Abstract
The abilities of an organism to cope with extrinsic stresses and activate cellular stress responses decline during aging. The signals that modulate stress responses in aged animals remain to be elucidated. Here, we discover that feeding Caenorhabditis elegans (C. elegans) embryo lysates to adult worms enabled the animals to activate the mitochondrial unfolded protein response (UPRmt) upon mitochondrial perturbations. This discovery led to subsequent investigations that unveil a hedgehog-like signal that is transmitted from the germline to the soma in adults to inhibit UPRmt in somatic tissues. Additionally, we find that the levels of germline-expressed piRNAs in adult animals markedly decreased. This reduction in piRNA levels coincides with the production and secretion of a hedgehog-like signal and suppression of the UPRmt in somatic cells. Building upon existing research, our study further elucidates the intricate mechanisms of germline-to-soma signaling and its role in modulating the trade-offs between reproduction and somatic maintenance within the context of organismal aging.
Similar content being viewed by others
Introduction
Aging, a time-related deterioration in organisms, is caused by changes in the homeostatic regulation of intrinsic processes, which can be accelerated by environmental stressors. Extensive studies in model organisms have demonstrated that during aging, the abilities to cope with different stresses and activate cellular stress responses are reduced1,2,3,4,5,6. Animals respond to mitochondrial perturbation by activating the mitochondrial unfolded protein response (UPRmt), a mitochondrion-to-nucleus communication that activates the transcription of mitochondrial stress response genes7,8. The UPRmt exhibits a distinct temporal activation profile in C. elegans, with the most significant induction observed prior to adulthood, particularly during developmental stages9. This suggests that once worms become adults and commit to reproduction, their ability to sense mitochondrial damage or respond to mitochondrial stress is diminished. Similarly, the decline of the mitochondrial stress response has also been reported in aged rats10. These phenomena correlate with the observations that mitochondrial disorders occur during aging, and mitochondria play essential roles in age-related diseases such as neurodegenerations.
In this work, we identify a potential piRNA-regulated germline-to-soma Hedgehog signaling pathway that enables the germline to communicate with key metabolic and stress response tissues, prioritizing resource allocation for reproduction over somatic maintenance.
Results
UPRmt is activated in adult worms upon ingestion of embryo lysates
To understand the molecular mechanisms that govern the age-related decline of UPRmt activation, we first verified this phenomenon by feeding C. elegans with different mitochondrial inhibitors (antimycin, carbonyl cyanide 3-chlorophenylhydrazone (CCCP), and paraquat), Escherichia coli (E. coli) strains expressing double-stranded RNAs (dsRNAs) that target nuclear-encoded mitochondrial genes atp-2, cco-1 or spg-7, or a Pseudomonas aeruginosa strain (PA14) reported to inhibit mitochondrial function11. The RNAi efficiency for each gene was confirmed by quantitative PCR (Supplementary Fig. 1). Activation of UPRmt, detected by expression of GFP driven by the promoter of a mitochondrial chaperone gene hsp-6 (hsp-6p::gfp), was notably observed at the L3 larval stage, but not in day 3 adults (Supplementary Fig. 2a, b). In addition, up-regulation of endogenous UPRmt transcripts was markedly observed in L3 worms, but not in day 3 adults, fed with antimycin (Supplementary Fig. 2c).
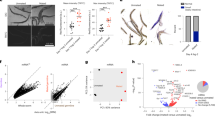
To identify the genes that modulate age-related decline of UPRmt activation, we then carried out several rounds of ethyl methane sulfonate (EMS) mutagenesis and forward genetic screens in C. elegans. Unfortunately, we failed to obtain any mutant worms capable of activating UPRmt in late adulthood. Inspired by previous studies demonstrating that establishing a shared circulatory system between young and aged mice can reverse specific aging phenotypes in older animals through factors present in young mice’s blood12,13, we fed C. elegans embryo lysates to adult worms to explore similar effects. Interestingly, we found that feeding adult worms with C. elegans embryo lysates, but not lysates from adult worms, activated UPRmt in adult worms (Fig. 1a–c and Supplementary Fig. 2d–f). Previous study has indicated that the age of parental worms can affect the traits of their offspring14. In light of this, we investigated whether embryo lysates from parental C. elegans at day 1, day 2, and day 3 of adulthood would have distinct effects. Our findings revealed that embryo lysates from worms at any of these days of adulthood equally induced UPRmt activation in adult worms (Supplementary Fig. 2g). Feeding adult worms with embryo lysates also promoted the survival of worms treated with mitochondrial inhibitors (Fig. 1d and Supplementary Fig. 2h). In addition, the effect of embryo lysate treatment was not specific to GFP fluorescent reporters, as the levels of endogenous UPRmt transcripts were also elevated upon embryo lysate feeding (Fig. 1e). UPRmt signaling activates transcriptional responses through the transcription factor ATFS-1, in conjunction with the chromatin remodeling protein DVE-1 and its interacting protein UBL-58. Knockdown of atfs-1 and dve-1, but not ubl-5 in adult worms prevented the activation of UPRmt and the elevated survival of embryo lysate-treated worms (Supplementary Fig. 2i, j). Moreover, we compared lysates from embryos, L3, young adult (YA) and day 6 adult worms. We found that embryo lysates were the most effective in activating UPRmt and promoting the survival of adult worms challenged with mitochondrial insults (Fig. 1c, d). To investigate whether embryo lysates affect a broader spectrum of stress responses in adult worms, we performed feeding assays with ER unfolded protein response (UPRER) and heat shock response (HSR) reporter strains (hsp-4p::gfp and hsp-16.2p::gfp, respectively). We found that embryo lysates specifically activated UPRmt in adult worms, they did not affect UPRER or HSR (Supplementary Fig. 3a–d). In addition, embryo lysates marginally improved survival under heat shock (Supplementary Fig. 3e, f).
a Schematic depicting the experimental procedure to treat day 7 hsp-6p::gfp adult worms with antimycin and lysates from embryos or day 6 adult worms. Embryo were harvested from day 1 adult worms. b Representative fluorescence images of hsp-6p::gfp worms with the indicated treatments. Antimycin and embryo/day 6 worm lysates were administered to day 7 adult worms for 24 h prior to visualization. Scale bar, 200 µm. c Quantification of GFP fluorescence intensity in panel (b). d Survival rate of day 9 N2 adults after 72 h exposure to antimycin and embryo/worm lysates. e qRT-PCR analysis of the indicated UPRmt genes in day 7 wild-type (N2) worms with the indicated treatments. f, g Quantification of GFP fluorescence intensity in hsp-6p::gfp worms with the indicated treatments. Antimycin and embryo/worm lysates were provided to day 7 adult worms for 24 h. h Survival rate of day 9 N2 adults after 72 h exposure to antimycin and embryo lysates (from N2, dcr-1, or prg-1 mutant worms). i, Quantification of GFP fluorescence intensity in hsp-6p::gfp worms with the indicated treatments. Antimycin and embryo lysates were provided to day 7 adult worms for 24 h. For panels (d, e) and (h) represents the number of independent experiments; for panels (c, f, g) and (i) represents the number of worms. Error bars indicate mean ± SD. p values were assessed using a two-tailed t test. RNAi treatment started at the L1 stage across all figure panels, unless specified otherwise. Source data are provided as a Source data file.
We next aimed to determine what substance in the embryo lysates enables UPRmt activation in adult worms. DNase-, RNase- and proteinase-treated embryo lysates were fed to adult worms while they were challenged with mitochondrial insults. RNase treatment greatly impaired the ability of embryo lysates to activate UPRmt and promote animal survival upon mitochondrial inhibition. In contrast, DNase or proteinase treatment had no such effect (Fig. 1f and Supplementary Fig. 3g). To further test the type of RNA required for UPRmt activation in adult worms, we fed adult worms with embryo lysates generated from different mutants. Interestingly, embryo lysates, or L3 worm lysates from prg-1 mutants that lack mature PIWI-interacting RNAs (piRNAs)15,16 were not able to activate UPRmt and promote animal survival under mitochondrial stress conditions (Fig. 1g, h and Supplementary Fig. 3h). In contrast, embryo lysates from dcr-1 mutants, which lack mature microRNAs (miRNAs) and small interfering RNAs (siRNAs)17,18,19, were still capable of activating UPRmt and promoting animal survival (Fig. 1g, h). In addition, RNAi knockdown of genes involved in various piRNA-related processes suppressed the ability of embryo lysates to activate UPRmt in adult worms. These processes include piRNA maturation (prg-1)15,16, piRNA biogenesis (prde-1)20, and the biogenesis of piRNA-dependent 22G-RNAs (secondary siRNAs produced when piRNAs interact with target mRNAs), such as drh-3. It should be noted that DRH-3 is involved in the production of both piRNA-dependent and piRNA-independent 22G-RNAs, as well as the CSR-1 pathways21,22,23. Other suppressed genes are involved in nuclear silencing (hpl-2 or hrde-1)24,25 and dsRNA transmembrane transport (sid-1) (Fig. 1i). In contrast, embryo lysates were still capable of promoting UPRmt activation in animals with RNAi knockdown of meg-1, meg-3 or meg-4, which are required for cytoplasmic but not perinuclear P granule formation26,27 (Fig. 1i). Consistently, embryo lysates from worms subjected to RNAi silencing of genes in the piRNA biogenesis pathway also failed to activate UPRmt and enhance survival under mitochondrial stress conditions (Supplementary Fig. 3i, j). piRNAs are predominantly expressed and function in the germline28. Therefore, when fed to a germline-deficient mutant (a temperature-sensitive glp-4 allele raised under the restrictive temperature29), the embryo lysates could not promote UPRmt activation (Supplementary Fig. 3k). These results suggest that piRNAs in the embryo lysates might function in the germline to modulate UPRmt activity in the somatic tissues of adult worms. Through examining a variety of germline mutants29,30,31,32, we found that the elimination of germline stem cells (GSCs) (glp-1 or glp-4)—rather than oocytes (fem-3) or sperm (fog-1)—contributes to the disruption of UPRmt in adult worms (Supplementary Fig. 3l).
Mitochondrial function is associated with worm lifespan33. Lifespan analysis revealed that adult worms fed with embryo lysates, but not day 6 worm lysates, experienced a ~20% lifespan extension (Fig. 2a–c). This extension reduces to ~10% in atfs-1 mutants (Fig. 2d–g), suggesting embryo lysates may also regulate lifespan through mechanisms beyond UPRmt regulation. To systematically evaluate the effect of embryo lysates feeding, we conducted RNA sequencing (RNA-seq) on adult worms that were either untreated or treated with embryo lysates (Supplementary Data 1). Our findings revealed that embryo lysates affected the expression of genes related to fatty acid metabolism, degradation of branched-chain amino acids, and lysosome functions (Fig. 2h–j, l). Notably, worms treated with embryo lysates showed increased fatty acid degradation gene expression and decreased fatty acid elongation gene expression (Fig. 2k, m). Given that enhanced fat degradation is linked to longer worm lifespans34, we used Nile Red staining to assess fat content. This revealed a significant decrease in lipid droplet intensity in embryo lysate-treated worms, consistent with their lifespan extension (Fig. 2n, o).
a–f Survival curves of wild-type (N2) worms (a-c) or atfs-1 mutants (d-f) treated with embryo lysates or day 6 worm lysates. Lifespan data from 3 biological replicates are shown. g Median lifespan extension of wild-type (N2) worms and atfs-1 mutant worms treated with embryo lysates or day 6 worm lysates. h, l Gene Ontology analysis of embryo lysate feeding-dependent upregulated (h) or downregulated (l) genes in day 7 wild-type worms. i, j, k, m Heat map plots illustrate the differentially expressed genes that depend on embryo lysate feeding, covering pathways such as valine, leucine, and isoleucine degradation (i), lysosome genes (j), fatty acid degradation (k), and fatty acid elongation (m). n Representative fluorescence images of adult day 7 N2 worms treated with Nile Red and/or lysates for 24 h. Scale bar, 200 µm. o Quantification of Nile Red intensity in panel (n). Error bars indicate mean ± SD. n represents the number of independent experiments for panel (g), and the number of worms for panel (o). p values were assessed using the Log-rank (Mantel–Cox) test for panels (a–f), and a two-tailed t test for panels (g, o). For KEGG in panels (h, i), a hypergeometric distribution test is used to assess enrichment of differentially expressed genes. Detailed methods are in the methods section. For heat maps in panels (i–k) and (m), a two-tailed t test is used to evaluate differential gene expression. Source data are provided as a Source Data file.
A hedgehog-like signal suppresses UPRmt in adult worms
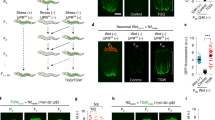
To identify the possible germline-to-soma signals modulated by piRNAs in the embryo lysates, we knocked down genes that have been reported to mediate the communications between the germline and the soma, including genes encoding putative secreted peptides/molecules associated with the insulin pathway35, the hedgehog pathway36, the Wnt signaling pathway37, the prostaglandin E2 pathway38, Cer retrotransposons39 and innate immunity40 (Supplementary Data 2). Interestingly, knockdown of wrt-5 and wrt-6, encoding hedgehog-like ligands, or ptr-8 and ptr-16, encoding homologs of the hedgehog receptor Patched, directly activated UPRmt in adult worms treated with antimycin, bypassing the requirement for ingestion of embryo lysates (Fig. 3a, b and Supplementary Fig. 4a–d). Accordingly, knockdown of wrt-5, wrt-6, ptr-8, or ptr-16 also elevated the transcript levels of endogenous UPRmt in adult worms challenged with mitochondrial insults (Fig. 3c and Supplementary Fig. 4e, f). More importantly, knockdown of other hedgehog-like family genes had no such effect (Supplementary Fig. 4g). Deficiency of wrt-5, wrt-6, ptr-8, or ptr-16 did not affect the levels of UPRmt activation in L4 stage larvae (Supplementary Fig. 4h, i). In adult worms, UPRmt induction via knockdown of wrt-5, wrt-6, ptr-8, or ptr-16 also required the transcription factor ATFS-1 and DVE-1, but not UBL-5, to elevate hsp-6p::gfp (Supplementary Fig. 5a, b). More interestingly, knockdown of wrt-5, wrt-6, ptr-8, or ptr-16 only enabled the adult worms to regain the ability to activate UPRmt, but not UPRER or the heat shock response (Supplementary Fig. 5c–f). Moreover, these knockdowns did not enhance survival in adult worms subjected to ER stress or heat shock (Supplementary Fig. 5g, h). These findings suggest a specific role for hedgehog-like signaling in regulating UPRmt.
a Representative fluorescence images of hsp-6p::gfp worms with the indicated treatments. Antimycin treatment occurred on day 3 of adulthood for 24 h. Scale bar, 200 µm. b Immunoblotting of GFP and tubulin in lysates collected from day 3 hsp-6p::gfp worms with the treatments described in panel (a). c qRT-PCR analysis of the indicated UPRmt genes in day 3 N2 worms subjected to the indicated treatments. d Schematic depicting the experimental procedure in panels (e, f). e qRT-PCR analysis of genes related to hedgehog signaling in day 7 N2 worms with the indicated treatments. f Survival rate of Day 7 N2 worms treated with embryo or worm lysates, followed by exposure to antimycin and the specified combinations of RNAi for 72 h. RNAi of individual genes started on day 7 of adulthood. Error bars indicate mean ± SD. n represents the number of independent experiments for panels (c, e, f). p values were assessed using a two-tailed t test. Source data are provided as a Source Data file.
Ingesting embryo lysates or knockdown of wrt-5, wrt-6, ptr-8, or ptr-16 enables adult worms to regain the ability to activate UPRmt upon mitochondrial perturbation. Therefore, we tested if the embryo lysate treatments exert their function by inhibiting the hedgehog-like signal. Day 7 worms were fed for one day with OP50, a bacterial strain conventionally used as a food source to culture C. elegans in the laboratory, or with OP50 plus embryo or day 6 worm lysates. RNAs were then extracted from a sub-population of the worms to test the expression levels of wrt-5, wrt-6, ptr-8, and ptr-16. At the same time, the other sub-population of the worms was treated with antimycin, together with control RNAi, or RNAi against each of the four genes, until day 10 to measure the survival rate (Fig. 3d). Interestingly, feeding adult worms with embryo lysates reduced the transcript levels of wrt-5 and wrt-6, but not ptr-8 and ptr-16 (Fig. 3e). Consistent with the previous report36, this result suggests that piRNAs in the embryo lysates transcriptionally regulate the hedgehog-like signal. However, no significant differences in the expression of wrt-5 and wrt-6 were observed between N2 and the piRNA pathway mutants (Supplementary Fig. 5i). We suspect that alternative mechanisms, potentially involving epigenetic regulation41, may compensate for the effects on wrt-5/6 expression in these mutants. In addition, embryo lysate treatment, or RNAi of wrt-5, wrt-6, ptr-8, or ptr-16, promoted animal survival under mitochondrial stress (Fig. 3f). More importantly, the embryo lysate-induced increase in animal survival upon mitochondrial perturbation was not further elevated by single RNAi of the four genes (Fig. 3f). This indicates that the increased animal survival caused by embryo lysates ingestion was primarily through suppression of the function of the hedgehog-like signaling proteins WRT-5 and WRT-6.
To explore the potential interactions between the hedgehog ligands WRT-5/6 and PTR-8/16, we conducted immunoprecipitation experiments using 293T cells overexpressing C. elegans wrt-5/6 or ptr-8/16. Although the observed protein sizes for PTR-8/16 were unexpected, siRNA knockdown experiments confirmed their identities (Supplementary Fig. 5j, k). We observed interactions between WRT-5 and PTR-8, as well as between WRT-6 and both PTR-8 and PTR-16 (Fig. 4a–d). These findings suggest that PTR-8 is a potential receptor for WRT-5, while both PTR-8 and PTR-16 may serve as receptors for WRT-6.
a-d Interactions of PTR-8 and PTR-16 with WRT-5 and WRT-6. HEK293T cells transfected with the indicated cDNAs and followed by the indicated immunoprecipitations. e qRT-PCR analysis of genes related to hedgehog signali ng in glp-4 worms at the indicated developmental stage. Worms were raised at the specified temperatures. f Quantification of GFP fluorescence intensity in hsp-6p::gfp worms treated with antimycin. Antimycin treatment started at indicated stage for 24 h. g Survival rate of N2 worms after 48 h of exposure to antimycin, started at the indicated developmental stage. Error bars indicate mean ± SD. n represents the number of independent experiments for panels (e, g), and the number of worms for panel (f). Source data are provided as a Source Data file.
To further investigate the precise timing of wrt-5/6 activation, we utilized the temperature-sensitive germline mutant glp-4. This approach enabled us to compare relative mRNA levels with and without a functional germline, across various developmental stages. In contrast to ptr-8 and ptr-16, our analysis revealed a significant increase in wrt-5 and wrt-6 expression in the germline during the L4 stage (Fig. 4e). Comparing to the control worms, knocking down wrt-5, wrt-6, ptr-8, or ptr-16 led to enhanced activation of the UPRmt and increased mitochondrial stress resistance when the worms enter adulthood (Fig. 4f, g). These results suggest that the activation of wrt-5 and wrt-6 is associated with the initiation of reproduction, highlighting a potential role for hedgehog-like signaling in early aging processes.
The age-related decline in UPRmt activation could be regulated by piRNAs targeting wrt-5 and wrt-6
To determine if piRNAs are essential for regulating wrt-5/6, we identified six piRNAs targeting each of wrt-5 and wrt-6 (piRTarBase42; Supplementary Fig. 6a). These piRNAs were added to a synthetic piRNA clusterE43 (Supplementary Fig. 6a), with six piRNAs targeting wrt-1 as controls. We found most targeted piRNAs were overexpressed, silencing wrt-5/6 in the germline (Supplementary Fig. 6b–e). Overexpressing piRNAs against wrt-5 or wrt-6 activated UPRmt in adult worms treated with antimycin (Fig. 5a, b and Supplementary Fig. 6f–i), but did not affect UPRmt in L4 larvae (Supplementary Fig. 6j, k). Importantly, there was a noticeable decline in levels of wrt-5/6 targeting piRNAs during germline development and aging (Fig. 5c). Furthermore, introducing synthesized wrt-5/6-targeting piRNAs into piRNA-devoid prg-1 embryo lysates activated UPRmt and improved survival under mitochondrial stress in lysate-fed adult worms (Fig. 5d, e). Adding wrt-5/6-targeting piRNAs to prg-1 lysates from embryos, L3 stages, and young adults—but not day 6 worms—effectively promoted UPRmt activation in lysate-fed adult worms (Supplementary Fig. 6l). This suggests that day 6 lysates might be missing essential components, beyond piRNAs, required to activate UPRmt.
a Representative fluorescence images of hsp-6p::gfp worms with the indicated treatments. Antimycin treatment occurred on day 3 of adulthood for 24 h. Scale bar, 200 µm. b Quantification of GFP fluorescence intensity in panel (a). c Heatmap showing the expression levels of piRNAs targeting wrt-5, wrt-6, and the control gene wrt-1 in lysates from different stages. d Quantification of GFP fluorescence intensity in hsp-6p::gfp worms with the indicated treatments. Antimycin, prg-1 embryo lysates and piRNAs were administered to day 7 worms for 24 h. e Survival rate of day 9 N2 worms after 72 h exposure to antimycin, prg-1 embryo lysates and piRNAs. Error bars indicate mean ± SD. n represents the number of independent experiments for panel (e), and the number of worms for panels (b) and (d). p values were assessed using a two-tailed t test. Source data are provided as a Source Data file.
Suppression of the hedgehog-like signal increases the resistance to mitochondrial stress and pathogen infection, but reduces brood size
Individual knockdown of the hedgehog-like genes wrt-5 and wrt-6, or the receptor genes ptr-8 and ptr-16, enables the adult worms to activate UPRmt under mitochondrial perturbation. Next, we tested if knockdown of these genes makes adult worms more resistant to mitochondrial stress. Indeed, knockdown of wrt-5, wrt-6, ptr-8, or ptr-16 during the whole life of the worms or only in adulthood promoted the survival of adult worms exposed to mitochondrial inhibitors (Fig. 6a, b and Supplementary Fig. 7a, b). In contrast, the survival rate of L4 worms was not affected by single deficiency of these genes (Supplementary Fig. 7c, d). A prior study identified two hedgehog ligands, WRT-1 and WRT-10, which are activated by germline overactivity, resulting in worm shrinkage and mortality36. Unlike wrt-5 or wrt-6, knocking down wrt-1 or wrt-10 did not activate UPRmt or impact resistance to mitochondrial stress in adult worms (Supplementary Fig. 4g, 7e). Knockdown of wrt-5, wrt-6, ptr-8, or ptr-16 elevated the transcript levels of stress response and innate immune response genes when the worms were exposed to mitochondrial inhibitor (Fig. 6c). Moreover, single gene deficiency also promoted the survival rate of adult worms exposed to pathogens such as Pseudomonas aeruginosa strains PA14 and PAO1, but not the survival of L4 worms under the same challenge (Fig. 6d, e and Supplementary Fig. 7f, g). Accordingly, lack of wrt-5, wrt-6, ptr-8, or ptr-16 elevated the expression levels of innate immune response genes (Fig. 6f, g and Supplementary Fig. 7h, i) and suppressed the accumulation of pathogen in adult worms exposed to PA14 or PAO1 (Fig. 6h, i). Moreover, overexpressing piRNAs that target either wrt-5 or wrt-6 enhanced resistance to mitochondrial inhibitor antimycin and PA14 infection in adult worms (Fig. 6j, k). Together, these results indicate that suppressing the hedgehog-like signal enhances the resistance to mitochondrial stress and pathogen infection in adult worms.
a, b Survival rate of day 9 wild-type (N2) worms treated with indicated RNAi and exposed to antimycin. RNAi treatment was applied either “whole-life” (starting at L1) or “adult-only” (starting on day 1 of adulthood). c qRT-PCR analysis of UPRmt genes in day 9 N2 adults treated with RNAi and exposed to antimycin. d, e Survival rate of day 5 N2 adults treated with RNAi and exposed to PA14. f Representative fluorescence images of day 5 irg-1p::gfp adults with the indicated treatments. Pathogen infections were done on day 5 of adulthood for 48 h unless otherwise noted. Scale bar, 200 µm. g qRT-PCR analysis of innate immune response genes in day 5 N2 animals exposed to PA14. h Representative fluorescence images of day 5 N2 worms treated with PAO1(GFP). Scale bar, 200 µm. i Quantification of PAO1 or PA14 CFU in day 5 N2 worms. j, k Survival rates of day 9 and day 5 hsp-6p::gfp adults overexpressing piRNAs targeting wrt-5, wrt-6, or a control gene, with antimycin treatment on day 9 and PA14 on day 5. l Brood size of N2 worms treated with RNAi from L1 stage (“whole life”) or day 1 adulthood (“adult-only”). m Brood size of hsp-6p::gfp worms overexpressing piRNAs targeting wrt-5, wrt-6, or a control gene. n Representative fluorescence images of day 5 vit-2::gfp worms treated with RNAi. Scale bar, 200 µm. o qRT-PCR analysis of the vit family genes in day 1 N2 adults treated with RNAi. Error bars indicate mean ± SD. n represents the number of independent experiments for panels (c, g, i, o), and the number of worms for panels (i, m). p values were assessed using a two-tailed t test for panels (c, g, i, l, m, o), and using the Log-rank (Mantel–Cox) test for panels (a, b, d, e, j, k). Source data are provided as a Source Data file.
When these four hedgehog pathway-related genes were singly knocked down starting at the L1 larval stage (during the whole life of the hatched animal), the brood size of the animals was greatly reduced (Fig. 6l). Overexpressing piRNAs that target either wrt-5 or wrt-6 also decreased brood size (Fig. 6m). Vitellogenin is a family of yolk proteins highly expressed in the intestine of the adult hermaphrodite and transported to the germline to support animal fertility and post-embryonic development44. The expression level of vit-2, the best-studied vitellogenin gene, was greatly reduced in worms with knockdown of wrt-5, wrt-6, ptr-8, or ptr-16 (Fig. 6n, o and Supplementary Fig. 8a, b). Additionally, we assessed yolk protein (YP) levels using gel electrophoresis45 and revealed that knockdown of wrt-5, wrt-6, ptr-8, or ptr-16 resulted in a significant reduction in YP content (Supplementary Fig. 8c–f). These data indicate that suppressing the hedgehog-like signal inhibits fertility and post-embryonic development.
Suppressing the hedgehog-like signal extends worm lifespan
Hedgehog signaling plays a crucial and evolutionarily conserved role in development. Worms carrying single mutant alleles of hedgehog pathway-related genes wrt-5, wrt-6, ptr-8, and ptr-16 exhibited developmental delays (Supplementary Fig. 8g) and commonly displayed the “exploded” phenotype during the early adult stage, due to defective vulval integrity46 (Supplementary Fig. 8h, i). However, worms fed with RNAi E. coli targeting wrt-5, wrt-6, ptr-8, and ptr-16 showed no developmental delays and a lower incidence of the “exploded” phenotype at the early adult stage (Supplementary Fig. 8j, k). This may be because the RNAi partially maintains hedgehog-like signaling capacity (Supplementary Fig. 1).
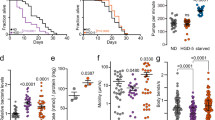
Knockdown of wrt-5, wrt-6, ptr-8, or ptr-16 caused approximately 30% of wild-type N2 worms to explode (Supplementary Fig. 9a). Interestingly, this phenotype occurred less frequently in germline and intestine-specific RNAi AMJ345 worms47 than in wild type N2 worms, suggesting that the hedgehog pathway’s role in tissues other than the germline and intestine contributes to the exploded phenotype (Supplementary Fig. 9b). Therefore, to avoid the exploded phenotype, we used AMJ345 worms for lifespan analysis. Knockdown of wrt-5, wrt-6, ptr-8, or ptr-16 in germline and intestine increased lifespan (Fig. 7a–c). Additionally, deficiency of atfs-1, which is essential for UPRmt induction, prevented the lifespan extension (Fig. 7d–f and Supplementary Fig. 9c). Accumulating evidence indicates that functional mitochondrial import machinery is crucial for UPRmt-mediated lifespan extension48. Although knockdown of wrt-5, wrt-6, ptr-8, or ptr-16 did not affect the expression of mitochondrial import machinery genes (Supplementary Fig. 9d), it enhanced the mitochondrial membrane potential (ΔΨ), as indicated by increased tetramethylrhodamine ethyl ester (TMRE) staining49 (Fig. 7g–i). Consistently, a fumarase activity assay showed an increase in the proportion of mitochondrial to total fumarase activity following these gene knockdowns (Fig. 7j and Supplementary Fig. 9e).
a–f Survival curves of control AMJ345 worms (a–c) or atfs-1-deficient AMJ345 worms (d–f) upon feeding with RNAi E. coli targeting a hedgehog pathway-related gene. Lifespan data from 3 biological replicates are shown. g-i Quantification of relative TMRE fluorescence in adult day 3 (g), day 5 (h), and day 7 (i) N2 worms. j Comparison of fumarase activity in different subcellular fractions in adult day 5 N2 worms. Fumarase activity was normalized to the quantity of protein used in the assay. Error bars indicate mean ± SD. n represents the number of independent experiments for panel j, and the number of worms for panels (g–i). p values were assessed using a two-tailed t test for panels (g–j) and the Log-rank (Mantel–Cox) test for panels (a–f). Source data are provided as a Source Data file.
Hedgehog-like signal-regulated phenotypes are mediated via ATFS-1-dependent and independent mechanisms
Compared to N2 worms, knockdown of wrt-5, wrt-6, ptr-8, or ptr-16 did not improve antimycin resistance in atfs-1 mutants, indicating that the hedgehog-like signal promotes mitochondrial stress resistance through the transcription factor ATFS-1 (Fig. 6a, b and Supplementary Fig. 10a, b). Additionally, these knockdowns decreased survival against PA14 infection in atfs-1 mutants relative to N2 worms (Fig. 6d, e and Supplementary Fig. 10c, d). Quantitative real-time PCR revealed that RNAi of hedgehog-like genes upregulated both ATFS-1-dependent and independent innate immunity genes50,51 (Supplementary Fig. 10e). The deficiency of atfs-1 did not alleviate the reduction in brood size caused by the knockdown of hedgehog-like signaling genes (Supplementary Fig. 10f, g), demonstrating that UPRmt is not essential for this reduction. These findings confirm that hedgehog-like signaling regulates innate immunity and developmental processes through ATFS-1-dependent and independent mechanisms.
A germline-to-soma hedgehog-like signal allows worms to re-invest their resources in reproduction rather than somatic maintenance
The hedgehog-like ligands have been reported to be expressed in the germline and secreted from the germline to the soma36. To explore which tissues receive the hedgehog-like ligands WRT-5 and WRT-6, we employed tissue-specific RNAi strains to singly knock down the Patched receptor genes ptr-8 and ptr-16 in different tissues. First, we demonstrated that germline-specific single knockdown of wrt-5 and wrt-6, but not ptr-8 and ptr-16, allowed the adult worms to activate UPRmt upon mitochondrial perturbation (Fig. 8a, b and Supplementary Fig. 11a–c). Deficiency of WRT-5 or WRT-6 in the germline also decreased the brood size and vitellogenin gene expression, and increased the resistance of animals to mitochondrial perturbations or pathogen infections (Fig. 8a, c–e and Supplementary Fig. 11d). The reduction in vitellogenin production, primarily in the intestine due to hedgehog pathway inhibition, may affect the levels of polyunsaturated fatty acids (PUFAs) in worms, and thereby affect brood size52,53. These results confirmed that the hedgehog-like ligands are generated in and secreted from the germline. Interestingly, single knockdown of the Patched receptor-like genes ptr-8 and ptr-16 in the somatic tissues or only in the intestine, but not in any other tissues such as the hypodermis, neurons, or muscles, allowed the adult worms to activate UPRmt upon mitochondrial perturbation (Fig. 8a, f and Supplementary Fig. 11e–g). Deficiency of PTR-8 or PTR-16 in the intestine also reduced brood size and vitellogenin gene expression, and increased resistance to mitochondrial perturbations or pathogen infection (Fig. 8a, g–i and and Supplementary Fig. 11h and Supplementary Fig. 12a, b). These results indicate that the hedgehog-like signal is transduced from the germline to the intestine to suppress the mitochondrial stress response. When worms enter adult stage, the hedgehog-like signal allows the reproductive tissue to communicate with the key metabolic and stress response tissue to allocate limited resources for investment in reproduction, rather than maintenance of the soma (Fig. 8j).
a Heat map showing the effect of tissue-specific knockdown of hedgehog pathway-related genes on the indicated phenotypes. b, f qRT-PCR analysis of hsp-6 mRNA levels in the day 3 germline- (b) and intestine-specific (f) RNAi strain treated with the indicated RNAi and antimycin. Antimycin treatment occurred on day 3 of adulthood for 24 h. c, g Brood size of the germline- (c) and intestine-specific (g) RNAi strain treated with the indicated RNAi. d, h Survival rate of the day 9 germline- (d) and intestine-specific (h) RNAi strain treated with the indicated RNAi and exposed to antimycin. e, i, Survival rate of the day 5 germline- (e) and intestine-specific (i) RNAi strain treated with the indicated RNAi and exposed to PA14. j A germline-to-soma hedgehog-like signal initiates resource reinvestment to promote reproduction while inhibiting somatic maintenance in adult worms. Error bars indicate mean ± SD. n represents the number of independent experiments for panels (b, f), and the number of worms for panels (c, g). p values were assessed using a two-tailed t test for panels (b, c, f, g), and the Log-rank (Mantel–Cox) test for panels (d, e, h, i). Source data are provided as a Source Data file.
It has been reported that certain tissue-specific RNAi strains may also affect other tissues. For example, MAH23, commonly used for germline-specific RNAi, also partially impacts the intestine54 (Supplementary Fig. 12c). Therefore, we generated transgenic worms that overexpress ptr-8/16 in the intestine or wrt-5/6 in the germline, and observed suppressed UPRmt (Fig. 9a–g). Additionally, intestine-specific overexpression of ptr-8/16 and germline-specific overexpression of wrt-5/6 decreased resistance to the mitochondrial inhibitor antimycin and PA14 infection, but increased brood size (Fig. 9h–j). These results confirm the specific roles of wrt-5/6 in the germline and ptr-8/16 in the intestine.
a, b Representative fluorescence images of hsp-6p::gfp worms with germline-specific wrt-5/6 or intestine-specific ptr-8/16 overexpression. Antimycin treatment occurred on L4 (a) or day 1 (b) of adulthood for 24 h. Scale bar, 200 µm. c, d Quantification of GFP fluorescence intensity in panels (a, b). e–g qRT-PCR analysis of the indicated UPRmt genes in day 3 hsp-6p::gfp worms with the indicated treatments. h, i Survival rate of the day 9 (h) and day 5 (i) hsp-6p::gfp worms with the indicated treatments. j Brood size of hsp-6p::gfp worms with germline-specific wrt-5/6 or intestine-specific ptr-8/16 overexpression. Ger germline, Int intestine, OE overexpression, GE germline expression, IE intestine expression. Error bars indicate mean ± SD. n represents the number of independent experiments for panels (e–g), and the number of worms for panels (c, d, j). p values were assessed using a two-tailed t test for panels (c–g, j), and the Log-rank (Mantel–Cox) test for panels (h, i). Source data are provided as a Source Data file.
The role of the hedgehog signaling pathway in mitochondrial stress response might be conserved in mammals
We also attempted to examine whether the role of the hedgehog signaling pathway in UPRmt activation and its associated phenotypes are conserved in mammals. Mice were treated with itraconazole, an FDA-approved drug that inhibits the hedgehog signaling pathway55 (Supplementary Fig. 13a, b). In both male and female mice, itraconazole treatment decreased mRNA levels of Gli1, Hhip, and Smo, indicating reduced hedgehog signaling56 (Fig. 10a, b). Notably, suppressing the hedgehog pathway increased the transcript levels of endogenous mitochondrial stress response genes and mitigated weight loss in mice challenged with the mitochondrial inhibitor oligomycin (Fig. 10c–f and Supplementary Fig. 13c–f). Fertility tests also showed that litter sizes in seven-month-old female mice significantly decreased following hedgehog pathway inhibition (Fig. 10g). Additionally, serum anti-mullerian hormone (AMH) levels in treated female mice and testosterone levels in treated male mice were lower than in controls (Fig. 10h, i).
a, b qRT-PCR analysis of Gli1, Hhip, and Smo mRNA levels in seven-month-old C57BL/6J male (a) and female (b) mice treated with DMSO or itraconazole. c, d qRT-PCR analysis of Chop and Asns mRNA levels in seven-month-old C57BL/6J male (c) and female (d) mice treated with DMSO or oligomycin. e, f Weight loss in seven-month-old C57BL/6J male (e) and female (f) mice during oligomycin treatment. g Litter size of seven-month-old C57BL/6J females treated with DMSO or itraconazole, mated with males of the same treatment group. h, i Serum AMH (h) or testosterone (i) levels assessed by ELISA in mice treated with DMSO or itraconazole. j–l Pearson’s correlation of GLI1, GLI2, and UPRmt mRNA levels in monkey (j), rat (k) and human (l) tissues. Blue indicates a positive correlation and white indicates a negative correlation. The intensity of the colors corresponds to the correlation coefficient. m Pearson’s correlation of GLI1, GLI2, GLI3, and human milk mRNA levels in human breast tissues. Blue indicates a positive correlation and white indicates a negative correlation. The intensity of the colors corresponds to the correlation coefficient. Error bars indicate mean ± SD. n represents the number of mice. p values were assessed using a two-tailed t test. Source data are provided as a Source Data file.
Mitochondria are essential organelles, particularly in the brain, one of the most energy-demanding organs. Utilizing the GeneNetwork database, we found negative correlations between mRNA levels of hedgehog pathway zinc finger proteins GLIs and UPRmt-related transcripts (HSPA9, HSPD1, HSPE1, LONP1, and TIMM17A) in various rat, monkey, and human brain tissues (Fig. 10j–l). Additionally, mRNA levels of GLI1, GLI2, and GLI3 negatively correlated with these UPRmt transcripts in multiple human tissues including adipose, esophageal, arterial, pulmonary, muscular, and hepatic tissues (Supplementary Fig. 13g). Conversely, in human breast tissue, GLI1, GLI2, and GLI3 expression positively correlated with transcripts of human milk genes (Fig. 10m). These findings indicate that the hedgehog signaling pathway may play a conserved role in regulating UPRmt and fertility across species.
Discussion
The ability to cope with different cellular stresses declines during animal aging. However, the underlying mechanisms remain elusive. Using C. elegans as a model organism, we found that a hedgehog-like signal, produced in the germline, suppressed the mitochondrial stress response in the intestine in adult animals. More importantly, this study uncovers the biological meaning of the age-related decline of mitochondrial stress response from an evolutionary point of view.
Several hypotheses have been proposed to explain why biological aging occurs. The “antagonistic pleiotropy” (multiple effects of a single gene) theory postulates that genes beneficial for early life are strongly selected but can be detrimental in later life when selection wanes after reproduction57. The “disposable soma” theory postulates that aging reflects the decision to allocate limited resources for investment in early life reproduction rather than maintenance of the soma to extend the lifespan58. The current findings support the “antagonistic pleiotropy” and the “disposable soma” theories. The genes related to hedgehog signaling are beneficial at the larval stages when they promote development and reproduction, but are detrimental in later life when they suppress the mitochondrial stress response. Moreover, the generation and transmission of the hedgehog-like signal from the germline to the soma suppresses the mitochondrial stress response in the somatic tissues, allowing the worms to invest more resources in reproduction rather than somatic maintenance.
The initiation of this project was stemming from an artificial experiment where we fed adult C. elegans with embryo lysates and noted the induction of UPRmt. This approach was instrumental in identifying potential factors capable of modulating UPRmt in adults. Subsequent investigations revealed that germline-expressed piRNAs played a significant role, with their levels markedly decreasing in young adults. This reduction may facilitate the production and secretion of a Hedgehog-like signal that suppresses somatic UPRmt. While the initial experiment with embryo lysates was artificial, it paved the way for discovering a naturally occurring germline-to-soma communication mechanism. Our findings demonstrate that the signal is primarily transmitted from the germline, underscoring its physiological relevance.
Our findings illustrate the role of wrt-5/6-tageting piRNAs in regulating UPRmt across various developmental stages of C. elegans. Notably, our analysis indicates a sustained expression of certain wrt-5/6-targeting piRNAs into the L3 and young adult phases (Fig. 5c), albeit at lower levels than observed in embryos, which appears to contribute to UPRmt activation. This is further corroborated by our experiments showing that UPRmt could not be activated in adult worms using L3 extracts from prg-1 mutants, underscoring the critical function of piRNAs in this context. piRNAs are thought to function by generating 22G-RNAs. We conducted small RNA sequencing on worms treated with embryo lysates but found no significant changes in 22G-RNAs following either embryo lysate feeding or antimycin treatment. This lack of significant changes may be due to the timing of the sequencing, performed 24 h post-treatment, which corresponds with UPRmt activation but may miss earlier events necessary for 22G-RNAs production. As a result, there is currently no strong evidence supporting 22G-RNAs in regulating wrt-5/6 transcripts. Further studies are required to uncover the exact mechanisms by which piRNAs regulate this process. Moreover, our exploration extends to identifying additional factors within the lysates from embryos, L3, and young adult stages that might enhance piRNA-mediated UPRmt induction. This premise gains support from our observations where the mere addition of piRNAs to day 6 prg-1 lysates was insufficient to trigger UPRmt (Supplementary Fig. 6l), suggesting the absence of other essential components in day 6 lysates that are pivotal for UPRmt induction. These insights collectively highlight the complex interplay between piRNAs and other molecular constituents in modulating the mitochondrial stress response, suggesting a broader regulatory landscape that extends beyond piRNA action alone.
Previous research demonstrated that PGL-1 aggregation in the germline, induced by the loss of CEY factors (Y-box binding proteins), affects not only the mitochondrial network in the germline but also triggers UPRmt activation in somatic tissues via Wnt signaling37. During the L4 and YA stages, mitochondrial DNA (mtDNA) copy number significantly increases in the germline59. Our findings suggest the L4 stage as critical for activating germline hedgehog-like signaling. Future research could explore how this surge in mitochondrial activity and protein synthesis during the L4 and YA stages influences hedgehog-like signaling and its coordination with the piRNA pathway for resource allocation between reproduction and soma maintenance. While this manuscript was being prepared, it was reported that the germline functions as a central signaling hub, regulating neuron-to-intestine cell-nonautonomous UPRmt activation, emphasizing the important role of the germline in maintaining mitochondrial health60.
Our study underscores the germline-to-soma signaling pathway’s role in modulating the UPRmt, yet it’s important to acknowledge that germline signals can also affect other proteostatic responses, such as the heat shock response (HSR)61,62. Whether UPRER is regulated in a similar manner requires further exploration. The selective regulatory mechanisms by which germline signals influence specific somatic stress responses are yet to be fully understood. Delving into the mechanisms through which germline signals selectively modulate distinct somatic stress pathways could offer profound insights into how organismal stress responses are coordinated, and the intricate dynamics between reproductive status and somatic health.
Methods
C. elegans strains and culture
Worms were grown and maintained at 20 °C and fed with E. coli OP50 on Nematode Growth Medium (NGM) (0.25% Bacto peptone, 0.3% sodium chloride, 1.7% agar, 5 μg/mL cholesterol, 25 mM potassium phosphate pH 6.0, 1 mM magnesium sulfate, 1 mM calcium chloride) unless otherwise indicated. Strains used in this study are detailed in Supplementary Data 3.
Bacterial strains and culture
Bacterial strains used in this study were Escherichia coli strains OP50 and HT115, and Pseudomonas aeruginosa strains PA14, PAO1 and PAO1(GFP). OP50 was cultured with LB medium at 37 °C unless otherwise indicated. HT115, PA14, PAO1 and PAO1(GFP) were cultured with LB medium containing ampicillin (50 mg/ml) at 37 °C unless otherwise indicated.
Induction of UPRmt
For induction of UPRmt in worms by treatment with mitochondrial inhibitor, unless otherwise stated, synchronized L1 worms were raised on NGM plates at 20 °C. Adult worms were transferred every day to new plates. Worms were transferred to NGM plates containing mitochondrial inhibitor (1 μg/ml antimycin, 20 μM DECA, 20 μM CCCP, 0.5 mM paraquat) at the indicated time stage. After 24 h, worms were imaged.
For induction of UPRmt by feeding worms with PA14, synchronized L1 worms were raised on NGM plates at 20 °C. Adult worms were transferred every day to new plates starting at day 3 of adulthood. Worms were transferred to PA14 slow-killing NGM plates. After 48 h, worms were imaged.
For the induction of UPRmt in worms by RNAi, synchronized L1 worms were cultured on NGM plates at 20 °C. Adult worms were transferred to new plates daily starting on day 3 of adulthood. The worms were synchronized and raised on NGM plates containing atp-2, cco-1, or spg-7 RNAi. After 48 h, the worms were imaged.
Induction of UPRER
Synchronized L1 worms were raised on NGM plates at 20 °C. Adult worms were transferred every day to new plates. Worms were transferred to NGM plates containing tunicamycin (4 μg/ml) at the indicated stage. After 24 h, GFP fluorescence intensity was quantified.
Induction of heat shock response
Synchronized L1 worms were raised on NGM plates at 20 °C. Adult worms were transferred every day to new plates. Worms were transferred to pre-heated NGM plates at the indicated stage, cultured at 33 °C for 2 h and then transferred to 20 °C. After 24 h, GFP fluorescence intensity was quantified.
Induction of innate immune response
Synchronized L1 worms were raised on NGM plates at 20 °C. Adult worms were transferred every day to new plates. At day 5 of adulthood, worms were transferred to PA14 slow killing plates. Wild-type worms were collected for RNA extraction after 48 h. irg-1p::gfp worms were imaged after 48 h.
Drug killing assay and Pseudomonas aeruginosa slow killing assay
Synchronized L1 worms were raised on NGM plates at 20 °C. Adult worms were transferred every day to new plates. 50-100 worms were transferred randomly to NGM plates containing different drugs (2 μg/ml antimycin, 30 μM DECA, 30 μM CCCP, 1 mM paraquat, 4 μg/ml tunicamycin) at the indicated stage. For Fig. 3m, worms were treated with 4 μg/ml antimycin. Living worms were counted every day, until all worms were dead. Animals that crawled off the plate or died due to vulva bursting were excluded. During the experiments, worms were transferred to new plates containing drug at the appropriate time if viable offspring were produced. To measure 72-h survival rates, the total number of worms was counted at the beginning of the experiment, and the number of surviving worms was counted 72 h later. For Fig. 3m, we measured 48-h survival rates.
Pseudomonas aeruginosa (PA) was cultured in LB containing 50 mg/ml ampicillin at 37 °C for 16 h. Concentrated PA were seeded onto air-dried slow killing plates, then incubated at 37 °C for 24 h and subsequently at room temperature for another 24 h. Synchronized L1 worms were raised on NGM plates at 20 °C. Adult worms were transferred every day to new plates. 50-100 worms were transferred to slow killing plates at the indicated stage. Living worms were counted every day until all worms were dead. Animals that crawled off the plate or died due to vulva bursting were excluded. During the experiments, worms were transferred to new plates containing drug at the appropriate time if viable offspring were produced.
The selection of Days 5 and 9 for the antimycin and PA14 survival experiments was aimed at identifying the most suitable time points for plotting survival curves. This choice helps avoid scenarios where median survival times are either too long or too short, which can obscure discernible differences.
Thermo-tolerance assay
Synchronized L1 worms were raised on NGM plates at 20 °C. Adult worms were transferred every day to new plates. The worms were transferred to pre-heated NGM plates at the indicated stage, cultured at 37 °C for 4 h and then transferred to 20 °C. After 72 h, living worms were counted.
Lifespan
For the lysate-treated lifespan assays, synchronized L1 worms were cultured on NGM plates at 20 °C. Starting from Day 1, worms were transferred daily to new plates. From Day 3 to Day 7, they were moved daily to fresh lysate-treated NGM plates, and then every other day to fresh NGM plates from Day 7 onwards. For the RNAi-treated lifespan assays, synchronized L1 worms were fed either control RNAi E. coli or RNAi E. coli targeting hedgehog-related genes. From Day 1 to Day 7, worms were transferred daily to fresh RNAi plates, and then every other day from Day 7 onwards. For all experiments, living worms were counted daily until all had died. Animals that crawled off the plates or died due to vulva bursting were censored. No 5-fluoro-2′-deoxyuridine (FUDR) was added to the plates.
Preparation of embryo/worm lysates
Day 1 hermaphrodites were collected from plates and washed 3 times in M9 buffer (42.3 mM Na2HPO4, 22.1 mM KH2PO4, 85.5 mM NaCl). The worms were then bleached to release the embryos at mixed developmental stages. Day 6 hermaphrodites were collected from plates and washed 3 times in M9 buffer. The embryo or worm pellet were washed and resuspended with PBS. Dounce tissue grinders were used to homogenize embryos or worms. A dissection microscope was used to monitor the level of homogenization to ensure no visible intact worms or embryos in the lysates. The homogenizing process was carried out on ice. Each lysate within an experiment was normalized to the starting volume of embryos/worm pellet. After homogenizing, lysates were cleared by centrifugation at 3000 g at 4 °C for 1 min. For treatment of the lysates with different enzymes, Proteinase K (0.1 mg per lysate sample derived from 2 million embryos), RNase AT1 (0.1 mg per lysate sample derived from 2 million embryos), or DNase I (0.1 mg per lysate sample derived from 2 million embryos) were added to the lysates. The lysates were then incubated at 37 °C and gently rotated at 500 rpm for 1 h. To feed worms with lysate, 300 µl of embryo lysate (derived from 2 million embryos) or 300 ul of worm lysate (derived from 100,000 worms) was immediately spread onto 3.5 cm NGM OP50 plates. Synchronized worms at the indicated stage were then transferred to the lysate-treated plates.
Germline dissection
For germline dissection, synchronized worms were placed in M9 buffer containing 2 mM levamisole. Using two syringe needles, worms were decapitated with a single scissoring motion, allowing one arm of the gonad to be fully released from the body cavity. The gut, appearing darker and uniform in width, contrasts with the clear gonad which has a distal tapered tip. Immediately after dissection, the germlines were placed into tubes containing 500 µl of TransZol Up reagent (TransGen Biotech, #ET111-01). Approximately 50 germlines were collected for each biological replicate.
Germline piRNA overexpression
For germline piRNA overexpression, the piRTarBase (http://cosbi6.ee.ncku.edu.tw/piRTarBase/) was utilized to predict piRNAs related to wrt-5 and wrt-6. The piRNA-mediated interference system (‘piRNAi’) was employed to overexpress endogenous piRNAs. Scaffold information for the piRNA expression cluster E was obtained from https://www.wormbuilder.org/piRNAi/. Overexpression clusters targeting wrt-5 and wrt-6, as well as a control piRNA overexpression cluster, were synthesized by Xianghong Biotech. Transgenic animals harboring piRNAi transgenes were created by Suny Biotech following standard protocols. The injection mix for creating transgenes included 10 ng/µl of synthetic dsDNA for piRNA overexpression cluster and 10 ng/µl of a co-injection marker (myo-2p::mCherry). The sequences of the piRNA overexpression clusters used in this study are listed below:
piRNA overexpression cluster targeting wrt-5 (uppercase: piRNAs)
cgcgcttgacgcgctagtcaactaacataaaaaaggtgaaacattgcgaggatacatagaaaaaacaatacttcgaattcatttttcaattacaaatcctgaaatgtttcactgtgttcctataagaaaacattgaaacaaaatattaagtTAATTTCTTCAATTTCTTCAActaattttgattttgattttgaaatcgaatttgcaaatccaattaaaaatcattttctgataattagacagttccttatcgttaattttattatatctatcgagttagaaattgcaacgaagataatgtcttccaaatactgaaaatttgaaaatatgttTTTGGCTCAAAATCTGAAAAAattgccagaactcaaaatatgaaatttttatagttttgttgaaacagtaagaaaatcttgtaattactgtaaactgtttgctttttttaaagtcaacctacttcaaatctacttcaaaaattataatgtttcaaattacataactgtgtGTAACGTGAAAAAATTGAGGAactgtagagcttcaatgttgataagatttattaacacagtgaaacaggtaatagttgtttgttgcaaaatcggaaatctctacatttcatatggtttttaattacaggtttgttttataaaataattgtgtgatggatattattttcagacctcatactaatctgcaaaccttcaaacaatatgtgaagtctactctgtttcactcaaccattcatttcaatttggaaaaaaatcaaagaaatgttgaaaaattttcctgtttcaacattatgacaaaaatgttatgattttaataaaaacaatTAAATATAAAAAAACGGAATAttctgtttttcttagaagtgttttccggaaacgcgtaattggttttatcacaaatcgaaaacaaacaaaaatttttttaattatttctttgctagttttgtagttgaaaattcactataatcatgaataagtgagctgcccaagtaaacaaagaaaatttggcagcggccgacaactaccgggttgcccgatttatcagtggaggaCTTTTTTCTTTTTATTTTTCAatctaatgtgatgtacacggttttcatttaaaaacaaattgaaacagaaatgactacattttcaaattgtctatttttgctgtgtttattttgccaccaacaatTTAGGAAGACATAAATAATTGtcaatctagtaaactcacttaatgcaattcctccagccacatatgtaaacgttgtatacatgcagaaaacggttttttggttttaatgggaacttttgacaaattgttcgaaaatcttaagctgtcccatttcagttgggtgatcgattt.
piRNA overexpression cluster targeting wrt-6 (uppercase: piRNAs)
cgcgcttgacgcgctagtcaactaacataaaaaaggtgaaacattgcgaggatacatagaaaaaacaatacttcgaattcatttttcaattacaaatcctgaaatgtttcactgtgttcctataagaaaacattgaaacaaaatattaagtTGTGAGAAGGGAATTTGTCGActaattttgattttgattttgaaatcgaatttgcaaatccaattaaaaatcattttctgataattagacagttccttatcgttaattttattatatctatcgagttagaaattgcaacgaagataatgtcttccaaatactgaaaatttgaaaatatgttCTCGGAAAATAAAATAATTAAattgccagaactcaaaatatgaaatttttatagttttgttgaaacagtaagaaaatcttgtaattactgtaaactgtttgctttttttaaagtcaacctacttcaaatctacttcaaaaattataatgtttcaaattacataactgtgtGAAAATCTGATAACTATGAAAactgtagagcttcaatgttgataagatttattaacacagtgaaacaggtaatagttgtttgttgcaaaatcggaaatctctacatttcatatggtttttaattacaggtttgttttataaaataattgtgtgatggatattattttcagacctcatactaatctgcaaaccttcaaacaatatgtgaagtctactctgtttcactcaaccattcatttcaatttggaaaaaaatcaaagaaatgttgaaaaattttcctgtttcaacattatgacaaaaatgttatgattttaataaaaacaatTTTTGTATTCGTATTCATTGTttctgtttttcttagaagtgttttccggaaacgcgtaattggttttatcacaaatcgaaaacaaacaaaaatttttttaattatttctttgctagttttgtagttgaaaattcactataatcatgaataagtgagctgcccaagtaaacaaagaaaatttggcagcggccgacaactaccgggttgcccgatttatcagtggaggaAAAATAAAGAATTCCATTCAAatctaatgtgatgtacacggttttcatttaaaaacaaattgaaacagaaatgactacattttcaaattgtctatttttgctgtgtttattttgccaccaacaatTATAATCGTGGTATTTGCTCGtcaatctagtaaactcacttaatgcaattcctccagccacatatgtaaacgttgtatacatgcagaaaacggttttttggttttaatgggaacttttgacaaattgttcgaaaatcttaagctgtcccatttcagttgggtgatcgattt.
piRNA overexpression cluster control (uppercase: piRNAs)
cgcgcttgacgcgctagtcaactaacataaaaaaggtgaaacattgcgaggatacatagaaaaaacaatacttcgaattcatttttcaattacaaatcctgaaatgtttcactgtgttcctataagaaaacattgaaacaaaatattaagtTTTATTGGGTATTGGAATTGActaattttgattttgattttgaaatcgaatttgcaaatccaattaaaaatcattttctgataattagacagttccttatcgttaattttattatatctatcgagttagaaattgcaacgaagataatgtcttccaaatactgaaaatttgaaaatatgttCCTTTTATAAAGCTGAAAATAattgccagaactcaaaatatgaaatttttatagttttgttgaaacagtaagaaaatcttgtaattactgtaaactgtttgctttttttaaagtcaacctacttcaaatctacttcaaaaattataatgtttcaaattacataactgtgtGGTCCTGAAAATAAAGAAAGAactgtagagcttcaatgttgataagatttattaacacagtgaaacaggtaatagttgtttgttgcaaaatcggaaatctctacatttcatatggtttttaattacaggtttgttttataaaataattgtgtgatggatattattttcagacctcatactaatctgcaaaccttcaaacaatatgtgaagtctactctgtttcactcaaccattcatttcaatttggaaaaaaatcaaagaaatgttgaaaaattttcctgtttcaacattatgacaaaaatgttatgattttaataaaaacaatTTCCATCACTCAGAAATCTGTttctgtttttcttagaagtgttttccggaaacgcgtaattggttttatcacaaatcgaaaacaaacaaaaatttttttaattatttctttgctagttttgtagttgaaaattcactataatcatgaataagtgagctgcccaagtaaacaaagaaaatttggcagcggccgacaactaccgggttgcccgatttatcagtggaggaTGTTCTAAAGATGAAGTTCTAatctaatgtgatgtacacggttttcatttaaaaacaaattgaaacagaaatgactacattttcaaattgtctatttttgctgtgtttattttgccaccaacaatTTTATTTTTCCTACGTTTTAAtcaatctagtaaactcacttaatgcaattcctccagccacatatgtaaacgttgtatacatgcagaaaacggttttttggttttaatgggaacttttgacaaattgttcgaaaatcttaagctgtcccatttcagttgggtgatcgattt.
piRNA feeding
For the piRNA feeding assay, piRNAs targeting wrt-5, wrt-6, and a control gene wrt-1 were synthesized by Xianghong Biotech. 60 µg of total piRNAs (10 µg per single piRNA) were combined with prg-1 embryo or worm lysates and then applied directly to OP50-seeded NGM plates. Synchronized worms at the indicated stage were subsequently transferred to these lysate-seeded plates. The piRNA oligos were 5′-monophosphorylated and 2′-O-methylation at their 3′ termini. The piRNA oligos used were:
21ur-9942: 3′ AACUUCUUUAACUUCUUUAAU 5′
21ur-9245: 3′ AAACCGAGUUUUAGACUUUUU 5′
21ur-13372: 3′ CAUUGCACUUUUUUAACUCCU 5′
21ur-10813: 3′ AUAAGGCAAAAAAAUAUAAAU 5′
21ur-9641: 3′ GAAAAAAGAAAAAUAAAAAGU 5′
21ur-6889: 3′ GUUAAUAAAUACAGAAGGAUU 5′
21ur-6059: 3′ AGCUGUUUAAGGGAAGAGUGU 5′
21ur-3765: 3′ GAGCCUUUUAUUUUAUUAAUU 5′
21ur-11925: 3′ CUUUUAGACUAUUGAUACUUU 5′
21ur-10851: 3′ UGUUACUUAUGCUUAUGUUUU 5′
21ur-265: 3′ UUUUAUUUCUUAAGGUAAGUU 5′
21ur-6555: 3′ GCUCGUUUAUGGUGCUAAUAU 5′
21ur-5847: 3′ AGUUAAGGUUAUGGGUUAUUU 5′
21ur-4659: 3′ GGAAAAUAUUUCGACUUUUAU 5′
21ur-12303: 3′ CCAGGACUUUUAUUUCUUUCU 5′
21ur-6464: 3′ UGUCUAAAGACUCACUACCUU 5′
21ur-32: 3′ ACAAGAUUUCUACUUCAAGAU 5′
21ur-1851: 3’ AAUUUUGCAUCCUUUUUAUUU 5′
Nile Red staining
For the Nile Red staining assay, Nile Red was added into OP50-seeded NGM plates to achieve a final concentration of 0.05 µg/ml in the agar. Synchronized worms were cultured on OP50-seeded NGM plates starting from the L1 stage. By day 7, the worms were transferred to OP50-seeded NGM plates containing Nile Red. After 48 h, the worms were imaged using a Zeiss Imager M2 microscope and the images were analyzed with ImageJ software.
TMRE staining
For the tetramethylrhodamine ethyl ester (TMRE) staining assay, TMRE was added to OP50-seeded NGM plates at a final concentration of 0.1 µM. Synchronized worms were cultured on RNAi plates starting from the L1 stage. At day 1, 3, or 5, the worms were transferred to RNAi plates that contained TMRE. After 48 h, the worms were imaged using a Zeiss Imager M2 microscope and the images were analyzed using ImageJ software.
Fumarase assay
Synchronized worms were harvested from the plates and washed at least three times with M9 buffer. The worms were then homogenized using Dounce tissue grinders. Cytosolic and mitochondrial fractions were separated via differential centrifugation using a Fumarate Hydratase Assay Kit (Grace Biotech, G0869F). Protein concentrations in each fraction were quantified using a BCA assay (Thermo Scientific, 23225). Fumarase activity in these fractions was measured with the Fumarate Hydratase Assay Kit.
Microscopy
Synchronized worms were randomly picked into 2 mM levamisole droplets on 2% agarose pads and imaged by a Zeiss Imager M2 microscope. Exposure time was the same in each experiment. At least three biologically independent experiments were performed and similar results were obtained.
RNA isolation and real-time PCR
Synchronized worms were collected from the plates, washed at least three times with M9 buffer, and resuspended in TransZol Up reagent (TransGen Biotech, #ET111-01). Worm samples were frozen in liquid nitrogen and freeze-thawed for at least five times. Total RNA was isolated by chloroform extraction followed by isopropanol precipitation, washed with 75% (v/v) ethanol and dissolved in RNase-free water. cDNA was then synthesized using EasyScript® One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech, #AE311-02) kits. Quantitative PCR was carried out using a SYBR® Green Premix Pro Taq HS qPCR Kit (Accurate Biology #AG11701). For quantification, mRNA levels were normalized to rpl32. For piRNA detection, cDNA was synthesized using a miRNA 1st Strand cDNA Synthesis Kit (Vazyme Biotech, MR101-01). PCR amplification was performed with a piRNA-specific forward primer and a universal reverse primer. Quantification of piRNA levels was normalized to U6. Supplementary Data 4 summarizes the primer sequences for quantitative RT-PCR.
Western blotting
Synchronized worms were collected from plates, washed at least three times with M9 buffer, and then resuspended with SDS loading buffer (100 mM Tris-HCl pH 6.8, 4% SDS, 20% glycerol, 10% β-mercaptoethanol, 0.004% bromophenol blue) and boiled at 100 °C for 15 min. Lysates containing the same amount of protein were loaded into an SDS-PAGE gel, followed by electrophoresis and transfer. 5% non-fat milk was used to block PVDF membranes. The membranes were then incubated with the designated primary and secondary antibodies. In this study, the following primary antibodies were used: anti-GFP (Sungene #KM8009, 1:2000 dilution), anti-tubulin (Abcam #ab6161, 1:1000 dilution), anti-Flag (Sigma #F7425, 1:2000 dilution), anti-HA (CST #3724, 1:2000 dilution), anti-b-actin (ABclonal #AC026, 1:1000 dilution), anti-HSP-60 (CST #4870, 1:1000 dilution). The membranes were developed with the enhanced chemiluminescence method (Thermo) and visualized by a Tanon 5200 chemical luminescence imaging system.
RNA interference
Some RNAi clones were obtained from the Ahringer library; other RNAi clones were generated by PCR amplification of worm cDNA or gDNA and ligated into L4440 vector. Supplementary Data 5 summarizes the information about all RNAi clones. After sequencing verification, RNAi plasmids were transformed into HT115 competent cells (ZOMANBIO, #ZC1233). HT115 clones were cultured in LB containing 50 mg/ml ampicillin at 37 °C for 12 h. IPTG was then added to the final concentration of 1 mM. After 12 h, E. coli was concentrated and then seeded onto a plate containing 1.2 mg/ml IPTG. The plates were air-dried and incubated at 37 °C overnight. Worms were transferred onto the plates when the plate temperature dropped to 20 °C. For double RNAi treatment, the concentrated RNAi clones were mixed at a 1:1 ratio and seeded on the plates. All RNAi treatments were started upon hatching and proceeded throughout post-embryonic development and adulthood, unless otherwise indicated. Control RNAi experiments were carried out using an empty vector plasmid L4440.
RNA screening
RNAi screening was performed by feeding worms with Escherichia coli HT115. RNAi clones were taken from the Ahringer library. Embryos from hsp-6p::gfp worms were collected by bleach synchronization, then 80-100 synchronized L1 worms were added to each well of 12-well plates containing RNAi bacteria. Control RNAi experiments were carried out using an empty vector plasmid L4440. Adult worms were transferred every day to new wells containing RNAi bacteria. Larval L4 or adult day 3 worms were transferred to RNAi NGM plates containing mitochondrial inhibitor (1 µg/ml antimycin). After 24 h, worms were scored. Scores were recorded from ns (no induction of GFP expression) to +++ (strong induction of GFP expression).
Quantification of PAO1(GFP) and PA14 colony-forming units (CFU)
Synchronized worms at the indicated stage were exposed to PAO1(GFP) or PA14. After 48 h, worms were washed at least three times with M9 buffer, then transferred to M9 buffer containing 1 mg/ml kanamycin and rotated for 20 min. Washing and kanamycin treatment were repeated for at least four times to kill external bacteria on the surface of worms. Worms were then spread on an empty plate and air-dried. 20 worms were randomly picked into 100 μl M9 buffer and ground with a motorized pestle. Worm lysates were serially diluted. For each dilution, triplicates of 10 μl suspension were dropped onto LB plates containing 50 mg/ml of ampicillin. After overnight cultivation at 37 °C, the colonies were counted
Brood size
Synchronized L4 worms were transferred to RNAi plates (one worm per plate). Worms were transferred to new RNAi plates every day until day 3 of adulthood, and then transferred to new RNAi plates every two days, until reproduction ceased. Offspring were allowed to grow at 20 °C for 24-48 h and then counted. Offspring from 10–20 individual worms were counted for each condition.
Larval developmental assay
To quantify developmental delay caused by knocking down hedgehog-related genes, synchronized L1 wild-type worms were fed with control RNAi E.coli or RNAi E.coli targeting hedgehog-related genes. To quantify developmental delays caused by knocking out hedgehog-related genes, wild-type worms and hedgehog-related mutants were fed with OP50 starting at the L1 stage. Worms were cultured at 20 °C for 55 h. The developmental stages of worms were analyzed. Larval stages were visually determined based on vulval development.
Exploded phenotype assay
To quantify the exploded (ruptured vulva) phenotype resulting from the knockdown of hedgehog-related genes, synchronized L1 wild-type worms were fed with control RNAi E. coli or RNAi E. coli targeting hedgehog-related genes. To assess the exploded phenotype upon knockout of hedgehog-related genes, wild-type worms and hedgehog-related gene mutants were fed with OP50 starting at the L1 stage. Data on the exploded phenotype were collected from day 1 to day 5 of adulthood. For the lifespan assay, data on the exploded phenotype were collected throughout all life stages.
PAO1(GFP) intestinal accumulation assay
PAO1(GFP) was cultured in LB containing 50 mg/ml ampicillin at 37 °C for 16 h. Concentrated PAO1(GFP) were seeded onto slow-killing plates, air-dried and incubated at 37 °C for 24 h and then at room temperature for another 24 h. Synchronized L1 worms were raised on control or hedgehog-related gene RNAi plates at 20 °C. Adult worms were transferred every day to new plates. At day 5 of adulthood, worms were transferred to PAO1(GFP) plates. After 48 h, worms were washed at least three times with M9 buffer and imaged.
vit-2::gfp observation assay
Synchronized L1 worms were raised on control or hedgehog-related gene RNAi plates at 20 °C. Adult worms were transferred every day to new plates. vit-2::gfp images were taken at day 5 of adulthood. Fluorescence intensity was quantified using ImageJ.
Yolk protein proportion measurements
For yolk protein proportion measurements, 100 day 1 N2 worms were picked into M9 buffer and washed at least three times with M9 buffer. Subsequently, 100 µL of 2x Laemmli sample buffer (Sigma) was added. The samples were incubated at 95 °C, vortexed continuously for 10 min, and then loaded onto Criterion XT precast gels (4%–12% Bis-Tris, Bio-Rad) using XT MOPS (Bio-Rad) as the running buffer. Gels were stained and destained following standard Coomassie blue dye protocols. Yolk proteins (YP170, YP115, and YP88) were identified based on published data and normalized to myosin.
mRNA-seq sample preparation and data analysis
To prepare the mRNA-seq samples, approximately 500 synchronized day 7 N2 worms were treated with either untreated or treated with embryo lysates for 24 h. After treatment, the worms were washed, collected with M9 buffer, and resuspended in TransZol Up reagent (TransGen Biotech, #ET111-01). Total RNA was then isolated using chloroform extraction followed by isopropanol precipitation. Sequencing was conducted by BGI Tech, with sequencing quality evaluation performed using FastQC (version 0.11.9) prior to mapping.
The reads were aligned to the Caenorhabditis elegans genome (Ensembl WBcel235/ce11 assembly) using HISAT2 (version 2.2.1) with default parameters, retaining only uniquely mapped reads for further analysis. Gene annotations for WBcel235/ce11 in GTF format were sourced from Ensembl (ftp://ftp.ensembl.org/pub/release-110/gtf/caenorhabditis_elegans/). Gene count matrices were generated using HTSeq-count from HTSeq (version 0.12.4).
Differential expression analysis was performed using DESeq2 (version 1.26.0). Genes with low expression (FPKM < 0.5 in all samples) were excluded from the analysis. Genes with an adjusted p-value below 0.05 were considered significantly differentially expressed. Finally, KEGG enrichment analysis of the differentially expressed genes was performed using the clusterProfiler R package (version 4.10.0). The hypergeometric distribution test is used to determine whether a known biological function or process is enriched for the differentially expressed genes. All expressed genes are taken as the total number of backgrounds N, the total number of genes annotated to a certain subset of known gene sets (KEGG) is M, the number of differentially expressed genes is n, and the number of differentially expressed genes belonging to M is k. P-value of the hypergeometric distribution test is calculated to determine whether k/n in each subcategory of KEGG is significantly higher than M/N. q-value is an estimation for false discovery rate control. p.adjust is Benjamini-Hochberg adjusted p-value for multiple hypothesis testing. For heat maps in Fig. 2i-k and m, a two-tailed t test is used to assess whether a gene is differentially expressed.
Cell lines and culture
HEK293T cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 4.5 g/L glucose, supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cultures were maintained in a humidified incubator at 37 °C with 5% CO2.
Transfection, cell lysis, IP and immunoblotting
Approximately 1×106 cells were seeded in each 6 cm dish and immediately transfected with 1 ng of p3.3-wrt-2/5/6-Flag combined with 1 ng of p3.3-ptr-8/16-HA plasmids using a PEIMax (Polysciences, 24765) transfection reagent. After 48 h, cells were lysed by adding 1 mL of Triton X-100 Lysis Buffer supplemented with protease and phosphatase inhibitors to each dish. The cell lysates were then collected and cleared by centrifugation at 20,000 g for 10 min at 4 °C.
For samples intended for immunoblottings, supernatants were mixed with 4× Laemmli sample buffer (Bio-Rad) and boiled for 5 min at 95 °C. For samples destined for immunoprecipitations (IP), supernatants were incubated with pre-washed Flag, HA, or agarose beads for 3 h at 4 °C with gentle rotation. The beads were subsequently washed three times with IP lysis buffer containing 500 mM NaCl, resuspended in 2× Laemmli sample buffer, and boiled at 95 °C for 5 min. Samples were then resolved by SDS-PAGE and analyzed by immunoblotting.
siRNA knockdown
For siRNA knockdown experiments, we utilized RNAiMAX (Thermo Scientific, 13778-150) transfection reagent to transfect siRNAs into cells. 8 h post-transfection, the culture medium was replaced with fresh DMEM. In experiments involving transfection of both siRNAs and cDNAs, cDNAs were first transfected into the cells, followed by the transfection of siRNAs. Cells were harvested 48 h after siRNA transfection. The sgRNA oligos used were:
siptr-8-sense-1: CCCUCCAACCAUUCCUCAUTT
siptr-8-antisense-1: AUGAGGAAUGGUUGGAGGGTT
siptr-8-sense-2: GCGGCUAUAUUCAUGGAUATT
siptr-8-antisense-2: UAUCCAUGAAUAUAGCCGCTT
siptr-8-sense-3: GCUCAUGUCGCAUUCCAUUTT
siptr-8-antisense-3: AAUGGAAUGCGACAUGAGCTT
siptr-16-sense-1: GGAAGACCCGUUUGUGCUUTT
siptr-16-antisense-1: AAGCACAAACGGGUCUUCCTT
siptr-16-sense-2: GCACCACAUUAAUGUCUAUTT
siptr-16-antisense-2: AUAGACAUUAAUGUGGUGCTT
siptr-16-sense-3: GCAAGAACUCAACCUCAAUTT
siptr-16-antisense-3: AUUGAGGUUGAGUUCUUGCTT
Mouse husbandry
Experiments were conducted with prior approval from the Institutional Animal Care and Use Committee (IACUC) at Peking University, IACUC number is FT-LiuY-8. Seven-month-old wild-type (WT) female and male C57BL/6 J mice were obtained from Biocytogen Pharmaceuticals (Beijing, China). All mice were maintained on a 12-h light/dark cycle at 25 ± 2 °C and relative humidity (50%). Food and water were provided ad libitum.
Oligomycin resistance assay
Seven-month-old C57BL/6 J female and male mice were randomly assigned to experimental groups. Starting from day 1, mice received daily intraperitoneal injections of Itraconazole (MedChemExpress, R51211) at a concentration of 10 mg/kg; control mice were administered a vehicle solution consisting of 0.1% DMSO in Sulfobutylether-β-Cyclodextrin (MedChemExpress, HY17031). Beginning on day 5, all mice were injected intraperitoneally with oligomycin (MedChemExpress, HY-N6782) at a concentration of 0.5 mg/kg once a day. Throughout the experiment, the weight of each mouse was recorded every 7 days. Any animals that died were censored.
Serum AMH and testosterone measurement
For AMH measurements, blood samples were drawn via the retro-orbital sinus using a serum separator tube (SST). Samples were allowed to clot for 2 h at room temperature before being centrifuged for 15 min at 1000 g. The serum was then stored at −80 °C until analysis. Serum AMH levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (CUSABIO, CSB-E13156m).
For testosterone measurements, 100 mg of testis tissue was rinsed with PBS, homogenized in 1 ml of PBS, and then centrifuged for 5 min at 5000 g, at 4 °C. The supernatants were aliquoted and stored at −80 °C. Tissue testosterone levels were measured using an ELISA kit (CUSABIO, CSB-E05101m).
Fertility test
Seven-month-old C57BL/6J female and male mice were randomly assigned to experimental groups. Starting on day 1, mice were injected intraperitoneally once a day with Itraconazole (MedChemExpress, R51211) at a concentration of 10 mg/kg; control mice received a vehicle of 0.1% DMSO in Sulfobutylether-β-Cyclodextrin (MedChemExpress, HY17031). One week after beginning the Itraconazole injections, mating trials were initiated. During these trials, one Itraconazole-injected female mouse was placed in a cage with one Itraconazole-injected male for one week. Similarly, one control female mouse was paired with one control male for one week. Approximately three weeks later, the number of offspring per female was counted.
Liver RNA isolation and cDNA synthesis
Seven-month-old C57BL/6J female and male mice were treated as indicated. Total RNA was isolated from the livers of these mice using TransZol Up reagent (TransGen Biotech, #ET111-01). The isolated RNA was then converted into cDNA using the EasyScript® One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech, #AE311-02) kits. This cDNA was used to detect the expression of hedgehog pathway-related genes.
Correlation analyses
For genetic correlation analyses, GeneNetwork (http://www.genenetwork.org) was used to download and analyze datasets. Pearson’s r was employed to measure the correlations.
Statistics and reproducibility
All statistical analyses were performed with GraphPad Prism 9.0. All survival curves were estimated with the log-rank (Mantel–Cox) test. For bar graphs and dot graphs, the two-tailed t test was used to determine statistical significance. Results are presented as mean ± SD. For statistical analysis, quantitative data were obtained from at least three biologically independent experiments. For detailed information of each assay, please see figures or figure legends. All images shown in the figures are representative of at least three biologically independent experiments. All western blots were repeated at least twice with different samples.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The RNA-seq data generated in this study have been deposited in the GEO under accession code GSE265886. All data supporting the findings of this study are available within this paper and its Supplementary Information files. Source data are provided with this paper.
References
Sheng, Y., Yang, G., Markovich, Z., Han, S. M. & Xiao, R. Distinct temporal actions of different types of unfolded protein responses during aging. J. Cell Physiol. 236, 5069–5079 (2021).
Ben-Zvi, A., Miller, E. A. & Morimoto, R. I. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc. Natl Acad. Sci. USA 106, 14914–14919 (2009).
Heydari, A. R. et al. Age-related alterations in the activation of heat shock transcription factor 1 in rat hepatocytes. Exp. Cell Res. 256, 83–93 (2000).
Blake, M. J., Fargnoli, J., Gershon, D. & Holbrook, N. J. Concomitant decline in heat-induced hyperthermia and HSP70 mRNA expression in aged rats. Am. J. Physiol. 260, R663–667, (1991).
Deguchi, Y., Negoro, S. & Kishimoto, S. Age-related changes of heat shock protein gene transcription in human peripheral blood mononuclear cells. Biochem. Biophys. Res. Commun. 157, 580–584 (1988).
Jurivich, D. A. et al. Human aging alters the first phase of the molecular response to stress in T-cells. Exp. Gerontol. 40, 948–958 (2005).
Benedetti, C., Haynes, C. M., Yang, Y., Harding, H. P. & Ron, D. Ubiquitin-like protein 5 positively regulates chaperone gene expression in the mitochondrial unfolded protein response. Genetics 174, 229–239 (2006).
Nargund, A. M., Pellegrino, M. W., Fiorese, C. J., Baker, B. M. & Haynes, C. M. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science 337, 587–590 (2012).
Durieux, J., Wolff, S. & Dillin, A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 144, 79–91 (2011).
Haak, J. L., Buettner, G. R., Spitz, D. R. & Kregel, K. C. Aging augments mitochondrial susceptibility to heat stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R812–820, (2009).
Liu, Y., Samuel, B. S., Breen, P. C. & Ruvkun, G. Caenorhabditis elegans pathways that surveil and defend mitochondria. Nature 508, 406–410 (2014).
Conboy, I. M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 (2005).
Loffredo, F. S. et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 153, 828–839 (2013).
Perez, M. F., Francesconi, M., Hidalgo-Carcedo, C. & Lehner, B. Maternal age generates phenotypic variation in Caenorhabditis elegans. Nature 552, 106–109 (2017).
Wang, G. & Reinke, V. A C. elegans Piwi, PRG-1, regulates 21U-RNAs during spermatogenesis. Curr. Biol. 18, 861–867 (2008).
Wang, J. J. et al. The influences of PRG-1 on the expression of small RNAs and mRNAs. BMC Genomics 15, 321 (2014).
Grishok, A. et al. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 106, 23–34 (2001).
Knight, S. W. & Bass, B. L. A role for the RNase III enzyme DCR-1 in RNA interference and germ line development in Caenorhabditis elegans. Science 293, 2269–2271 (2001).
Ketting, R. F. et al. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 15, 2654–2659 (2001).
Weick, E. M. et al. PRDE-1 is a nuclear factor essential for the biogenesis of Ruby motif-dependent piRNAs in C. elegans. Genes Dev. 28, 783–796 (2014).
Bagijn, M. P. et al. Function, targets, and evolution of Caenorhabditis elegans piRNAs. Science 337, 574–578 (2012).
Gu, W. et al. Distinct argonaute-mediated 22G-RNA pathways direct genome surveillance in the C. elegans germline. Mol. Cell 36, 231–244 (2009).
Claycomb, J. M. et al. The Argonaute CSR-1 and its 22G-RNA cofactors are required for holocentric chromosome segregation. Cell 139, 123–134 (2009).
Ashe, A. et al. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell 150, 88–99 (2012).
Buckley, B. A. et al. A nuclear Argonaute promotes multigenerational epigenetic inheritance and germline immortality. Nature 489, 447–451 (2012).
Ouyang, J. P. T. et al. P granules protect RNA interference genes from silencing by piRNAs. Dev. Cell 50, 716–728 e716 (2019).
Chen, W. et al. GLH/VASA helicases promote germ granule formation to ensure the fidelity of piRNA-mediated transcriptome surveillance. Nat. Commun. 13, 5306 (2022).
Klattenhoff, C. & Theurkauf, W. Biogenesis and germline functions of piRNAs. Development 135, 3–9 (2008).
Beanan, M. J. & Strome, S. Characterization of a germ-line proliferation mutation in C. elegans. Development 116, 755–766 (1992).
Maine, E. M. & Kimble, J. Suppressors of glp-1, a gene required for cell communication during development in Caenorhabditis elegans, define a set of interacting genes. Genetics 135, 1011–1022 (1993).
Schedl, T. & Kimble, J. fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. Genetics 119, 43–61 (1988).
Hodgkin, J. Sex determination in the nematode C. elegans: analysis of tra-3 suppressors and characterization of fem genes. Genetics 114, 15–52 (1986).
Sun, N., Youle, R. J. & Finkel, T. The mitochondrial basis of aging. Mol. Cell 61, 654–666 (2016).
Qu, Z., Zhang, L., Huang, W. & Zheng, S. Vitamin K2 enhances fat degradation to improve the survival of C. elegans. Front Nutr. 9, 858481 (2022).
Burton, N. O. et al. Insulin-like signalling to the maternal germline controls progeny response to osmotic stress. Nat. Cell Biol. 19, 252–257 (2017).
Shi, C. & Murphy, C. T. piRNAs regulate a Hedgehog germline-to-soma pro-aging signal. Nat. Aging 3, 47–63 (2023).
Calculli, G. et al. Systemic regulation of mitochondria by germline proteostasis prevents protein aggregation in the soma of C. elegans. Sci. Adv. 7, eabg3012 (2021).
Lee, H. J. et al. Prostaglandin signals from adult germ stem cells delay somatic aging of Caenorhabditis elegans. Nat. Metab. 1, 790–810 (2019).
Moore, R. S. et al. The role of the Cer1 transposon in horizontal transfer of transgenerational memory. Cell 184, 4697–4712 e4618 (2021).
Ermolaeva, M. A. et al. DNA damage in germ cells induces an innate immune response that triggers systemic stress resistance. Nature 501, 416–420 (2013).
Barucci, G. et al. Small-RNA-mediated transgenerational silencing of histone genes impairs fertility in piRNA mutants. Nat. Cell Biol. 22, 235–245 (2020).
Wu, W. S. et al. piRTarBase: a database of piRNA targeting sites and their roles in gene regulation. Nucleic Acids Res. 47, D181–D187 (2019).
Priyadarshini, M., Ni, J. Z., Vargas-Velazquez, A. M., Gu, S. G. & Frokjaer-Jensen, C. Reprogramming the piRNA pathway for multiplexed and transgenerational gene silencing in C. elegans. Nat. Methods 19, 187–194 (2022).
Perez, M. F. & Lehner, B. Vitellogenins-Yolk gene function and regulation in Caenorhabditis elegans. Front. Physiol. 10, 1067 (2019).
Ezcurra, M. et al. C. elegans eats its own intestine to make yolk leading to multiple senescent pathologies. Curr. Biol. 28, 2544–2556.e2545 (2018).
Leiser, S. F. et al. Age-associated vulval integrity is an important marker of nematode healthspan. Age (Dordr.) 38, 419–431 (2016).
Marre, J., Traver, E. C. & Jose, A. M. Extracellular RNA is transported from one generation to the next in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 113, 12496–12501 (2016).
Xin, N. et al. The UPRmt preserves mitochondrial import to extend lifespan. J. Cell Biol. 221, e202201071 (2022).
Berry, B. J. et al. Optogenetic rejuvenation of mitochondrial membrane potential extends C. elegans lifespan. Nat. Aging 3, 157–161 (2023).
Pellegrino, M. W. et al. Mitochondrial UPR-regulated innate immunity provides resistance to pathogen infection. Nature 516, 414–417 (2014).
Soo, S. K. & Van Raamsdonk, J. M. High confidence ATFS-1 target genes for quantifying activation of the mitochondrial unfolded protein response. MicroPubl. Biol 2021, https://doi.org/10.17912/micropub.biology.000484 (2021).
Edmonds, J. W. et al. Insulin/FOXO signaling regulates ovarian prostaglandins critical for reproduction. Dev. Cell 19, 858–871 (2010).
Kubagawa, H. M. et al. Oocyte signals derived from polyunsaturated fatty acids control sperm recruitment in vivo. Nat. Cell Biol. 8, 1143–1148 (2006).
Kumsta, C. & Hansen, M. C. elegans rrf-1 mutations maintain RNAi efficiency in the soma in addition to the germline. PLoS ONE 7, e35428 (2012).
Kim, J. et al. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell 17, 388–399 (2010).
Zhang, Y. & Beachy, P. A. Cellular and molecular mechanisms of Hedgehog signalling. Nat. Rev. Mol. Cell Biol. 24, 668–687 (2023).
Olson, C. B. A review of why and how we age: a defense of multifactorial aging. Mech. Ageing Dev. 41, 1–28 (1987).
Kirkwood, T. B. Evolution of ageing. Nature 270, 301–304 (1977).
Tsang, W. Y. & Lemire, B. D. Mitochondrial genome content is regulated during nematode development. Biochem. Biophys. Res. Commun. 291, 8–16 (2002).
Shen, K. et al. The germline coordinates mitokine signaling. Cell 187, 4605–4620 e4617 (2024).
Labbadia, J. & Morimoto, R. I. Repression of the heat shock response is a programmed event at the onset of reproduction. Mol. Cell 59, 639–650 (2015).
Shemesh, N., Shai, N. & Ben-Zvi, A. Germline stem cell arrest inhibits the collapse of somatic proteostasis early in Caenorhabditis elegans adulthood. Aging Cell 12, 814–822 (2013).
Acknowledgements
The authors thank the Caenorhabditis Genetics Center (CGC) and the National BioResource Project (NBRP) for worm strains. We thank Profs. Mengqiu Dong, Di Chen and Qinghua Zhou for generously providing worm strains. Ying Liu was supported by grants from the National Natural Science Foundation of China (grants no. 32293212, 31925012, 92254305, 92157301), and the Ministry of Science and Technology of China (National Key Research and Development Program of China, grant no. 2022YFA0806502). Ying Liu acknowledges support from the Tencent Foundation through the New Cornerstone Investigator Program.
Author information
Authors and Affiliations
Contributions
L.Z. and Y.L. conceived the study and designed the experiments. L.Z., L.J., L.L., C.M., and P.X. performed the experiments. W.D. conducted the bioinformatics analysis. L.Z., W.D., and Y.L. analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks David Vilchez and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, L., Jiang, L., Li, L. et al. A germline-to-soma signal triggers an age-related decline of mitochondrial stress response. Nat Commun 15, 8723 (2024). https://doi.org/10.1038/s41467-024-53064-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-53064-0