis was used. The area under the curve (AUC) was calculated, and the optimal ccPWV cutoff value was determined as the level with the highest Youden index. Furthermore, ROC curve analysis was performed for the multivariate model to measure the improvement in predictive ability achieved by adding the ccPWV. We compared the AUC between the models with and without the ccPWV using the z-statistic, calculated by the method described by DeLong et al. [22] To identify predictors of a poor functional outcome, we performed multivariate logistic regression analyses with adjustments for sex, age, risk factors, medication during admission, and other variables with P < 0.05 in three different models on univariate analysis, and odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Statistical significance was established at P < 0.05. Statistical analyses were performed using SPSS 17.0 software for Windows (SPSS, Inc., Chicago, IL, USA).
Results
A total of 336 patients were enrolled in this study; the mean age was 60.58 ± 9.89 years,
and the proportion of men was 73.81% (Fig. 1 and Table 1). The mean ccPWV was 6.81 ± 2.37 m/s. Among all patients, 120 (35.71%) were classified as having small-artery occlusion, which was the most common stroke subtype in the present study, and seven (2.08%) of the eligible patients were treated with intravenous tissue plasminogen activator (tPA) according to international guidelines. The baseline demographics are shown in Table 1.
At 3 months after stroke onset, 110 (32.74%) patients had a poor functional outcome (mRS score > 2). A higher ccPWV was associated with a poor functional outcome; this association was found to be significant in all patients (Table 2). When stratified according to sex, age, initial stroke severity, risk factors, large artery atherosclerosis, and the small-vessel occlusion stroke subtype, the ccPWV was consistently higher in the poor outcome group than in the good outcome group (Table 2). Receiver operating characteristic curve analysis (Fig. 2) showed that when the continuous value of ccPWV only was considered a predictor of a poor functional outcome (Fig. 2a), the AUC was 85.27% (95% CI, 80.95%–89.58%; P < 0.001). The optimal ccPWV cutoff point was 6.66 m/s; at this point, the sensitivity was 82.73%, the specificity was 77.88%, and the Youden index was 0.61. As shown in Fig. 2b, sex, age, risk factors, and the significant variables determined by univariate analyses (Table 1 in the Supplementary Information) were combined in a multivariate model. When we compared the AUC of multivariate models with and without the ccPWV, the AUC was significantly increased (Z = 2.94, P = 0.048) from 86.42% (95% CI, 82.02–90.82%; P < 0.001) to 87.35% (95% CI, 83.00%–91.70%; P < 0.001). As shown in Fig. 3, patients with ccPWV ≥ 6.66 m/s more frequently suffered from disabling stroke than those with ccPWV < 6.66 m/s (P < 0.001). These findings suggest that the ccPWV could provide useful information for predicting the functional outcome after acute ischemic stroke.
Results of receiver operating characteristic analysis using the carotid–cerebral pulse wave velocity (ccPWV; continuous variable). a Receiver operating characteristic curve using the ccPWV alone for identifying a poor functional outcome after acute stroke. b Change in the area under the curve (AUC) after the addition of the ccPWV to the multivariate model for identifying a poor functional outcome. Adjustments were made for the following variables: sex, age, risk factors, National Institute of Health Stroke Scale score on admission, use of thrombolytic treatment, stroke subtype, antihypertensive medication, cholesterol, low density lipoprotein, and systolic blood pressure. CI confidence interval
In the univariate analysis (Table 1 in the Supplement Information), the factors associated with a poor functional outcome (P < 0.05) were the male sex, an older age, a higher NIHSS score on admission, higher cholesterol, low density lipoprotein, systolic blood pressure, diastolic blood pressure, mean blood pressure, and pulse pressure levels, a higher ccPWV, ccPWV ≥ 6.66 m/s, stroke subtype and antihypertensive medication use. Among them, ccPWV ≥ 6.66 m/s was the strongest predictor of a poor functional outcome [OR (95% CI): 16.86 (9.39–30.28); P < 0.001]. In the multivariate logistic regression analysis, ccPWV ≥6.66 m/s remained an independent predictor of a poor functional outcome (Table 3). After adjusting for age, sex, and significant (P < 0.05) variables from the univariate analyses in Model 1, only ccPWV ≥6.66 m/s and a higher NIHSS score on admission were independently associated with a poor 3-month functional outcome after acute ischemic stroke. After additionally adjusting for the risk factors (hypertension, diabetes mellitus, coronary artery disease, hypercholesterolemia, current smoking, systolic BP, and BMI), in Model 2, ccPWV ≥ 6.66 m/s [OR (95% CI): 6.27 (2.07–18.99); P = 0.001] and a higher NIHSS score [OR (95% CI): 1.50 (1.25–1.81); P < 0.001] remained independent predictors, as well as diabetes mellitus [OR (95% CI): 2.82 (1.38–5.75); P = 0.004]. Further inclusion of medication use during admission (hypoglycemic agents, antiplatelet agents, anticoagulants, and statins) did not improve the model and was not independently associated with the outcome. Not surprisingly, ccPWV ≥ 6.66 m/s and a higher NIHSS score on admission were remained independent predictors in Model 3.
Discussion
To the best of our knowledge, this is the first systematic evaluation of the association between cerebral arterial stiffness and the long-term functional outcome after AIS. As key findings, we observed that cerebral arterial stiffness, measured using the ccPWV, was independently associated with a poor 3-month functional outcome in patients with AIS. The ccPWV was higher in patients with a poor prognosis than in those with a good prognosis when the patients were classified into different subpopulations by their demographics, initial severity, risk factors and stroke subtypes. In the multivariate analysis, adjusting for possible confounding factors in three different models, ccPWV ≥ 6.66 m/s was still the strongest independent predictor of a poor functional outcome after AIS. These findings suggest that cerebral arterial stiffness may play a significant role in the outcome after AIS, independent of the stroke mechanism and known vascular risk factors.
There are some mechanisms that could explain the association between a higher ccPWV and a poor clinical outcome after AIS. The ccPWV, reflecting cerebral arterial stiffness, mainly measures the C-M segment stiffness [19]. Increased arterial stiffness may lead to the transmission of a higher pulse pressure to distal organs, including the brain, resulting in circumferential stretching of the arterial wall and leading to intimal fibrosis, necrosis, remodeling, atherosclerosis, and even plaque rupture [3, 5,6,7,8,9, 20]. Moreover, the C-M segment has been verified as a specific region more prone to atherosclerosis [23], and atherosclerosis of the C-M segment is regarded as the most frequent cause of anterior circulation ischemic stroke [23,24,25,26,27]. All of these factors have the potential to prompt cerebral ischemia, disturb brain repair, and cause new vascular events.
Many previous studies have demonstrated that the cfPWV and baPWV are both independent predictors of cardiovascular morbidity and mortality, but whether they are prognostic indicators of AIS is still controversial [3,4,5,6,7,8,9,10,11]. Two previous studies reported that the cfPWV and baPWV did not predict the functional outcome or in-hospital mortality rate of AIS; [5, 7] however, some studies have suggested that they are independent prognostic predictors of AIS [3, 8, 9]. Jinkwon Kim et al. reported that the AUC of the baPWV for predicting the 3-month functional outcome after AIS was 67.21% (sensitivity, 62.43%; specificity, 66.48%) [9]. Likewise, Kentaro Ishizuka et al. reported that the sensitivity and specificity of the baPWV for predicting the 3-month functional outcome was 80.6 and 48.6%, respectively [6]. In the present study, however, we observed that the AUC of the ccPWV for predicting the 3-month functional outcome after AIS was 85.27% (sensitivity, 82.73%; specificity, 77.88%), and addition of the ccPWV significantly improved the ability of the model to predict the functional outcome. These findings suggest that the ccPWV may be a more accurate indicator for predicting the functional outcome of AIS. Unlike the cfPWV and baPWV, the ccPWV reflects the stiffness of cerebral large arteries. Therefore, these results potentially indicate that regional arterial stiffness may be more suitable as a prognostic predictor of corresponding vascular events. However, this hypothesis needs to be verified in future studies.
The present study has both strengths and limitations. The strengths include the following: First, this study systematically verified the association between cerebral arterial stiffness and the long-term functional outcome after AIS for the first time. The present results demonstrate that cerebral arterial stiffness is an independent predictor of the functional outcome, which may guide us to adopt more therapeutic interventions to reduce poor outcomes after IS. Second, all patients were subjected to careful phenotyping for both the origin and consequences of AIS using validated scales and imaging techniques. Third, the ccPWV was measured by experienced operators with intensive training using gold standard techniques. However, the present study was performed at a single center and included a population with a single ethnicity. Meanwhile, the waveform of the flow wave may be slightly changed in cases with carotid stenosis, although it may not affect the accuracy of the ccPWV measurements. Furthermore, patients who died acutely or had a stroke of the most severe type might have been excluded because they could not participate in the ccPWV measurements or mRS evaluations. These conditions could have led to a selection bias. In addition, because of the observational study design, we cannot prove a causal relationship between the ccPWV and functional outcome after AIS.
In conclusion, cerebral arterial stiffness, measured using the ccPWV on admission, has value an independent prognostic factor for predicting the long-term functional outcome in patients with AIS.
References
Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:743–800.
Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498–504.
Gasecki D, Rojek A, Kwarciany M, Kowalczyk K, Boutouyrie P, Nyka W, et al. Pulse wave velocity is associated with early clinical outcome after ischemic stroke. Atherosclerosis. 2012;225:348–52.
Tuttolomondo A, Casuccio A, Della Corte V, Maida C, Pecoraro R, Di Raimondo D, et al. Endothelial function and arterial stiffness indexes in subjects with acute ischemic stroke: Relationship with TOAST subtype. Atherosclerosis. 2017;256:94–9.
Matsushima H, Hosomi N, Hara N, Yoshimoto T, Neshige S, Kono R, et al. Ability of the ankle brachial index and brachial-ankle pulse wave velocity to predict the 3-month outcome in patients with non-cardioembolic stroke. J Atheroscler Thromb. 2017;24:1167–73.
Ishizuka K, Hoshino T, Shimizu S, Shirai Y, Mizuno S, Toi S, et al. Brachial-ankle pulse wave velocity is associated with 3-month functional prognosis after ischemic stroke. Atherosclerosis. 2016;255:1–5.
Tziomalos K, Bouziana SD, Spanou M, Giampatzis V, Papadopoulou M, Kazantzidou P, et al. Increased augmentation index is paradoxically associated with lower in-hospital mortality in patients with acute ischemic stroke. Atherosclerosis. 2014;236:150–3.
Kim J, Song TJ, Song D, Lee KJ, Kim EH, Lee HS, et al. Brachial-ankle pulse wave velocity is a strong predictor for mortality in patients with acute stroke. Hypertension. 2014;64:240–6.
Kim J, Song TJ, Kim EH, Lee KJ, Lee HS, Nam CM, et al. Brachial-ankle pulse wave velocity for predicting functional outcome in acute stroke. Stroke. 2014;45:2305–10.
Tuttolomondo A, Di Sciacca R, Di Raimondo D, Serio A, D’Aguanno G, Pinto A, et al. Arterial stiffness indexes in acute ischemic stroke: relationship with stroke subtype. Atherosclerosis. 2010;211:187–94.
Kim DH, Kim J, Kim JM, Lee AY. Increased brachial-ankle pulse wave velocity is independently associated with risk of cerebral ischemic small vessel disease in elderly hypertensive patients. Clin Neurol Neurosurg. 2008;110:599–604.
Sugawara J, Tanaka H. Brachial-ankle pulse wave velocity: myths, misconceptions, and realities. Pulse. 2015;3:106–13.
Palombo C, Kozakova M. Arterial stiffness, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. Vasc Pharmacol. 2016;77:1–7.
Wang X, Keith JC Jr., Struthers AD, Feuerstein GZ. Assessment of arterial stiffness, a translational medicine biomarker system for evaluation of vascular risk. Cardiovasc Ther. 2008;26:214–23.
Lane HA, Smith JC, Davies JS. Noninvasive assessment of preclinical atherosclerosis. Vasc Health Risk Manag. 2006;2:19–30.
Coutinho T, Turner ST, Kullo IJ. Aortic pulse wave velocity is associated with measures of subclinical target organ damage. JACC Cardiovasc imaging. 2011;4:754–61.
Matsumoto C, Tomiyama H, Yamada J, Yoshida M, Shiina K, Yamashina A. Brachial-ankle pulse wave velocity as a marker of subclinical organ damage in middle-aged patients with hypertension. J Cardiol. 2008;51:163–70.
Tanaka A, Tomiyama H, Maruhashi T, Matsuzawa Y, Miyoshi T, Kabutoya T, et al. Physiological diagnostic criteria for vascular failure. Hypertension. 2018;72:1060–71.
Fu X, Huang C, Wong KS, Chen X, Gao Q. A new method for cerebral arterial stiffness by measuring pulse wave velocity using transcranial doppler. J Atheroscler Thromb. 2016;23:1004–1010.
Fu X, Liu Q, Zeng X, Huang S, Huang R, Gao Q. Association between cerebral arterial stiffness and large artery atherosclerosis in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2018;27:2993–3000.
Fure B, Wyller TB, Thommessen B. TOAST criteria applied in acute ischemic stroke. Acta Neurol Scand. 2005;112:254–8.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45.
Vilela P, Goulao A. Ischemic stroke: carotid and vertebral artery disease. Eur Radiol. 2005;15:427–33.
Kim JS, Kim YJ, Ahn SH, Kim BJ. Location of cerebral atherosclerosis: Why is there a difference between East and West? Int J Stroke 2016;13:35–46.
Qureshi AI, Caplan LR. Intracranial atherosclerosis. Lancet. 2014;383:984–8.
Chaturvedi S, Bhattacharya P. Large artery atherosclerosis: carotid stenosis, vertebral artery disease, and intracranial atherosclerosis. Continuum. 2014;20(2 Cerebrovascular Disease):323–34.
Battistella V, Elkind M. Intracranial atherosclerotic disease. Eur J Neurol. 2014;21:956–62.
Funding
This study was funded by the Key Project of the National Natural Science Foundation of China (81530036); the National Key Scientific Instrument and Equipment Development Project (31627803); the Mission Program of Beijing Municipal Administration of Hospitals (SML20150801); the Beijing Scholars Program and Beijing Brain Initiative of the Beijing Municipal Science & Technology Commission (Z161100000216137); the Beijing Municipal Commission of Health and Family Planning; the Program of National Natural Science Foundation of China (Grant No. 81371573); the Science and Technology Planning Project of Guangdong Province (Grant No. 2014A020212328); and the Project of Guangzhou Science and Technology Information Bureau (Grant No. 2014Y2-00046), China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Fu, X., Chu, C., Li, X. et al. Cerebral arterial stiffness for predicting functional outcome in acute ischemic stroke. Hypertens Res 42, 1916–1922 (2019). https://doi.org/10.1038/s41440-019-0313-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-019-0313-8
Keywords
This article is cited by
-
PUM2 aggravates the neuroinflammation and brain damage induced by ischemia–reperfusion through the SLC7A11-dependent inhibition of ferroptosis via suppressing the SIRT1
Molecular and Cellular Biochemistry (2023)