Abstract
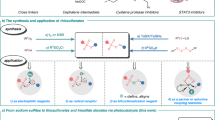
Unsymmetrical disulfides are widely found in the areas of food chemistry, pharmaceutical industry, chemical biology and polymer science. Due the importance of such disulfides in various fields, general methods for the nondirected intermolecular disulfuration of C-H bonds are highly desirable. In this work, the conversion of aliphatic C(sp3)-H bonds and aldehydic C(sp2)-H bonds into the corresponding C-SS bonds with tetrasulfides (RSSSSR) as radical disulfuration reagents is reported. The decatungstate anion ([W10O32]4−) as photocatalyst is used for C-radical generation via intermolecular hydrogen atom transfer in combination with cheap sodium persulfate (Na2S2O8) as oxidant. Herein a series of valuable acyl alkyl disulfides, important precursors for the generation of RSS-anions, and unsymmetrical dialkyl disulfides are synthesized using this direct approach. To demonstrate the potential of the method for late-stage functionalization, approved drugs and natural products were successfully C-H functionalized.
Similar content being viewed by others
Introduction
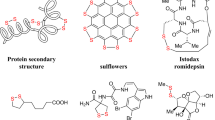
Owing to the unique properties of disulfides in folding, stability, and biological functions of peptides and proteins, the disulfide moiety is of high importance in such biomacromolecules1,2,3,4. Moreover, disulfides are also involved in biological systems in maintaining the cellular redox balance in cell survival5,6. Due to their unique pharmacological and physiochemical properties, disulfide moieties also occur in lower molecular weight biologically active natural products and in drugs7,8,9, such as in members of the antifungal polycarpamine family, gombamide A, polycarpamine B and C, Z-ajoene, (-)-emethallicin E, psammaplin F and kottamide E (Fig. 1). More generally, compounds bearing a disulfide moiety are widely found in the realm of food chemistry, pharmaceutical industry, chemical biology and also in polymer science10,11,12. Consequently, the development of synthetic strategies for the preparation of disulfides, in particular unsymmetrical disulfides, has gained considerable attention in recent years8,13,14,15,16,17.
However, efficient and selective synthetic methods for disulfide bond construction remain limited, especially considering the formation of unsymmetrical disulfides. Disulfides have been prepared by following either the S-S or the C-SS bond construction pathway (Fig. 2). Unsymmetrical disulfides can directly be accessed by oxidative cross coupling of two different thiols through electro-oxidation18 or using photocatalysis19,20,21. However, these methods suffer from a low chemoselectivity providing unavoidable homocoupling products (symmetrical disulfides). This selectivity problem was solved by using the ionic approach choosing sulfur derivatives bearing an anionic leaving group (LG) as electrophiles in combination with thiols as nucleophiles22,23. Along these lines, the disulfide exchange reaction represents the currently most commonly applied method for the preparation of unsymmetrical disulfides24. Considering C-SS bond formation, that approach has benefited from the development of electrophilic and nucleophilic “masked” disulfuration reagents25,26. These transformations proceed through metal-catalyzed cross-coupling27,28 or substitution reactions15,29,30,31. It is worth mentioning that in these disulfuration processes, one reaction component carries both sulfur atoms rendering such methods valuable for the preparation of unsymmetrical disulfides. Nevertheless, these approaches remain limited due to the tedious preparation of the requested rather complex disulfuration reagents.
Radical chemistry has become highly popular and offers alternative retrosynthetic routes for bond construction under mild conditions, and has therefore significantly influenced the reaction design in modern organic chemistry32,33,34,35,36,37,38. Recently, Pratt and co-workers have successfully prepared a diverse set of unsymmetrical disulfides via radical substitution with tetrasulfides39 or trisulfide-1,1-dioxides40 as C-radical acceptors, profiting from the inherent cleavage tendency of S-S bonds to generate thermodynamically stable perthiyl (RSS•) or sulfonyl radicals. The C-radicals engaging in such disulfurations were generated from O-acyl oximes, Barton PTOC esters or from carboxylic acids. Meanwhile, Wang and co-workers introduced a metal-free disulfuration of anilines via their corresponding diazonium salts41. In these reactions, the C-radical intermediates are generated through the transformation of a functional group which is accompanied by the generation of additional waste. It is important to note that Pratt and co-workers disclosed very recently an elegant regioselective radical C-H disulfuration that proceeds via Hofmann-Löffler-Freytag type chemistry, where C-radical generation is achieved by an intramolecular 1,5-HAT to an N-centered radical40. However, methods that allow the formation of C − SS bonds via an intermolecular HAT process are underdeveloped.
In this work, we explore a more atom-economic radical disulfuration strategy that operates without the need of a pre-functionalized radical precursor via direct C-H bond functionalization using a hydrogen atom transfer (HAT) catalyst. This route significantly expands the substrate scope for radical disulfuration, especially considering late-stage functionalization of pharmaceuticals.
Result and discussion
Reaction design
In recent years, photocatalysis has been successfully applied for hydrogen-atom transfer (HAT) mediated radical functionalization of C-H bonds. Among the photocatalysts that operate via HAT, the decatungstate anion ([W10O32]4−), featuring electron-deficient oxygen atoms, has been widely explored due to its ability to catalytically cleave even strong inert aliphatic C(sp3)-H bonds42,43,44,45,46,47,48,49,50,51,52. We therefore selected the tetrabutylammonium decatungstate 1 as the photocatalyst to realize the direct intermolecular radical C-H disulfuration. Our reaction design and mechanistic hypothesis are depicted in Fig. 3, exemplified for the functionalization of cyclohexane (2a). Irradiation of the decatungstate anion of 1 will lead to the reactive excited state 1*, which is known to abstract an H atom from an alkane such as 2a to give the cyclohexyl radical 2a’ along with H[W10O32]4−. Such alkyl radicals are readily trapped by a tetrasulfide to give the targeted disulfuration product 4 and the corresponding thermodynamically stable perthiyl radical (RSS•) which can dimerize (k = 6 × 109 M−1 s−1, for R = t-Bu) to reform the starting tetrasulfide53. The photocatalysis cycle would be closed by deprotonation of H[W10O32]4− to give [W10O32]5− which should then be oxidized via single-electron transfer (SET) to regenerate the starting decatungstate 1. Since the perthiyl radical (RSS•) is thermodynamically stable, hydrogen abstraction of this RSS-radical from H[W10O32]4− to regenerate 1 is not very likely. Therefore, an external stoichiometric oxidant is probably required to drive the process. Moreover, the RSSH formed in such a HAT would be a very good hydrogen atom donor that would competitively react with the alkyl radical 2a’ suppressing the desired disulfuration. A challenge associated with our design is the potential unwanted direct oxidation of the tetrasulfide with the terminal oxidant. We selected cheap Na2S2O8, that is known to oxidize [W10O32]5−54, as the oxidant in the targeted C-H disulfuration. In this redox reaction, the NaSO4-radical will be generated that may also act as an electrophilic reagent engaging in C-H abstraction from the substrate55. Another problem that might appear is the potential direct reduction or oxidation of the tetrasulfide by the redox catalyst which must also be circumvented.
Investigation of the reaction conditions
Optimization studies were performed with cyclohexane 2a and the tetrasulfide 3a as the limiting reagent targeting the unsymmetrical disulfide 4a (Table 1). Careful experimentation revealed that this reaction is best conducted with TBADT 1 (2 mol%) and sodium persulfate Na2S2O8 (1.5 equiv.) in acetonitrile/water (2:1) upon irradiation with a Kessil 40 W 390 nm lamp for 12 h at 60 °C to provide the disulfuration product 4a in 86% isolated yield (entry 1). The reaction did not occur in the absence of the photocatalyst 1 (entry 2) and showed a large solvent dependence. In a CH2Cl2/water mixture, only traces of 4a were formed (entry 3) and in acetonitrile a low yield was noted (entry 4). Decreasing the reaction temperature to 25 °C resulted in a lower conversion (46%, entry 5). Interestingly, without added Na2S2O8, the disulfide 4a could still be obtained, albeit in low 12% yield (entry 6). We currently assume that the tetrasulfide 3a is also capable of reoxidizing the photocatalyst, though at a much lower efficiency. This catalyst reoxidation is slightly more efficient in aqueous acidic acetonitrile, where 4a was formed in 28% yield in the absence of the terminal oxidant (entry 7). An alternative is the reaction of the dithyl radical with H[W10O32]4− to regenerate the catalyst and the dithiol. However, tBuSSH was not detected in the reaction mixture. Replacing Na2S2O8 with Na2S2O4, the product 4a was formed in trace amounts (entry 8). Upon replacing the tetrasulfide 3a by a trisulfide-1,1-dioxide (p-TolSO2SSt-Bu), the disulfide 4a was observed in traces only (entry 9). When using cyclohexane 2a as the limiting reagent (2a 0.3 mmol, 3a 0.45 mmol) under otherwise identical conditions, 4a was obtained in 29% GC yield.
Substrate Scope
With the optimized conditions in hand, we turned our attention to explore the substrate scope (Fig. 4). The tetrasulfides were readily obtained from the corresponding thiols upon treatment with S2Cl2 (see Supplementary Information). Tert-butyl (3a), cyclohexyl (3b), n-nonyl (3c), benzyl (3d-3g), heterocyclic (3 h), phenyl (3i), benzoyl (3j) and ester (3 l) substituted tetrasulfides were included into these investigations. In addition, the more complex cysteine-derived tetrasulfide 3k was also prepared. Scope with respect to the tetrasulfide component was tested first with cyclohexane (2a) as the C-radical precursor. All tetrasulfide reagents 3a-3l performed well to provide the disulfuration products 4a-4k’ with moderate to high yields. Some reactions were repeated using cyclohexane as the limiting reagent; however, low yields were obtained in these cases (see 4b-4d). Replacement of the tert-butyl substituent in the parent reagent 3a with cyclohexyl or a long-chain alkyl group was well tolerated and the products 4b and 4c were obtained in high yields (89% and 81%). Bisbenzyl-substituted tetrasulfides bearing either electron-donating or electron-withdrawing para-substituents at the arene moiety showed good reactivity (4d-4g, 76–83%). Moreover, the tetrasulfide 3 h reacted smoothly to afford the 2-furyl-substituted disulfide 4 h in 71% isolated yield. However, with the diphenyltetrasulfide 3i the targeted 4i was not formed. Instead the monosulfide 4i’ was obtained (72%). We found that the reaction in the absence of Na2S2O8-oxidant gave the targeted disulfide 4i as minor product in 26% yield besides the monosulfide 4i’ (37%). A similar outcome was noted for the bisbenzoyltetrasulfide 3j providing under the standard conditions exclusively the monosulfide 4j’ (53%). In this case, the oxidant-free protocol afforded the monosulfide 4j’ (37%) and the targeted disulfide 4j (41%). We found that diaryl, diacyl tetrasulfides and the corresponding targeted disulfides are not perfectly stable under the reaction conditions eventually leading to the isolated monosulfides. It is worth mentioning that acyl alkyl disulfides (see 4j and also congeners depicted in Fig. 5) are valuable precursors for the preparation of difficult-to-access hydropersulfides (RSSH)56,57,58, which are excellent hydrogen-atom donors59 playing a regulatory role in redox processes in biology60,61,62.
Isolated yields are provided. Selectivity determined by gas chromatography is reported as the percentage of the major regioisomer. GC yields with alkanes as the limiting reagent in parentheses: substrate 2 (0.3 mmol) and tetrasulfide 3 (0.45 mmol). aMethod A: tetrasulfide 3 (0.3 mmol, 1.0 equiv.), 2 (3 mmol, 10 equiv.), TBADT 2 mol%, Na2S2O8 (0.45 mmol, 1.5 equiv.), solvent 3 mL (CH3CN/H2O, v/v, 2/1), 390 nm, Ar, 60 °C, and 12 h. bMethod B: tetrasulfide 3 (0.3 mmol, 1.0 equiv.), 2 (3 mmol, 10 equiv.), TBADT 2 mol%, solvent 3 mL [CH3CN/(HCl aq. 1.0 M), v/v, 2/1], 390 nm, Ar, 60 °C, and 12 h. cUsing CH3CN/H2O (v/v, 2/1) as solvent. d2 (0.3 mmol, 1.0 equiv.), tetrasulfide 3 (0.45 mmol, 1.5 equiv.), TBADT 2 mol%, solvent 3 mL [CH3CN/(HCl aq. 1.0 M), v/v, 2/1], 390 nm, Ar, 60 °C, and 12 h. eTetrasulfide 3 (0.3 mmol, 1.0 equiv.), substrate 2 (1.5 mmol, 5.0 equiv.), TBADT 2 mol%, Na2S2O8 (0.45 mmol, 1.5 equiv.), solvent 3 mL (CH3CN/H2O, v/v, 2/1), 390 nm, Ar, 60 °C, and 12 h. fDetermined by 1H NMR spectroscopy. gTetrasulfide 3 (0.45 mmol, 1.5 equiv.), substrate 2 (0.3 mmol, 1.0 equiv.), TBADT 2 mol%, Na2S2O8 (0.45 mmol, 1.5 equiv.), solvent 3 mL (CH3CN/H2O, v/v, 2/1), 390 nm, Ar, room temperature, and 12 h.
Isolated yields are provided. Reaction conditions: substrate 5 (0.3 mmol, 1.0 equiv.), tetrasulfide 3a (0.45 mmol, 1.5 equiv.), TBADT 2 mol%, Na2S2O8 (0.45 mmol, 1.5 equiv.), solvent 3 mL (CH3CN/H2O, v/v, 2/1), 390 nm, Ar, 60 °C, and 12 h. aReaction time: 4 h. If the reaction time is extended to 12 h, some of the products will decompose into aroylmonosulfides (see SI for details). bReaction conducted at room temperature. cWith tetrasulfide 3b (1.5 equiv.). dDetermined by 1H NMR spectroscopy. eReaction run in the absence of Na2S2O8 in 3 mL [CH3CN/(HCl aq. 1.0 M), v/v, 2/1]. fReaction conditions: substrate 5m (0.2 mmol, 1.0 equiv.), 5 h. gReaction conditions: substrate 5n (0.1 mmol, 1.0 equiv.), room temperature, 12 h.
The scope with respect to the C-radical precursor was addressed next and the direct disulfuration of unactivated C(sp3)-H bonds in various systems was investigated. Larger-ring cycloalkanes were also converted into the corresponding disulfuration products 4l-4n in good yields (74–76%). Surprisingly, for the reactions of adamantane derivatives we found that better yields can be obtained in the absence of Na2S2O8. In these cases, the adamantane derivative was used as the limiting component in combination with 1.5 equivalents of the tetrasulfide. The reaction of adamantanes bearing an electron-withdrawing group at the 1-position proceeded predominantly at the tertiary sites in 51–68% yield (see 4o-4r). For 1-hydroxy- and 1-bromoadamantane, reaction occurred with complete regioselectivity, whereas the 1-adamantyl methyl ketone provided three isomers. The regioselectivities observed agree well with reported selectivities for TBADT mediated HAT processes on adamantanes and is a result of steric as well as thermodynamic effects50,63. Substrates bearing methyl, methylene and methine moieties react preferably at the tertiary position. Thus, the disulfides 4 s and 4t were isolated with good regioselectivities with little disulfuration at the terminal methyl groups. No reaction occurred at the two methylene moieties that are both deactivated by the neighboring cyano group due to polar effects. It is known that the excited decatungstate reacts as an electrophilic H-abstractor which engages preferably with nucleophilic C-H σ-bonds42. Accordingly, the α- and β-positions of the ketone 3 u are deactivated and disulfuration occurred with good regioselectivity at the terminal methyl groups (4 u, 50% yield, 80% regioselectivity). Note that in addition to polar effects, steric effects have to be also considered in these intermolecular HATs. The disulfuration of ketone 3 u’ gave the product 4 u’ in 42% yield with 78% regioselectivity. In analogy to the open chain ketones, the electrophilic α-position in cyclic ketones is deactivated and disulfuration proceeds at the β and γ-sites (4 v and 4w, 72% and 82%). Again, with the cyclic ketone as the limiting reagent only moderate yields were obtained. As expected, a similar outcome was noted for the reaction of a cyclic sulfone (4x, 68%). Consistent with the hydridic nature of C-H bonds next to oxygen or sulfur atoms, functionalization of 3 y afforded the disulfides 4 y (62%) with excellent selectivity as single regioisomers. For the reaction with tetrahydrofuran 3z, 4z was obtained in 32% yield besides some unidentified byproducts. For benzylic substrates, disulfurated products 4aa (82%) and 4bb (86%) could be obtained in high yields at room temperature using the benzylic compound as the limiting reagent. Thus, for reactive substrates with activated C-H bonds the disulfuration works well with the alkane as the limiting reagent.
As mentioned above, S-acylated disulfides are precursors for hydropersulfides RSSH that can be used in nucleophilic substitution or metal-catalyzed cross-coupling reactions for the preparation of unsymmetrical disulfides27,30,64. Reported methods for the preparation of acyl alkyl disulfides generally require to synthesize complex sulfur-containing reagents or highly unstable sulfur chloride moieties27,60,65,66. Moreover, bisacyltetrasulfides are rather unstable and lead to acylated monsulfide products (see above). We therefore tested aldehydes as acyl radical precursors for the direct preparation of S-acylated disulfides (Fig. 5). Pleasingly, with the aldehyde as the limiting reagent using 1.5 equivalents of the tetrasulfide, functionalization of aldehydic C-H bonds worked well. For example, the aromatic 3-thienylcarbaldehyde 5a could be transformed in a very good yield to the S-aroylated disulfide 6a (89%). For the reaction of benzaldehyde (5b) and 2-naphthaldehyde (5c), the targeted disulfides 6b (30%) and 6c (46%) were formed in moderate yields besides the corresponding aroylmonosulfides (51% and 31%). However, upon shortening reaction time to 4 h, the disulfides 6b and 6c were obtained with excellent yields (82% and 76%, respectively). Due to product instability, decomposition to give the corresponding monosulfides is occurring at longer reaction times in these cases. Primary aliphatic aldehydes bearing a phenyl (5d) or a furanyl moiety (5e) afforded the disulfuration products 6d and 6e in 50% and 82% yields, respectively. Of note, aliphatic aldehydes reacted slower and 12 h reaction time was required to complete the disulfuration. As expected, primary long-chain aliphatic aldehydes worked equally well (6f and 6g, 69–91% yields). The α-branched aliphatic aldehyde 5h was converted to the disulfide 6h (52%) and the corresponding decarbonylated dialkyl sulfide was not identified. However, with pivalaldehyde (5i) as a member of the tert-alkyl carbaldehyde family, the C-H functionalization reaction with the tetrasulfide 3b provided the unsymmetrical dialkyl disulfide 6i (89%) resulting from decarbonylation of the intermediate acyl radical. Such decarbonylation processes are often used to generate tertiary carbon radicals67.
Synthetic applications
To demonstrate the utility of this methodology for late-stage functionalization of natural products and pharmaceuticals, more complex substrates were tested (Fig. 5). For example, the lurasidone derivative 5j underwent a highly regioselective radical disulfuration to afford 6j in 72% yield with high diastereoselectivity (dr = 10:1). Sclareolide, a well-studied sesquiterpenoid, reacted with tetrasulfide 3a to provide 6k as a single regio- and diastereoisomer (52%). The disulfuration process gave direct access to the memantine derivative 6 l that was isolated in 71% yield and good regioselectivity. Moreover, dehydrocholic acid and indomethacin derivatives worked to give the corresponding disulfuration products 6m and 6n in 80% and 42% yields, respectively.
To document the practicality of the transformations, larger scale experiments were conducted at lower catalyst loading (1 mol% of 1 was used). As shown in Fig. 6a, the product 4a was obtained in 84% yield (1.72 g) on a 5.0 mmol scale and the disulfide 6 g was isolated in 88% yield on a 10 mmol scale, documenting the robustness of these procedures.
Mechanistic Investigations
The radical nature of the disulfuration was supported by control experiments. When adding 2.0 equivalents of the radical scavenger TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy), yield of the disulfuration product decreased and the TEMPO-trapping product 7 was detected by HRMS analysis (Fig. 6b). Considering the fast rate of the reaction of TEMPO with an acyl radical27, the low amount of TEMPO trapping product is surprising. This is likely due to the fact that TEMPO gets oxidized by Na2S2O8 under the applied conditions. We therefore ran a trapping experiment with cyclohexane in the absence of Na2S2O8. Indeed, TEMPO adduct 8 was isolated in 21% isolated yield along with traces of the disulfide 4a. These results indicate that the reaction proceeds through a radical-based mechanism and that the corresponding alkyl and acyl radicals were generated under the photocatalysis conditions. Moreover, repeating the disulfuration of cyclohexane (2a) in the presence of 2.0 equivalents of methyl acrylate, the radical cascade product 9 was detected by HMRS analysis along with 4a, further supporting the radical nature of the transformation (Fig. 6b).
Decatungstate photocatalysis has enabled the development of a practical radical C-H bond disulfuration strategy, that allows to access a broad range of unsymmetrical disulfides. The method works with readily prepared tetrasulfides as C-radical trapping reagents. Steered by steric and electronic effects, C-H functionalization via HAT occurs with high regioselectivity on simple and also more complex alkanes. In addition, reaction also works on aromatic and aliphatic aldehydes, where disulfuration proceeds with complete regioselectivity at the aldehydic C-H bond to afford unsymmetrical acyl disulfides. Importantly, such acylated disulfides are valuable precursors for hydropersulfides RSSH. Successful application of our strategy for late-stage C-H functionalization of more complex compounds documents its potential for the preparation of biologically important disulfide-containing compounds.
Methods
Representative procedures for the alkane and aldehyde disulfuration
Alkane disulfuration. To an oven dried Schlenk tube with a magnetic stirring bar, the tetrasulfide 3 (0.3 mmol, 1.0 equiv.), the substrate 2 (3 mmol, 10 equiv.), Na2S2O8 (0.45 mmol, 1.5 equiv.), photocatalyst TBADT (2 mol%), and 3.0 mL mixed solvent (CH3CN/H2O, v/v, 2/1) were added under argon atmosphere using standard Schlenk techniques at ambient temperature. After backfilling with nitrogen, the tube was placed in a photoreactor, stirred and irradiated with a Kessil 40 W 390 nm lamp for 12 h at 60 oC. The solvent was removed under reduced pressure and the crude residue was purified by silica gel column chromatography with hexane as the eluent to afford the desired product.
Aldehyde disulfuration. To an oven dried Schlenk tube with a magnetic stirring bar, the aldehyde 5 (0.3 mmol, 1.0 equiv.), the tetrasulfide 3 (0.45 mmol, 1.5 equiv.), Na2S2O8 (0.45 mmol, 1.5 equiv.), photocatalyst TBADT (2 mol%) and 3.0 mL mixed solvent (CH3CN/H2O, v/v, 2/1) were added under argon atmosphere using standard Schlenk techniques at ambient temperature. After backfilling with nitrogen, the tube was placed in a photoreactor, stirred and irradiated with a Kessil 40 W 390 nm lamp for 12 h at 60 oC. The solvent was removed under reduced pressure and the crude residue was purified by silica gel column chromatography with hexane as the eluent to afford the desired product.
Data availability
Supplementary information and chemical compound information accompany this paper at www.nature.com/ncomms. The data supporting the results of this work are included in this paper or in the Supplementary Information and are also available upon request from the corresponding author.
References
Narayan, M., Welker, E., Wedemeyer, W. J. & Scheraga, H. A. Oxidative Folding of Proteins. Acc. Chem. Res. 33, 805–812 (2000).
Hogg, P. J. Disulfide Bonds as Switches for Protein Function. Trends Biochem. Sci. 28, 210–214 (2003).
Liu, T. et al. Enhancing Protein Stability with Extended Disulfide Bonds. Proc. Natl Acad. Sci. USA. 113, 5910–5915 (2016).
Góngora-Benítez, M., Tulla-Puche, J. & Albericio, F. Multifaceted Roles of Disulfide Bonds. Peptides as Therapeutics. Chem. Rev. 114, 901–926 (2014).
Chari, R. V. J., Miller, M. L. & Widdison, W. C. Antibody–Drug Conjugates: An Emerging Concept in Cancer Therapy. Angew. Chem. Int. Ed. 53, 3796–3827 (2014).
Staben, L. R. et al. Targeted Drug Delivery Through the Traceless Release of Tertiary and Heteroaryl Amines from Antibody–drug Conjugates. Nat. Chem. 8, 1112–1119 (2016).
Wang, N., Saidhareddy, P. & Jiang, X. Construction of Sulfur-containing Moieties in the Total Synthesis of Natural Products. Nat. Prod. Rep. 37, 246–275 (2020).
Wang, M. & Jiang, X. Sulfur–Sulfur Bond Construction. Top. Curr. Chem. 376, 14 (2018).
Jiang, C.-S., Müller, W. E. G., Schröder, H. C. & Guo, Y.-W. Disulfide- and Multisulfide-Containing Metabolites from Marine Organisms. Chem. Rev. 112, 2179–2207 (2012).
Silva, F. et al. Short Total Synthesis of Ajoene. Angew. Chem. Int. Ed. 57, 12290–12293 (2018).
Black, S. P., Sanders, J. K. M. & Stefankiewicz, A. R. Disulfide Exchange: Exposing Supramolecular Reactivity through Dynamic Covalent Chemistry. Chem. Soc. Rev. 43, 1861–1872 (2014).
Dong, R. et al. Persulfurated Coronene: A New Generation of “Sulflower”. J. Am. Chem. Soc. 139, 2168–2171 (2017).
Mai, S. & Song, Q. Divergent Synthesis of Disulfanes and Benzenesulfonothioates Bearing 2-Aminofurans From N-Tosylhydrazone-Bearing Thiocarbamates. Angew. Chem. Int. Ed. 56, 7952–7957 (2017).
Li, X.-B. et al. Mechanistic Insights into the Interface-Directed Transformation of Thiols into Disulfides and Molecular Hydrogen by Visible-Light Irradiation of Quantum Dots. Angew. Chem. Int. Ed. 53, 2085–2089 (2014).
Zhang, Q., Li, Y., Zhang, L. & Luo, S. Catalytic Asymmetric Disulfuration by a Chiral Bulky Three-Component Lewis Acid-Base. Angew. Chem. Int. Ed. 60, 10971–10976 (2021).
Parida, A., Choudhuri, K. & Mal, P. Unsymmetrical Disulfides Synthesis via Sulfenium Ion. Chem. Asian J. 14, 2579–2583 (2019).
Qiu, X., Yang, X., Zhang, Y., Song, S. & Jiao, N. Efficient and Practical Synthesis of Unsymmetrical Disulfides via Base-catalyzed Aerobic Oxidative Dehydrogenative Coupling of Thiols. Org. Chem. Front. 6, 2220–2225 (2019).
Huang, P., Wang, P., Tang, S., Fu, Z. & Lei, A. Electro-Oxidative S−H/S−H Cross-Coupling with Hydrogen Evolution: Facile Access to Unsymmetrical Disulfides. Angew. Chem. Int. Ed. 57, 8115–8119 (2018).
Dethe, D. H., Srivastava, A., Dherange, B. D. & Kumar, B. V. Unsymmetrical Disulfide Synthesis through Photoredox Catalysis. Adv. Synth. Catal. 360, 3020–3025 (2018).
Spiliopoulou, N. & Kokotos, C. G. Photochemical Metal-free Aerobic Oxidation of Thiols to Disulfides. Green. Chem. 23, 546–551 (2021).
Oka, M., Katsube, D., Tsuji, T. & Iida, H. Phototropin-Inspired Chemoselective Synthesis of Unsymmetrical Disulfides: Aerobic Oxidative Heterocoupling of Thiols Using Flavin Photocatalysis. Org. Lett. 22, 9244–9248 (2020).
Xiao, X., Feng, M. & Jiang, X. Transition-metal-free Persulfuration to Construct Unsymmetrical Disulfides and Mechanistic Study of the Sulfur Redox Process. Chem. Commun. 51, 4208–4211 (2015).
Soleiman-Beigi, M. & Arzehgar, Z. A Novel Method for the Direct Synthesis of Symmetrical and Unsymmetrical Sulfides and Disulfides from Aryl Halides and Ethyl Potassium Xanthogenate. Synlett 29, 986–992 (2018).
Arisawa, M. & Yamaguchi, M. Rhodium-Catalyzed Disulfide Exchange Reaction. J. Am. Chem. Soc. 125, 6624–6625 (2003).
Xiao, X., Xue, J. & Jiang, X. Polysulfurating Reagent Design for Unsymmetrical Polysulfide Construction. Nat. Commun. 9, 2191 (2018).
Xue, J. & Jiang, X. Unsymmetrical Polysulfidation via Designed Bilateral Disulfurating Reagents. Nat. Commun. 11, 4170 (2020).
Xiao, X., Feng, M. & Jiang, X. New Design of a Disulfurating Reagent: Facile and Straightforward Pathway to Unsymmetrical Disulfanes by Copper-Catalyzed Oxidative Cross-Coupling. Angew. Chem. Int. Ed. 55, 14121–14125 (2016).
Zou, J. et al. Phthalimide-Carried Disulfur Transfer To Synthesize Unsymmetrical Disulfanes via Copper Catalysis. ACS Catal. 9, 11426–11430 (2019).
Guo, J. et al. PdCl2/DMSO-Catalyzed Thiol–Disulfide Exchange: Synthesis of Unsymmetrical Disulfide. Org. Lett. 23, 3167–3172 (2021).
Park, C.-M. et al. 9-Fluorenylmethyl (Fm) Disulfides: Biomimetic Precursors for Persulfides. Org. Lett. 18, 904–907 (2016).
Gao, W.-C., Tian, J., Shang, Y.-Z. & Jiang, X. Steric and Stereoscopic Disulfide Construction for Cross-linkage via N-Dithiophthalimides. Chem. Sci. 11, 3903–3908 (2020).
Wang, X. & Studer, A. Iodine(III) Reagents in Radical Chemistry. Acc. Chem. Res. 50, 1712–1724 (2017).
Taniguchi, T. Advances in Chemistry of N-Heterocyclic Carbene Boryl Radicals. Chem. Soc. Rev. 50, 8995–9021 (2021).
Xiong, P. & Xu, H.-C. Chemistry with Electrochemically Generated N-Centered Radicals. Acc. Chem. Res. 52, 3339–3350 (2019).
Zhang, W. & Li, A. A Radical Step Forward. Nat. Chem. 9, 198–199 (2017).
Matsubara, H., Kawamoto, T., Fukuyama, T. & Ryu, I. Applications of Radical Carbonylation and Amine Addition Chemistry: 1,4-Hydrogen Transfer of 1-Hydroxylallyl Radicals. Acc. Chem. Res. 51, 2023–2035 (2018).
Bhunia, A. & Studer, A. Recent Advances in Radical Chemistry Proceeding through Pro-aromatic Radicals. Chem 7, 2060–2100 (2021).
Péter, Á., Agasti, S., Knowles, O., Pye, E. & Procter, D. J. Recent Advances in the Chemistry of Ketyl Radicals. Chem. Soc. Rev. 50, 5349–5365 (2021).
Wu, Z. & Pratt, D. A. Radical Substitution Provides a Unique Route to Disulfides. J. Am. Chem. Soc. 142, 10284–10290 (2020).
Wu, Z. & Pratt, D. A. A Divergent Strategy for Site-Selective Radical Disulfuration of Carboxylic Acids with Trisulfide-1,1-Dioxides. Angew. Chem. Int. Ed. 60, 15598–15605 (2021).
Chen, S. et al. Sandmeyer-Type Reductive Disulfuration of Anilines. Org. Lett. 23, 7428–7433 (2021).
Ravelli, D., Fagnoni, M., Fukuyama, T., Nishikawa, T. & Ryu, I. Site-Selective C–H Functionalization by Decatungstate Anion Photocatalysis: Synergistic Control by Polar and Steric Effects Expands the Reaction Scope. ACS Catal. 8, 701–713 (2018).
Fukuyama, T., Nishikawa, T. & Ryu, I. Site-Selective C(sp3)–H Functionalization of Fluorinated Alkanes Driven by Polar Effects Using a Tungstate Photocatalyst. Eur. J. Org. Chem. 2020, 1424–1428 (2020).
Laudadio, G. et al. Selective C(sp3)−H Aerobic Oxidation Enabled by Decatungstate Photocatalysis in Flow. Angew. Chem. Int. Ed. 57, 4078–4082 (2018).
Sarver, P. J. et al. The Merger of Decatungstate and Copper Catalysis to Enable Aliphatic C(sp3)–H Trifluoromethylation. Nat. Chem. 12, 459–467 (2020).
Sarver, P. J., Bissonnette, N. B. & MacMillan, D. W. C. Decatungstate-Catalyzed C(sp3)–H Sulfinylation: Rapid Access to Diverse Organosulfur Functionality. J. Am. Chem. Soc. 143, 9737–9743 (2021).
Mazzarella, D., Pulcinella, A., Bovy, L., Broersma, R. & Noël, T. Rapid and Direct Photocatalytic C(sp3)−H Acylation and Arylation in Flow. Angew. Chem. Int. Ed. 60, 21277–21282 (2021).
Wan, T. et al. Decatungstate-Mediated C(sp3)–H Heteroarylation via Radical-Polar Crossover in Batch and Flow. Angew. Chem. Int. Ed. 60, 17893–17897 (2021).
Schultz, D. M. et al. Oxyfunctionalization of the Remote C−H Bonds of Aliphatic Amines by Decatungstate Photocatalysis. Angew. Chem. Int. Ed. 56, 15274–15278 (2017).
Cao, H. et al. Photoinduced Site-selective Alkenylation of Alkanes and Aldehydes with Aryl Alkenes. Nat. Commun. 11, 1956 (2020).
Halperin, S. D., Fan, H., Chang, S., Martin, R. E. & Britton, R. A Convenient Photocatalytic Fluorination of Unactivated C-H Bonds. Angew. Chem. Int. Ed. 53, 4690–4693 (2014).
West, J. G., Huang, D. & Sorensen, E. J. Acceptorless Dehydrogenation of Small Molecules through Cooperative Base Metal Catalysis. Nat. Commun. 6, 10093 (2015).
Chauvin, J.-P. R., Haidasz, E. A., Griesser, M. & Pratt, D. A. Polysulfide-1-oxides React with Peroxyl Radicals as Quickly as Hindered Phenolic Antioxidants and Do So by A Surprising Concerted Homolytic Substitution. Chem. Sci. 7, 6347–6356 (2016).
Wang, X., Yu, M., Song, H., Liu, Y. & Wang, Q. Radical Transformation of Aliphatic C–H Bonds to Oxime Ethers via Hydrogen Atom Transfer. Org. Lett. 23, 8353–8358 (2021).
Ueda, M. et al. Site-Selective Alkenylation of Unactivated C(sp3)−H Bonds Mediated by Compact Sulfate Radical. Angew. Chem. Int. Ed. 60, 3545–3550 (2021).
Khodade, V. S., Pharoah, B. M., Paolocci, N. & Toscano, J. P. Alkylamine-Substituted Perthiocarbamates: Dual Precursors to Hydropersulfide and Carbonyl Sulfide with Cardioprotective Actions. J. Am. Chem. Soc. 142, 4309–4316 (2020).
Hamsath, A. et al. Acyl Selenyl Sulfides as the Precursors for Reactive Sulfur Species (Hydrogen Sulfide, Polysulfide, and Selenyl Sulfide). Org. Lett. 21, 5685–5688 (2019).
Kang, J., Ferrell, A. J., Chen, W., Wang, D. & Xian, M. Cyclic Acyl Disulfides and Acyl Selenylsulfides as the Precursors for Persulfides (RSSH), Selenylsulfides (RSeSH), and Hydrogen Sulfide (H2S). Org. Lett. 20, 852–855 (2018).
Chauvin, J.-P. R., Griesser, M. & Pratt, D. A. Hydropersulfides: H-Atom Transfer Agents Par Excellence. J. Am. Chem. Soc. 139, 6484–6493 (2017).
Khodade, V. S. & Toscano, J. P. Development of S-Substituted Thioisothioureas as Efficient Hydropersulfide Precursors. J. Am. Chem. Soc. 140, 17333–17337 (2018).
Meng, S. et al. Thiocysteine Lyases as Polyketide Synthase Domains Installing Hydropersulfide into Natural Products and A Hydropersulfide Methyltransferase. Nat. Commun. 12, 5672 (2021).
Saund, S. S. et al. The Chemical Biology of Hydropersulfides (RSSH): Chemical Stability, Reactivity and Redox Roles. Arch. Biochem. Biophys. 588, 15–24 (2015).
Kim, K., Lee, S. & Hong, S. H. Direct C(sp3)–H Cyanation Enabled by a Highly Active Decatungstate Photocatalyst. Org. Lett. 23, 5501–5505 (2021).
Dai, Z., Xiao, X. & Jiang, X. Nucleophilic Disulfurating Reagents for Unsymmetrical Disulfides Construction via Copper-catalyzed Oxidative Cross Coupling. Tetrahedron 73, 3702–3706 (2017).
Mizuta, M., Katada, T., Itoh, E., Kato, S. & Miyagawa, K. A Convenient, High-Yield Preparation of Novel Unsymmetrical Bis[acyl] Disulfides via N-(Aroylthio)-succinimides. Synthesis 1980, 721–722 (1980).
Huang, M.-Q. et al. Switchable Copper-Catalyzed Approach to Benzodithiole, Benzothiaselenole, and Dibenzodithiocine Skeletons. Org. Lett. 22, 3454–3459 (2020).
Xuan, J., Zhang, Z.-G. & Xiao, W.-J. Visible-Light-Induced Decarboxylative Functionalization of Carboxylic Acids and Their Derivatives. Angew. Chem. Int. Ed. 54, 15632–15641 (2015).
Acknowledgements
We thank the Alexander von Humboldt Foundation (Z.J.) for supporting this work.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
J.J.Z. and A.S. conceived and designed the experiments. J.J.Z. performed the experiments and analyzed the data. J.J.Z. and A.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, J., Studer, A. Decatungstate-catalyzed radical disulfuration through direct C-H functionalization for the preparation of unsymmetrical disulfides. Nat Commun 13, 3886 (2022). https://doi.org/10.1038/s41467-022-31617-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-31617-5
This article is cited by
-
Generation of perthiyl radicals for the synthesis of unsymmetric disulfides
Nature Communications (2025)
-
SO2F2 mediated click chemistry enables modular disulfide formation in diverse reaction media
Nature Communications (2024)
-
Bioinspired zinc-mediated umpolung thiolation of alkyl electrophiles: reaction development, scope and mechanism
Science China Chemistry (2024)
-
Radical approaches to C–S bonds
Nature Reviews Chemistry (2023)