Abstract
Sir2-HerA is a widely distributed antiphage system composed of a RecA-like ATPase (HerA) and an effector with potential NADase activity (Sir2). Sir2-HerA is believed to provide defense against phage infection in Sir2-dependent NAD+ depletion to arrest the growth of infected cells. However, the detailed mechanism underlying its antiphage activity remains largely unknown. Here, we report functional investigations of Sir2-HerA from Staphylococcus aureus (SaSir2-HerA), unveiling that the NADase function of SaSir2 can be allosterically activated by the binding of SaHerA, which then assembles into a supramolecular complex with NADase activity. By combining the cryo-EM structure of SaSir2-HerA in complex with the NAD+ cleavage product, it is surprisingly observed that Sir2 protomers that interact with HerA are in the activated state, which is due to the opening of the α15-helix covering the active site, allowing NAD+ to access the catalytic pocket for hydrolysis. In brief, our study provides a comprehensive view of an allosteric activation mechanism for Sir2 NADase activity in the Sir2-HerA immune system.
Similar content being viewed by others
Introduction
To protect themselves from infection by their viral predators, known as phages, bacteria have developed extremely sophisticated and diverse immune systems1. Recently, an arsenal of previously unknown antiviral systems, in addition to the well-known CRISPR‒Cas and restriction-modification (RM) systems2,3, have been discovered due to the development of high-throughput bioinformatic and experimental screening methods4,5,6,7. Recent studies have shown that prokaryotic antiviral systems utilize complex strategies and mechanisms to confer resistance against phage infection1. Among the various mechanisms, bacteria frequently use nucleotide-modifying effectors to target molecules that are essential for cellular signaling8. For example, ATP molecules can be deaminated by the RADAR defense system to inhibit phage replication9,10. Similarly, ATP can be degraded by the conserved CBASS effector Cap17 with ATP nucleosidase to provide immunity against phage infection10,11, and deoxynucleotide triphosphates (dNTPs) can be consumed by bacterial deoxycytidine triphosphate (dCTP) deaminase proteins and deoxyguanosine triphosphatase (dGTPase)12. In addition, cellular NAD+ molecules can be rapidly degraded by proteins with NADase activity13.
NAD+ is an important cellular molecule in energy metabolism, and loss of NAD+ can cause cell dormancy or host death14. It is not surprising that the cellular NAD+ pool can be hijacked in prokaryotes and eukaryotes, ultimately leading to host growth arrest13. It has been reported that 7% of sequenced bacterial genomes carry defense systems that limit phage propagation by degrading NAD+7. To date, several families of proteins with the ability to hydrolyze NAD+ have been characterized. For example, TIR ___domain-containing proteins in plants15,16,17,18, animals19 and bacteria20,21,22,23,24,25,26,27,28,29 can serve as NADase effectors. Additionally, Sir2 ___domain-containing proteins provide antiphage protection via Sir2-dependent NAD+ depletion30,31. Intriguingly, prokaryotic
Sir2 NADases in bacteria generally exhibits an autoinhibited conformation, and its enzymatic activity is tightly regulated by auxiliary components in response to viral infection. For example, the activation of the N-terminal Sir2 ___domain in ThsA requires the binding of 3’ cADPR, which is produced by the neighboring ThsB5,32. In the SPARSA system, the NADase activity of the pAgo-associated partner is triggered upon the recognition of ssDNA by pAgo and its APAZ ___domain25,33. Defense-associated sirtuin (DSR2) also possesses a conserved Sir2 ___domain at its N-terminus with putative NAD+ cleavage activity5, which becomes an active NADase effector that requires the direct binding of phage tail tube proteins34.
In prokaryotes, prior work had noticed that the genomic neighborhood of Sir2 genes encode proteins belonging to the ASCE (additional strand conserved E) family protein, HerA. Although the specific functions are unknown, a functional link connecting these two proteins has been proposed35. Recent findings have revealed their high frequency of co-occurrence in the so-called defense system, and their role in phage protection has been established4,36. As exemplified in E. coli, simultaneous overexpression of Sir2-HerA of Paenibacillus sp. 435MF (PsSir2-HerA) can protect the cells against phage infection, which is attributed to Sir2-mediated NAD+ depletion34. A similar antiphage role was also observed in Sir2-HerA of E. coli NCTC 11129 (EcSir2-HerA) to combat λ phage5. Recent studies on the EcSir2-HerA and PsSir2-HerA have revealed the assembly-mediated activation of Sir2 NADase activity and anti-phage defense in the Sir2-HerA37,38,39. However, the accurate mechanisms on the activation of Sir2 NADase function of the dual-component bacterial antiphage defense system remain obscure.
In the present study, we demonstrated that Sir2 of S. aureus (SaSir2) alone is in the autoinhibited state, which can specifically interact with the associated partner SaHerA to form a giant catalytic complex with NAD+ hydrolytic activity in response to phage infection. Furthermore, we obtained high-resolution cryo-electron microscopy (cryo-EM) structures of the trimeric SaHerA and the ADPR-bound SaSir2-HerA complex. We found that SaSir2 and SaHerA can assemble into a 900 kDa supramolecular complex with a stoichiometry of 12:6. Importantly, SaSir2 molecules in the two-layered hexamers display different states that the six SaSir2 protomers interacted with SaHerA are active NADase due to the opening of the NAD+-binding pocket compared with the inactive SaSir2 in the other layer, which failed to be discovered in the recent studies on EcSir2-HerA and PsSir2-HerA37,38,39. Collectively, the structural findings, complemented by the results of the biochemical analyses, revealed a distinct mechanism by which SaSir2 NADase is allosterically triggered by the binding of the accessory HerA, to our knowledge, which has not been described before.
Results
SaHerA induces SaSir2 NADase activity in the SaSir2-HerA antiphage system
The Sir2-HerA locus of S. aureus encodes a potential NADase (SaSir2) and a predicted ATPase protein (SaHerA) (Fig. 1a). We hypothesized that SaSir2 and SaHerA cooperate to fulfill their antiphage functions based on the cooccurrence of the Sir2/HerA operon in the bacterial genome and recent findings showing that coexpression of Sir2 and HerA in bacteria lacking this system enables the depletion of cellular NAD+ to protect against phage infections33,34.
a Schematic diagram of the Sir2 and HerA operon of S. aureus. b In vitro NADase activity of SaSir2 and SaSir2-HerA; SaSir2 could not hydrolyze NAD+; in contrast, a mixture of SaSir2 and SaHerA exhibited NADase activity. c The gel filtration profile and SDS‒PAGE results of copurified SaSir2-HerA. d AUC result for apo SaSir2, suggesting that it behaved as a homodimer in solution. e Effects of different concentrations of ATP on the NADase activity of SaSir2-HerA. Data were presented as mean ± SD in histograms; n = 3 independent experiments. P-value determined by paired two-tailed t-tests is indicated in the figure. Source data are provided as a Source Data file.
All known Sir2 NADases in previously characterized antiphage systems are inactivated in the absence of viral infection30,32,33; thus, we wondered whether SaSir2 is capable of hydrolyzing NAD+ or whether its NAD+ degradation activity requires other stimuli. Subsequently, in vitro NADase assays were utilized to test whether SaSir2 has intrinsic NADase activity. To this end, SaSir2 and SaHerA were individually cloned and purified (Supplementary Fig. 1). Next, twenty microliters of purified SaSir2 (1 mM) and 10 mM NAD+ were incubated at 37 °C for 1 h. After the reaction was terminated, the residual NAD+ levels were determined by measuring the absorbance at 450 nm. As a negative control, the amount of NAD+ was not reduced in SaHerA. There was no decrease in the level of NAD+ in the SaSir2 group compared with the control group (Fig. 1b). This result suggested that SaSir2 alone was unable to degrade NAD+, which is similar to other Sir2 NADases in SPARSA30,33 and the Theoris antiphage system40.
Previous studies established that antiphage functions were achieved through supramolecular complex assemblies in multiple-component antiphage systems9,10,41,42. We were curious whether SaSir2 and SaHerA could form a functional unit to perform their antiphage functions. To test this hypothesis, the recombinant plasmid pET21a-Sir2-HerA (with a ribosome binding site, AGGAGG, introduced between the two genes) was constructed to coexpress N-terminally 6 x His-tagged SaSir2 and untagged SaHerA43. Ni affinity chromatography and the size-exclusion chromatography revealed the coelution of SaHerA, confirming its direct interactions with SaSir2 (Fig. 1c). Next, the NADase activity of the mixture of SaSri2 and SaHerA was further measured to investigate the effect of SaHerA binding to Sir2. As expected, robust NADase activity was observed on the basis of the significant decrease in the level of NAD+ after the addition of HerA (Fig. 1b). Multiple sequence alignments of SaSir2 homologs revealed that N226 and H287 were completely conserved (Supplementary Fig. 2) and that they may constitute the catalytic dyad for NAD+ hydrolysis. A point mutation at H287 completely abolished the ability of SaSir2-HerA to hydrolyze NAD+, confirming that H287 is the key catalytic residue for NAD+ degradation (Fig. 1b). Interestingly, SaSir2 lacked the C-terminal helix responsible for the dodecamer assembly of Sir2 in E. coli, in contrast, SaSir2 existed as a homodimer in solution according to the result of analytic ultracentrifugation (Fig. 1d). Collectively, these observations implied that the NADase activity of SaSir2 can be triggered by HerA. This result also corresponded with findings of recent studies on the Sir2-HerA complex of E. coli (EcSir2-HerA)37,38.
The SaSir2-HerA complex could hydrolyze NAD+ in vitro; however, we did not detect growth arrest in E. coli when SaSir2 and SaHerA were coexpressed (Supplementary Fig. 3), which seemed to contradict the robust NADase activity observed in vitro. We reasoned that the NADase activity of SaSir2-HerA was probably inhibited in vivo in the absence of phage infection. Recent studies have shown that cellular ATP can inhibit the enzymatic activities of associated effectors by binding to the predicted ATPase in some antiphage systems, including EcSir2-HerA37,38 and Septu44. In the EcSir2-HerA antiphage system, a concentration of ATP lower than the physiological concentration (0.5 mM vs. 3 mM)45 can effectively inhibit NADase activity in vitro37,38. Thus, we hypothesized that the NADase activity of SaSir2-HerA could also be inhibited by cellular ATP. To confirm this hypothesis, the purified SaSir2-HerA was incubated with different concentrations of ATP (ranging from 0.5 to 2 mM, the same concentrations of ATP used for Ec. Sir2-HerA)45 for 30 min to test the effect on SaSir2-HerA NADase activity. As expected, the addition of ATP indeed efficiently inhibited the NADase activity of SaSir2-HerA in vitro (Fig. 1e). Hence, these results revealed that SaSir2 and SaHerA can form a complex with NADase activity and the enzymatic activity of this complex can be repressed by cellular ATP.
The homotrimeric structure of the apo form SaHerA
Unlike other RecA-like ATPases, SaHerA alone did not exhibit ATPase activity in vitro. The apo structure of EcHerA in EcSir2-HerA has not been successfully solved because of its heterogeneity37,38, which limits our knowledge of the mechanism underlying its inability to hydrolyze ATP. However, SaHerA behaved well and we finally obtained a 2.80 Å cryo-EM structure of apo SaHerA (Supplementary Fig. 4, Table 1). The electron density map indicated that SaHerA in solution is a homotrimer (Fig. 2a, b), unlike the hexameric RecA-like ATPases46. According to the structure of SaHerA predicted by AlphaFold47, each SaHerA protomer comprises three domains, namely, the N-terminal β-barrel ___domain, the central ATPase ___domain and the helical-bundle ___domain (Fig. 2c)47. However, we exclusively observed densities corresponding to parts of the central ATPase ___domain and the helical-bundle ___domain (Fig. 2d). Structural analysis via a DALI search revealed that HerA of Sofolobus solfataricus (SsHerA) closely resembled SaHerA48. Like in SsHerA, the N-terminus of SaHerA forms a six-stranded β-barrel ___domain with an extra β-hairpin, which is assembled on top of the central RecA-like ATPase ___domain. Unlike the four-helix ___domain of SsHerA, SaHerA possesses an extended helical-bundle ___domain composed of nine α-helices but lacks the α-helical extension from the barrel ___domain that interacts with the NurA nuclease (Fig. 2c).
a Cryo-EM density maps of the trimeric SaHerA from different views. b Ribbon diagram of the structure of the homotrimeric SaHerA from the same views as in (a). c Structure of SaHerA generated by AlphaFold; the HAS ___domain, ATPase ___domain and helical bundle are colored orange, cyan and blue, respectively. d The portions discernible in the apo SaHerA structure are boxed. e, f Detailed interactions among the protomers mediated by the helical-bundle ___domain of SaHerA.
In SsHerA, the four-helix bundle ___domain forms the entrance to the pore for the DNA48; however, the tripartite SaHerA molecules are pulled together by the helical-bundle ___domain (Fig. 2b), causing pore closure and separation of the central ATPase ___domain and the N-terminus of SaHerA. The trimerization of SaHerA relies predominantly on the helical-bundle ___domain, unlike the structure of SsHerA, in which all three domains are involved in hexameric assembly via interactions with the corresponding domains of neighboring chains48. The interfaces are mediated by extensive hydrogen bonds among individual protomers with buried areas of 675–850 Å2 (Fig. 2e, f). Additionally, the interactions among the three protomers are nearly identical; thus, the interactions between protomers A and B were used to elucidate the intersubunit contacts. Residues Q358, S361 and Y362 of the small helix in protomer A form hydrogen bonds with S353, Y240 and L323 of the adjacent chain, respectively (Fig. 2e). In addition, the loop connecting the α-helices also contributes to interactions in which residues Q266 and Q267 form hydrogen bonds with the side chains Q266 and N265 (Fig. 2f).
The C-terminal brace in the ATPase ___domain plays an important role in interactions with adjoining protomers, contributing to hexamer assembly. In addition, the trans-acting arginine fingers from neighboring protomers are responsible for ATP catalysis48. However, the distances among the ATPase domains of the trimeric SaHerA proteins are >30 Å from each other (Fig. 2b), which may account for the conformation being unfavorable for ATP hydrolysis in the apo form of SaHerA.
Architecture of the SaSir2-HerA phage defense system
Given that SaHerA can bind to and induce the NADase function of SaSir2, to better understand the relationship between the assembly of SaSir2-HerA and NAD+ hydrolysis, we aimed to solve the structure of the catalytically inactive SaSir2-HerA H287A in complex with NAD+ via cryo-EM. We therefore incubated NAD+ with the purified SaSir2-HerA H287A complex prior to cryo-EM grid preparation. It was found that substitution on H287 caused its instability, luckily, we finally obtained a three-dimensional reconstruction of SaSir2-HerA supplemented with NAD+ at an overall resolution of 2.81 Å (Supplementary Fig. 5a–c, Table 1).
The overall structure of SaSir2-HerA assumes a pin-like configuration that contains twelve copies of SaSir2 and six sets of SaHerA molecules with dimensions of ~193 Å across and ~193 Å in height (Fig. 3a, b). The tip is composed of a hexameric SaHerA, which is significantly distinct from the trimeric architecture of apo SaHerA (Fig. 2b). The basement is composed of two layers of SaSir2 with six SaSir2 molecules per layer, in all of which the large domains are packed against the pore of the hexameric SaHerA assembly. The N-terminal electron density of SaHerA, which was not observed in apo SaHerA, was greatly improved in the SaSir2-HerA complex. In contrast, the density of the C-terminal α-bundle ___domain in the SaSir2-HerA assembly was low (Fig. 3a).
a Different views of the cryo-EM density map of the SaSir2-HerA complex, SaSir2 and SaHerA are shown with the protomers color coded. b Atomic model built into the cryo-ME map, which is colored as (a). c, d Cartoon mode and surface mode of the N-terminal ___domain of SaHerA. e Top view of the SaHerA hexamer; the two clefts between the two trimers are illustrated.
Assembly of the HerA hexamer
There are many similarities between SsHerA and SaHerA48, including the hexameric assembly, a C-terminal brace that links neighboring subunits (Supplementary Fig. 6a), and the dependence of the assembly on extensive interfaces mediated by the three domains in adjacent subunits. However, SaHerA has several distinct features.
First, compared with the symmetric SsHerA, SaHerA assumes an asymmetric hexameric assembly. The loop connecting β6 and β7 protrudes from the β-barrel ___domain, forming a pore with a diameter of ~16 Å inserted into the ring of the two-layered Sir2 hexamer (Fig. 3c–d, Supplementary Fig. 6b, c). Second, the asymmetric hexamer of SaHerA is composed of dimers of trimers in which two clefts can be observed between the trimers (Fig. 3e), which is distinct from the symmetric SsHerA48 but somewhat similar to EcHerA37,38, although the two clefts are less evident than those of EcHerA37,38. Moreover, the interfaces mediated trimer-trimer complex formation are comparable to the protomers within each trimer (4200 and 3472 Å2 vs. 3400 Å2) (Supplementary Fig. 6d). These findings differ significantly from those for SsHerA (in which the interaction interfaces were identical among the protomers48) and EcHerA (in which the number of interfaces between the trimers was less than that between the protomers37,38). Finally, all the N-terminal barrel domains are packed against the physically interacted SaHerA, in contrast, only five EcHerA protomers were observed to interact with EcSir237,38.
Structural analysis of SaSir2 NADase and assembly of the two-layered SaSir2 hexamer
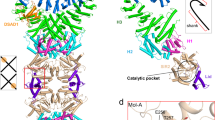
The overall structure of each SaSir2 protomer resembles that of homologs of typical Sir2 proteins, exhibiting a bilobed structure that consists of a large Rossmann fold ___domain and a small cap-like ___domain (Fig. 4a)49. The small ___domain comprises eight α helices, which are composed of two α bundles (denoted α bundles 1 and 2), formed by α4-α7 and α2, α8 and α9, which are separated by α3. The large ___domain adopts a Rossmann fold, which is composed of eight β-sheet cores sandwiched between two layers of α helices and an extra β-hairpin. A cleft is formed by the large Rossmann fold ___domain and the small cap ___domain, which is typical of the canonical Sir2 ___domain proteins and is predicted to accumulate NAD+ for hydrolysis (Fig. 4a).
a SaSir2 exhibits a typical Rossmann fold. b The closest structurally similar SaSir2 NADase (EcSir2, PDB 8SXX) to SaSir2. c Overlay of SaSir2 and EcSir2 highlighting the differences among SaSir2, EcSir2 and other Sir2 ___domain-containing proteins. The additional loop and α helix bundle are highlighted in blue. d Cartoon model of the SaSir2 dodecamer. e The hexameric layer of SaSir2 protomers that interact directly with SaHerA (upper layer). f The bottom layer of SaSir2 protomers that do not interact with SaHerA. g Homodimer units of SaSir2. h-i Enlarged view of the interactions shown in (g). j Neighboring SaSir2 dimer unit were pulled together by Sir2 molecules in the bottom layer (Molecule B) and the top layer (Molecule C). k Enlarged view of the β-hairpin formed in (j). l Detailed interactions of the interfaces shown in (j).
SaSir2 exhibits a classic fold similar to that of Sir2 proteins in eukaryotes and prokaryotes, and a Dali server search revealed that SaSir2 is structurally similar to the homologous Sir2 from E. coli (EcSir2) (Fig. 4b)32. Superposition of SaSir2 and EcSir2 revealed several distinct features of SaSir2 (Fig. 4c). First, SaSir2 contains an additional α-bundle 1 in the small ___domain compared with the known Sir2 proteins in eukaryotes and prokaryotes. Second, Sir2 of the Sir2-HerA antiphage system possesses an extra β4β5-hairpin protruding from the large ___domain (Fig. 4c), which is essential for the supramolecular complex formation (see the next section), distinguishing it from other Sir2 proteins. In addition, SaSir2 lacks the C-terminal α-helix essential for oligomer formation (Fig. 4c).
Unlike the dimeric SaSir2 in solution, a dodecameric assembly was further formed by the two-layered hexameric ring in the SaSir2-HerA supermolecule (Fig. 4d). Only the top layer of SaSir2 directly interacted with SaHerA (SaSir2top); however, there were no direct interactions among the SaSir2top protomers. As observed in SaSir2top, at their closest, the protomers were ~ 10 Å apart (Fig. 4e). The structure lacked the C-terminal α16 stretch of the Rossmann fold ___domain, which contributes to the assembly of the EcHerA-interacting Sir2 assembly in EcSir2-HerA37,38. In SaSir2top, the inserted loop pointed toward the center of the pore, with an inner cavity diameter of ~54.5 Å. In contrast, in the bottom-layer SaSir2 (SaSir2bottom), the corresponding loops were bent toward the neighboring SaSir2 protomers, leading to tight assembly with an inner cavity diameter of ~ 59 Å (Fig. 4f).
Two SaSir2 protomers of different layers form a head-to-head dimer unit, which primarily through a combination of hydrogen bond contacts and charge‒charge interactions, mediates both the small cap-like ___domain and the Rossmann-fold ___domain (Fig. 4g). Both the small ___domain (interface I) and the Rossmann fold ___domain (interface II) are responsible for the formation of the individual dimeric interfaces (Fig. 4h, i). Moreover, the dimer units are held together by the antiparallel β-sheet formed by the additional β4-β5 hairpin that protrudes from the large ___domain of SaSir2 (Fig. 4j, k). Sir2bottom establishes interactions with the adjacent Sir2top of the dimer unit (interface III), which contributes to the intra-layer assembly (Fig. 4i).
Structural basis for the interaction between SaSir2 and SaHerA
To determine the mechanism of SaSir2-HerA assembly, the interfaces between SaSir2 and SaHerA were analyzed. The structure revealed that the N-terminal β-barrel domains of SaHerA protomers were inserted into the pore of the SaSir2 dodecameric ring, and interactions between the SaHerA N-terminal ___domain and the large ___domain established the structural basis for supramolecular assembly (Fig. 5a). Moreover, all six SaHerA protomers were involved in interactions with the SaSir2top protomers, unlike in EcSir2-HerA.
a Surface and cartoon representation of SaSir2-HerA, showing the minimal SaSir2-HerA unit. b, c Specific interactions between SaSir2 and SaHerA; the residues involved in their interactions are shown as sticks. d Deletion of the N-terminal residues of Sir2 and HerA, encompassing residues 1–34 and 1–35, simultaneously abolished the NADase activity of Sir2-HerA. Data were presented as mean ± SD in histograms from three replicates. P-values were determined by paired two-tailed t-tests. e In vitro pull-down assays of SaSir2 with an N-terminal 1–34 deletion and HerA with an N-terminal 1–35 deletion, His-tagged HerA was used as bait, Sir2 was used as a pray. Source data are provided as a Source Data file.
The structure showed that each SaHerA protomer is engaged in the gap between two neighboring SaSir2top molecules with buried surface areas of 1050 Å2, which predominantly results from the large domains of SaSri2 and the N-terminal ___domain of SaHerA. Specifically, the α1-helix of the SaSir2top is bracketed by the β3-β4 hairpin that protrudes from the N-terminal β-barrel ___domain of SaHerA, as well as a loop connected by the β-sheet core (Fig. 5b). The residues located in β3 and β4 of SaHerA form contacts with the residues in the loops connected to the close parallel β-sheets. The loops at the tip on the other side that protrude from the β-barrel are close to the α-helices of SaSir2top (Fig. 5c). To investigate the functional importance of these residues in the interactions between SaSir2top and SaHerA, we simultaneously truncated the N-terminal helix (residues 1–35) of SaHerA and the N-terminus of SaSir2 (residues 1–36), and the purified proteins were subjected to in vitro NADase assays and pull-down experiments. The NADase activity of the mutant was significantly lower than that of wild-type SaSir2-HerA (Fig. 5d), and the truncation disrupted the interactions between SaSir2 and HerA (Fig. 5e). Taken together, these results emphasize the importance of these residues for SaSir2-HerA assembly.
Structural basis for NAD+ hydrolysis by SaSir2
To characterize the basis for NAD+ hydrolysis by SaSir2-HerA, we focused on the active sites and found strong density near the catalytic residues N226 and H287 of all six SaSir2top protomers that interacted with SaHerA. However, instead of NAD+, the density was compatible with adenosine diphosphate ribose (ADPR), the cleavage product of NAD+, confirming the ability of this enzyme to hydrolyze NAD+ (Fig. 6a). Thus, the SaSir2-HerA complex represented a posthydrolyzed state in which the nicotinamide (NAM)-ribosyl bond of NAD+ was cleaved, generating NAM and ADPR. This result also confirmed the previous finding that the enzymatic activity of SaSir2 is activated upon HerA binding (Fig. 1c). However, no discernible density could be found for any of the SaSir2bottom molecules (Supplementary Fig. 7a, b).
a Cut-open sliced view of the NAD+ binding pocket of SaSir2top, the NAD+ cleavage product is shown in sticks. b An enlarged view of ADPR coordination by the key residues of SaSir2. c Effects of mutations on the ADPR-coordinated residues in the in vitro NADase assays. Data were presented as mean ± SD in histograms from three replicates. P-values were determined by paired two-tailed t-tests. Source data are provided as a Source Data file.
ADPR is situated at the cleft formed by the small cap ___domain and Rossmann-like fold, akin to typical Sir2 proteins50, which makes extensive contacts with SaSir2top via an ~450 Å2 buried surface area. The nicotinamide-ribose is positioned at the catalytic residue H287 and is coordinated by the NE atom of H287 and the hydroxy group of T225, E152 and Y352. The pyrophosphate group forms hydrogen bonds with S55. In addition, adenine is packed against the aromatic residue Y413, and the NH2 group forms hydrogen bonds with T48 and Y413. The hydroxy group of the ribose is stabilized by the side chain of H412 and E382 (Fig. 6b). To investigate the abovementioned residues involved in ADPR coordination and NAD+ catalysis, we carried out site-directed mutagenesis to evaluate their importance in NAD+ catalysis. These residues were individually substituted with alanine, and all the mutants were subsequently purified and used for in vitro NADase activity assays. A significant decrease in the NAD+ level was observed in SaSir2 with mutation of E152 and Y352, similar to that observed for wild-type SaSir2-HerA, suggesting that their NADase activity was not affected. In contrast, when other residues were mutated, the residual amount of NAD+ in the reaction system was significantly greater than that observed with wild-type SaSir2-HerA (Fig. 6c), suggesting that the NAD+ depletion ability was affected in in vitro NAD+ degradation assays. Taken together, these results explain the importance of these residues in NAD+ hydrolysis.
A helix-to-loop transition in the Rossmann fold ___domain activates SaSir2 NADase activity
To explore why the NADase activity of SaSir2 requires SaHerA, we carefully compared all SaSir2 molecules in both layers of the SaSir2 hexamer. Noteworthy, ADPR moieties were exclusively visualized in SaSir2top, whereas no densities of ADPR were visible in the other layer of the SaSir2 hexamer. All SaSir2 molecules in the same layer exhibited nearly identical conformations when the structures of SaSir2 in the different layers were compared (Supplementary Fig. 8). Unlike the known Sir2 NADases, such as ThsA and PsSir2, the cores of the α-helices (especially α6) in the small cap ___domain of ADPR-bound Sir2top moved toward the Rossmann-fold ___domain instead of shifting away from the large ___domain, indicating that SaSir2 in Sir2-HerA may be activated in a distinct manner32. In contrast, subtle local conformational changes in α15 (residues 316-325) covering the catalytic pocket were observed in SaSir2bottom, which turned into a loop in SaSir2top (Fig. 7a).
a Overlay of SaSir2 in the bottom layer (ADPR-free) and the upper layer (ADPR-bound) in cartoon representation. The SaSir2 molecules in the bottom and upper layers are colored white and cyan, respectively. α15 (residues 316-325) is colored blue, and the corresponding loop is highlighted in red. b Structural alignment of SaSir2top docked into NAD+ and SaSir2bottom (left) and enlarged view of the steric clashes between the NAD+ and SaSir2bottom, the NAD+ is shown in dotted surface mode (right). c, d BLI sensorgrams of SaSir2, SaSir2-HerA and NAD+. From the top curve to the bottom curve, NAD+ was subjected to twofold serial dilutions starting at 16.5 µM. e In vitro NADase activity of wild-type Sir2 and Sir2 with α15 deletion, as similar to the complex of SaSir2-HerA, the NAD+ level in SaSir2 with α15 deletion is significant lower that the wild-type SaSir2. Data were presented as mean ± SD in histograms from three replicates. Unpaired two-tailed Student’s tests were performed. f The proposed mechanism of the antiphage function of SaSir2-HerA. Source data are provided as a Source Data file.
The conformational change in the α15-helix attracted our attention because this structural change caused the NAD+ binding pocket to become enlarged, which led to the exposure of the active sites. Interestingly, similar to that in SaSir2bottom, AlphaFold also predicted an α-helix in that region with the same orientation in the apo form of SaSir2 (Supplementary Fig. 9a, b). These observations prompted us to suspect that SaSir2 alone was unable to hydrolyze NAD+, probably because of the steric hindrance between the α15-helix and NAD+. To verify this hypothesis, NAD+ was docked into SaSir2top protomers, then the structural alignment was performed by SaSir2top-NAD+ and SaSir2bottom. Steric clashes were clearly observed between the nicotinamide group and the residues of α15 of SaSir2bottom, especially the side chain of K320 (Fig. 7b). We conducted biolayer interferometry (BLI) to measure the affinity between SaSir2-HerA and NAD+, SaSir2 and NAD+, respectively. The results revealed that the affinity between SaSir2 alone and NAD+ was undetectable. In contrast, the affinity of SaSir2-HerA for NAD+ was 4.39 µM (Fig. 7c, d). Thus, it is reasonable to conclude that the helix-to-loop transition of SaSir2top is critical for the activation of its NADase to ensure the access to the active site for NAD+. To further test this hypothesis, we deleted this region and performed an NADase activity assay. If the NADase activity in apo SaSir2 is inhibited because of the blockage of NAD+ binding, deletion of the α15-helix may trigger its enzymatic activity without HerA. In support with this idea, robust NADase activity was obtained in SaSir2 with α15-helix deletion, suggesting the NADase activity of SaSir2 was unleashed even without the assistance of SaHerA (Fig. 7e).
These results satisfactorily support the hypothesis that SaSir2 alone is unable to bind the substrate NAD+, while the binding of HerA in turn confers the ability to bind NAD+. In summary, the results of the present study demonstrated that the helix-to-loop change in the α15 helix occurred in SaSir2top is induced by SaHerA binding, which enables NAD+ to access the catalytic site and undergo hydrolysis; this reaction ultimate leads to the depletion of NAD+ in the infected bacteria, which protects against phage infection (Fig. 7f).
Discussion
Sir2 proteins are a conserved protein family found across all domains of life. In eukaryotes, functional studies have extensively revealed that Sir2 proteins are NAD+-dependent deacetylases49,50 or ADP ribosyltransferases51. However, recent studies have suggested that multiple Sir2 proteins in prokaryotes deplete NAD+ as their defensive mechanism, including ThsA in the Theoris antiphage system4,32, the prokaryotic Argonaute system SPARSA33, DSR1/2 and antiviral ATPase/NTPase of the STAND superfamily 5 (AVAST5)5. Additionally, it seems that the enzymatic activities of known Sir2 NADases are controlled by their associated partners. For example, the NADase activity of ThsA depends on the small molecule produced by ThsB32,52, and the Sir2 NAD+ depletion activity is promoted by the recognition of tDNA and the gRNA in SPARSA33. Moreover, the NAD+ cleavage activity of DSR2 is induced by the phage tail protein to consume the cellular concentration of NAD+. Similarly, in the Sir2-HerA antiphage system, both Sir2 and the adjacent HerA are necessary for antiphage activity34.
To date, the mechanisms underlying the activation of prokaryotic Sir2 NADases have been elucidated in Theoris32, the pAgo-related SPARSA system30 and DSR253,54,55. The activation of some Sir2 NADases depends on conformational changes in their small domains. In the Theoris system, the NADase activity of ThsA is triggered by the destabilization of the tetramers, which leads to the exposure of the catalytic sites through the movement of the α3 helix in the small ___domain32. In addition, the activated ThsA from Bacillus cereus MSX-D12 forms a filament assembly to stabilize the active conformation of the Sir2 ___domain40. In contrast, Sir2 NADase activation in SPARSA depends on the movement of the catalytic loop30. Although the molecular mechanisms involved in the assembly of the PsSir2-HerA system39 and E. coli37,38 have been reported, the mechanism of Sir2 NADase activation has not been completely elucidated. PsSir2 may be allosterically activated by the movement of its small ___domain, because a steric clash was observed in the α2-α3 loop of PsSir2 and the Sir2 ___domain in Sir2-Af1 in complex with NAD+, which is thought to be analogous to ThsA39. Despite the two-gene antiphage system Sir2-HerA is supposed to employ a similar mechanism involving the deletion of cellular NAD+ via Sir2-dependent NADase4, the exact mechanism on the activation of Sir2 NADase activity remains unknown.
Our results support a model of the SaSir2-HerA antiphage system in which the SaSir2-HerA complex detects phage infections by sensing the decrease in cellular ATP, thereby releasing Sir2 NADase and nuclease activities to kill infected cells. We detected only NADase and nuclease activities for the SaSir2-HerA complex; however, we did not detect ATPase or helicase activity in EcSir2-HerA37,38 (Supplementary Fig. 10). There are some differences between SaSir2-HerA and the described PsSir2-HerA and EcSir2-HerA. First, unlike the dodecameric assembly of PsSir2 and EcSir2, SaSir2 is a homodimer owing to the lack of a C-terminal helix that participates in oligomerization. Second, in contrast to apo EcHerA and the PsSir2-HerA complex, which can induce ATP hydrolysis, neither SaHerA nor SaSir2-HerA possesses ATPase activity. Third, SaSir2 and SaHerA form a supramolecule composed of a hexameric SaHerA and dodecameric SaSir2 assembly. However, upon the binding of PsHerA, PsSir2 transforms from the dodecameric state to the tetradecameric state. Finally, the structures described here revealed that the activation of SaSir2 relies on a local conformational change in the large ___domain of SaSir2, which has never been observed in other NADases, including ThsA, PsSir2-HerA and EcSir2-HerA37,38,39.
At present, some questions related to the two-component Sir2-HerA antiphage system remain incompletely answered. For example, the specific mechanism by which ATP levels decrease during phage infection remains unclear. In addition, although cellular ATP can inhibit NADase in the absence of phage infection, the precise molecular mechanism by which ATP represses NADase activity is also not clear. Whatever, the ATPase HerA may detect viral infection by sensing a decrease in ATP and then activate the NADase activity of Sir2-HerA. Thus, the current study focused on the mechanistic basis for the activation of the NADase activity of SaSir2 in the SaSir2-HerA antiphage system. Nevertheless, this study revealed a distinct mechanism that, to the best of our knowledge, is different from those of other known Sir2-mediated NAD+-depleting defense systems.
Methods
Protein expression and purification
The genes encoding SaSir2 (WP_000513494.1) and SaHerA (WP_000555614.1) were synthesized, cloned and inserted into the pET28a (+) and pGEX-6p-1 vectors to express the 6x His-tagged SaSir2 and the GST-tagged SaHerA, respectively. The corresponding recombinant plasmids were transformed into E. coli BL21(DE3) cells to overexpress the recombinant target proteins. E. coli BL21(DE3) was cultivated in LB medium at 37 °C in the presence of Kan or Amp at a concentration of 25 µM or 100 µM until the OD600 reached 0.6–0.8. The temperature was subsequently decreased to 16 °C, and IPTG was added at a final concentration of 0.3 mM. After cultivation for 20 h, the cells were harvested via centrifugation and resuspended in buffer containing 25 mM Tris-HCl (pH 8.0) and 150 mM NaCl. The cells were then lysed by ultrasonication, and the lysate was centrifuged at 17,000 × g at 4 °C for 30 min. The supernatant was then loaded onto a Ni2+-NTA column (Qiagen) for further purification. The target proteins were washed with buffer supplemented with 25 mM imidazole and eluted with buffer supplemented with 200 mM imidazole.
To form the SaSir2-HerA complex, a ribosomal binding site (RBS, AGGAGA)43 was added between the termination codon of SaSir2 and the initiation codon of SaHerA, and the sequence was cloned and inserted into a modified pET-21a with an N-terminal 6xHis tag (SaSir2) and SaHerA without a 6xHis tag, which was purified via a procedure similar to that used for 6xHis-SaSir2. Fractions containing the target protein were pooled and concentrated to obtain a 0.5 mL sample, which was subjected to purification with a Superdex 200 Increase column (Cytiva Life Sciences) equilibrated with buffer that contained 20 mM Tris-HCl (pH 8.0) and 150 mM NaCl.
Analytic ultracentrifugation
The sedimentation velocity of SaSir2 was measured using a Beckman Opima XL-I analytical ultracentrifuge. The test proteins were diluted to 0.8 mg/ml in gel filtration buffer before use. The software SEDFIT56 was employed to calculate the sedimentation coefficient distribution.
In vitro NADase assays
The in vitro NADase assays were performed according to previously described methods22,33. The samples of the purified proteins were concentrated to 1 mM for use. Briefly, a 20 μl reaction mixture was prepared, containing a final concentration of 1 μM purified target proteins and 10 μM NAD+ dissolved in buffer containing 10 mM HEPES (pH 7.5) and 125 mM KCl. The reaction was performed at 37 °C for 3 h. An NAD/NADH quantitation kit (Sigma Aldrich, MAK307) was used to measure the levels of NAD+ in each sample by recording the absorbance at 450 nm after the reaction was terminated. In this study, all in vitro NADase assays were performed at least three times.
To inhibit the NADase activity of Sir2-HerA with ATP, various concentrations of ATP, ranging from 0 mM to 2 mM, were added to the same reaction system used for the NADase activity assay. NADase activity assays were conducted under the same conditions as those described above.
Cryo-EM grid preparation and data collection
The grids were glow-discharged for 30 s (GloQube). For apo-SaHerA, 3 µL of SaHerA at a concentration of 1.9 mg/mL was supplemented with 0.05% octyl-glucoside before it was applied to glow-discharged Quantifoil Au R1.2/1.3 grids (300 mesh). For the SaSir2-HerA complex, 3 µL of protein at a concentration of 2 mg/mL incubated with NAD+ was applied to a glow-discharged grid (Quantifoil Cu R1.2/1.3, 300 mesh). Using a ThermoFisher Vitrobot at 4 °C and 100% humidity, the grids were frozen in liquid ethane with a blot time of 2.5 s and a blot force of 4 s.
Datasets were collected using a Titan Krios G4 microscope (Thermo Fisher Scientific) equipped with a Falcon4 direct electron detector equipped with a SelectrisX energy filter with a 10 eV slit width. Each image was automatically collected using the EPU software (Thermo Fisher Scientific) at a magnification of 165,000×, corresponding to a pixel size of 0.71 Å/pix. The defocus range was between −0.8 and −2.4 µm, with an accumulated total dose of ~50 e − /Å2.
Cryo-EM data processing
In cryoSPARC Live57, movies were subjected to alignment, and contrast transfer function (CTF) estimation was performed58. Subsequently, the particles were blob picked and subjected to 2D classification. For apo-SaHerA, a total of 153,294 particles were chosen for ab initio reconstruction, followed by heterogeneous refinement. From the heterogeneous refinement, Class 3 was selected for homogeneous refinement. Topaz training was executed59, resulting in the selection of 486,514 particles for picking and 2D classification. Ultimately, after ab initio reconstruction, 273,014 particles were selected for heterogeneous refinement, and Class 2 was chosen for homogeneous refinement, yielding a 2.8 Å-resolution map.
For the SaSir2-HerA complex, 487,509 particles were selected and subjected to 2D classification. A total of 117,506 particles were subsequently chosen for ab initio reconstruction. From the ab initio reconstruction, Class 3 was selected for homogeneous refinement under C1 symmetry. Following Topaz training, 303,376 particles were selected and subjected to 2D classification. Finally, Class 3 from ab initio reconstruction was chosen for homogeneous refinement, resulting in a 2.81 Å-resolution map.
Model building and refinement
The model-building and refinement procedures involved the use of Chimera60, ChimeraX61, COOT62, and the cryo-EM module of the Phenix package63. Initial models for both apo-SaHerA and the SaSir2-HerA complex were obtained directly from AlphaFold247. These initial models were then fitted into the EM maps via the “fit in the map” function in UCSF ChimeraX61. The fitted initial models were subsequently subjected to manual adjustments in COOT62. Multiple rounds of real-space refinement were carried out via Phenix and Namdinator. NAD+ was manually added using COOT, followed by further real-space refinement. Finally, structure figures were generated via PyMOL (https://pymol.org/2/), Chimera60, and ChimeraX61.
Structural prediction
The apo SaSir2 and SaHerA were predicted with AlphaFold47, and the outputs were used as templates to construct the models, which were compared to the structures determined by cryo-EM or were searched via a DALI search. All the structural figures in this study were drawn via PyMol (https://pymol.org/2/) and ChimeraX60.
Biolayer interferometry (BLI) assays
The binding affinity between SaSir2, SaSir2-HerA and NAD+ was measured via BLI using Octet R8 (Sartorius). The experiments were performed at 25 °C. The SA sensor tips were preequilibrated in buffer containing 20 mM HEPES and 150 mM NaCl (pH 8.0) in PBS for 10 min. The target proteins were captured in the Ni-NTA sensor for 60 s, and the loaded tips were dipped into a 2-fold dilution series of NAD+, with concentrations of 15.6 nM, 31.3 nM, 62.5 nM, 125 nM, 250 nM, and 500 nM. The curves were processed via Gator software at a ratio of 1:1 after background subtraction. The plots were generated in Prism 9.
Site-directed mutagenesis
PCR-based site-directed mutagenesis was used to generate point mutants using wild-type SaSir2 or SaHerA as the template and oligonucleotide primers containing the desired mutations, which were designed via SnapGene. The oligonucleotide primers used in this study are listed in Supplementary Table 3. All the mutants were verified via DNA sequencing.
Nuclease activity assay
The purified SaSir2 was used to perform a nuclease activity assay, in which 400 nM purified SaSir2 and the pUC19 plasmids were incubated in reaction buffer containing 25 mM Tris-HCl (pH 8.0), 150 mM NaCl and 2 mM MgCl2 at 37 °C for 30 min. The mixture was subsequently separated on a 15% urea gel.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The cryo-EM maps of SaSir2 and SaSir2-HerA-ADPR from this study have been deposited in the Electron Microscope Data Bank (EMDB) with the accession codes EMD-39290 [https://www.ebi.acuk/pdbe/entry/emdb/EMD-39209] and EMD-39302. The atomic coordinates and structure factors for the structures determined in this study have been deposited in the Protein Data Bank under the accession codes 8YHO and 8YHX. The following previously published PDB codes were used for comparison: 4D2I, 8SXX, 8SU9, 6LHX, 8WLD. Source data are provided with this paper.
References
Georjon, H. & Bernheim, A. The highly diverse antiphage defence systems of bacteria. Nat. Rev. Microbiol. 21, 686–700 (2023).
Watson, B. N. J., Steens, J. A., Staals, R. H. J., Westra, E. R. & van Houte, S. Coevolution between bacterial CRISPR-Cas systems and their bacteriophages. Cell Host Microbe 29, 715–725 (2021).
Hille, F. et al. The biology of CRISPR-Cas: backward and forward. Cell 172, 1239–1259 (2018).
Doron, S. et al. Systematic discovery of antiphage defense systems in the microbial pangenome. Science 359, eaar4120 (2018).
Gao, L. et al. Diverse enzymatic activities mediate antiviral immunity in prokaryotes. Science 369, 1077–1084 (2020).
Goldfarb, T. et al. BREX is a novel phage resistance system widespread in microbial genomes. EMBO J. 34, 169–183 (2015).
Millman, A. et al. An expanded arsenal of immune systems that protect bacteria from phages. Cell Host Microbe 30, 1556–1569.e1555 (2022).
Mayo-Munoz, D., Pinilla-Redondo, R., Birkholz, N. & Fineran, P. C. A host of armor: pokaryotic immune strategies against mobile genetic elements. Cell Rep. 42, 112672 (2023).
Duncan-Lowey, B. et al. Cryo-EM structure of the RADAR supramolecular anti-phage defense complex. Cell 186, 987–998.e915 (2023).
Gao, Y. et al. Molecular basis of RADAR anti-phage supramolecular assemblies. Cell 186, 999–1012.e1020 (2023).
Rousset, F. et al. A conserved family of immune effectors cleaves cellular ATP upon viral infection. Cell 186, 3619–3631.e3613 (2023).
Tal, N. et al. Bacteria deplete deoxynucleotides to defend against bacteriophage infection. Nat. Microbiol. 7, 1200–1209 (2022).
Wang, M., Ji, Q., Liu, P. & Liu, Y. NAD(+) depletion and defense in bacteria. Trends Microbiol. 31, 435–438 (2023).
Belenky, P., Bogan, K. L. & Brenner, C. NAD+ metabolism in health and disease. Trends Biochem. Sci. 32, 12–19 (2007).
Horsefield, S. et al. NAD(+) cleavage activity by animal and plant TIR domains in cell death pathways. Science 365, 793–799 (2019).
Jia, A. et al. TIR-catalyzed ADP-ribosylation reactions produce signaling molecules for plant immunity. Science 377, eabq8180 (2022).
Wan, L. et al. TIR domains of plant immune receptors are NAD(+)-cleaving enzymes that promote cell death. Science 365, 799–803 (2019).
Yu, D. et al. TIR domains of plant immune receptors are 2’,3’-cAMP/cGMP synthetases mediating cell death. Cell 185, 2370–2386.e2318 (2022).
Jiang, Y. et al. The NAD(+)-mediated self-inhibition mechanism of pro-neurodegenerative SARM1. Nature 588, 658–663 (2020).
Kottur, J., Malik, R. & Aggarwal, A. K. Nucleic acid mediated activation of a short prokaryotic argonaute immune system. Nat. Commun. 15, 4852 (2024).
Morehouse, B. R. et al. Cryo-EM structure of an active bacterial TIR-STING filament complex. Nature 608, 803–807 (2022).
Ni, D., Lu, X., Stahlberg, H. & Ekundayo, B. Activation mechanism of a short Argonaute-TIR prokaryotic immune system. Sci. Adv. 9, eadh9002 (2023).
Ofir, G. et al. Antiviral activity of bacterial TIR domains via immune signalling molecules. Nature 600, 116–120 (2021).
Shen, Z. et al. Oligomerization-mediated activation of a short prokaryotic argonaute. Nature 621, 154–161 (2023).
Wang, X. et al. Structural insights into mechanisms of Argonaute protein-associated NADase activation in bacterial immunity. Cell Res. 33, 699–711 (2023).
Zhang, J. T., Wei, X. Y., Cui, N., Tian, R. & Jia, N. Target ssDNA activates the NADase activity of prokaryotic SPARTA immune system. Nat. Chem. Biol. 20, 503–511 (2023).
Koopal, B. et al. Short prokaryotic Argonaute systems trigger cell death upon detection of invading DNA. Cell 185, 1471–1486.e1419 (2022).
Coronas-Serna, J. M. et al. The TIR-___domain containing effectors BtpA and BtpB from Brucella abortus impact NAD metabolism. PLoS Pathog. 16, e1007979 (2020).
Finocchio, G. et al. Target DNA-dependent activation mechanism of the prokaryotic immune system SPARTA. Nucleic Acids Res. 52, 2012–2029 (2024).
Zhen, X. et al. Structural basis of antiphage immunity generated by a prokaryotic Argonaute-associated SPARSA system. Nat. Commun. 15, 450 (2024).
Ka, D., Oh, H., Park, E., Kim, J. H. & Bae, E. Structural and functional evidence of bacterial antiphage protection by Thoeris defense system via NAD(+) degradation. Nat. Commun. 11, 2816 (2020).
Manik, M. K. et al. Cyclic ADP ribose isomers: production, chemical structures, and immune signaling. Science 377, eadc8969 (2022).
Zaremba, M. et al. Short prokaryotic Argonautes provide defence against incoming mobile genetic elements through NAD(+) depletion. Nat. Microbiol. 7, 1857–1869 (2022).
Garb, J. et al. Multiple phage resistance systems inhibit infection via SIR2-dependent NAD(+) depletion. Nat. Microbiol. 7, 1849–1856 (2022).
Iyer, L. M., Makarova, K. S., Koonin, E. V. & Aravind, L. Comparative genomics of the FtsK-HerA superfamily of pumping ATPases: implications for the origins of chromosome segregation, cell division and viral capsid packaging. Nucleic Acids Res. 32, 5260–5279 (2004).
Tesson, F. et al. Systematic and quantitative view of the antiviral arsenal of prokaryotes. Nat. Commun. 13, 2561 (2022).
Tang, D. et al. Multiple enzymatic activities of a Sir2-HerA system cooperate for anti-phage defense. Mol. Cell. 83, 4600–4613.e6 (2023).
Shen, Z., Lin, Q., Yang, X. Y., Fosuah, E. & Fu, T. M. Assembly-mediated activation of the SIR2-HerA supramolecular complex for anti-phage defense. Mol. Cell. 83, 4586–4599.e5 (2023).
Liao, F. et al. Structural basis for the concerted antiphage activity in the SIR2-HerA system. Nucleic Acids Res. 52, 11336–11348 (2024).
Tamulaitiene, G. et al. Activation of Thoeris antiviral system via SIR2 effector filament assembly. Nature 627, 431–436 (2024).
Antine, S. P. et al. Structural basis of Gabija anti-phage defence and viral immune evasion. Nature 625, 360–365 (2023).
Bravo, J. P. K., Aparicio-Maldonado, C., Nobrega, F. L., Brouns, S. J. J. & Taylor, D. W. Structural basis for broad anti-phage immunity by DISARM. Nat. Commun. 13, 2987 (2022).
Zhou, B. et al. Structural and functional insights into a novel two-component endolysin encoded by a single gene in Enterococcus faecalis phage. PLoS Pathog. 16, e1008394 (2020).
Li, Y. et al. PtuA and PtuB assemble into an inflammasome-like oligomer for anti-phage defense. Nat Struct Mol. Biol. 31, 413–423 (2024).
Cheng, R. et al. A nucleotide-sensing endonuclease from the Gabija bacterial defense system. Nucleic Acids Res. 49, 5216–5229 (2021).
Khan, Y. A., White, K. I. & Brunger, A. T. TheA. A. A. + superfamily: a review of the structural and mechanistic principles of these molecular machines. Crit. Rev. Biochem Mol. Biol. 57, 156–187 (2022).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Rzechorzek, N. J. et al. Structure of the hexameric HerA ATPase reveals a mechanism of translocation-coupled DNA-end processing in archaea. Nat. Commun. 5, 5506 (2014).
Min, J., Landry, J., Sternglanz, R. & Xu, R. M. Crystal structure of a SIR2 homolog-NAD complex. Cell 105, 269–279 (2001).
Avalos, J. L. et al. Structure of a Sir2 enzyme bound to an acetylated p53 peptide. Mol. Cell 10, 523–535 (2002).
Imai, S., Armstrong, C. M., Kaeberlein, M. & Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800 (2000).
Bayless, A. M. et al. Plant and prokaryotic TIR domains generate distinct cyclic ADPR NADase products. Sci. Adv. 9, eade8487 (2023).
Zhang, J. T. et al. Structural basis for phage-mediated activation and repression of bacterial DSR2 anti-phage defense system. Nat. Commun. 15, 2797 (2024).
Yin, H. et al. Insights into the modulation of bacterial NADase activity by phage proteins. Nat. Commun. 15, 2692 (2024).
Huang, J. et al. Molecular basis of bacterial DSR2 anti-phage defense and viral immune evasion. Nat. Commun. 15, 3954 (2024).
Schuck, P. On the analysis of protein self-association by sedimentation velocity analytical ultracentrifugation. Anal. Biochem. 320, 104–124 (2003).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Bepler, T. et al. Positive-unlabeled convolutional neural networks for particle picking in cryo-electron micrographs. Nat. Methods 16, 1153–1160 (2019).
Pettersen, E. F. et al. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput Chem. 25, 1605–1612 (2004).
Meng, E. C. et al. UCSF ChimeraX: tools for structure building and analysis. Protein Sci. 32, e4792 (2023).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. Biol. Crystallogr. 60, 2126–2132 (2004).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D. Struct. Biol. 74, 531–544 (2018).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2021YFC2301403 to S.O., 2021YFA1300903 to X.X.), National Nature Science Foundation of China grants (32170045 to X.Z., 82225028 and 82172287 to S.O., 32300035 to X.W., and 82341085 to X.X.), and the Nature Science Foundation of Fujian Province (2023J06026 to X.Z. 2023J0123 to X.W.). We thank the staff at the Cryo-EM Facilities of GIBH-CAS, Guangzhou National Laboratory Bio-Imaging Technology, for data collection.
Author information
Authors and Affiliations
Contributions
X.Z. and S.O. conceived the project and designed the experiments. Z.L., S.W., Q.Z., J.L. and Z.L. prepared the samples. W.Z. performed the oligomeric analysis of the apo form SaSir2. B.Z., Z.H. and X.W. collected the EM data. B. Z., Z.H and X.W. analyzed and calculated the EM map and built and regained the atomic model. X. Z., S.O., B.Z., H.J. and X.X. discussed and analyzed the results. X.Z. and S.O. wrote the manuscript with support from all the authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhen, X., Zhou, B., Liu, Z. et al. Mechanistic basis for the allosteric activation of NADase activity in the Sir2-HerA antiphage defense system. Nat Commun 15, 9269 (2024). https://doi.org/10.1038/s41467-024-53614-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-53614-6