Abstract
Diabetic wounds are usually entangled in a disorganized and self-perpetuating microenvironment and accompanied by a prolonged delay in tissue repair. Sustained and coordinated microenvironment regulation and tissue regeneration are key to the healing process of diabetic wounds, yet they continue to pose a formidable challenge. Here we report a rational double-layered dressing design based on chitosan and a degradable conjugated polymer polydiacetylene, poly(deca-4,6-diynedioic acid) (PDDA), that can meet this intricate requirement. With an alternating ene-yne backbone, PDDA degrades when reacting with various types of reactive oxygen species (ROS), and more importantly, generates proliferative succinic acid as a major degradant. Inheriting from PDDA, the developed PDDA-chitosan double layer dressing (PCD) can eliminate ROS in the microenvironment of diabetic wounds, alleviate inflammation, and downregulate gene expression of innate immune receptors. PCD degradation also triggers simultaneous release of succinic acid in a sustainable manner, enabling long-term promotion on tissue regeneration. We have validated the biocompatibility and excellent performance of PCD in expediting the wound healing on both diabetic mouse and porcine models, which underscores the significant translational potential of this microenvironment-modulating, growth-promoting wound dressing in diabetic wounds care.
Similar content being viewed by others
Introduction
Diabetic wounds are open sores or ulcers that occur in 15–25% of patients with diabetes1,2, and they are prevalent and highly morbid—approximately 20% of the patients with diabetic wounds will undergo lower extremity amputation3 and 13% of them will pass away within one year of diagnosis without proper care4. In diabetic wounds, inflammation persists throughout the remodeling stage5, preventing the wounds from healing the way they should. The continuing inflammation in diabetic wounds is linked to the infiltration of immune cells that secrete inflammatory cytokines and proteases6, initiating the production of reactive oxygen species (ROS)7 and tissue damage8. The elevated oxidative stress thus impairs crucial wound healing processes including angiogenesis, collagen deposition, and tissue regeneration, so that the pace of wound healing is hindered9,10. The prolonged and intricate course of diabetic wounds is characterized by the recurrent localized inflammation and the delayed tissue repair. As a result, treating diabetic wounds presents significant challenges, which requires sustained and effective modulation of the erratic local inflammatory microenvironment over the long term, and at the same time, precisely regulates tissue growth based on real-time variations in the microenvironment.
Functional dressings are prevalently used to facilitate wound healing and prevent infection11,12,13,14. Many existing dressings for diabetic wounds are devoted to mitigating inflammation15,16,17, involving ROS elimination18,19,20, chronic infection reduction21,22, or direct immunity modulation23,24. Other dressing strategies focus on enhancing tissue regeneration25,26,27, usually by incorporating proliferative molecules28,29 or embracing mechanical modulation30 and electric stimulation31 to promote proliferation. Moreover, for dressings that integrate multiple functional components to realize simultaneous anti-inflammatory and tissue regeneration32,33,34, they are facing difficulties in achieving long-term coordination of individual components within the chaotic microenvironment of diabetic wounds. Therefore, dressings that can modulate the wound microenvironment while generating proliferative molecules in a correlated manner are essential for diabetic wound treatment.
Here, we create a double-layered, microenvironment-modulating dressing that can accurately eliminate ROS and release proliferative degradants during the healing course of diabetic wounds. The dressing is composed of a layer of hydrophilic conjugated polymer poly(deca-4,6-diynedioic acid) (PDDA, Fig. 1a)35, which can react with ROS gradually due to its alternating ene-yne chemical structure36,37,38,39,40,41. The dressing therefore possesses a long-acting ability to scavenge ROS in the wound microenvironment. More strikingly, the major degradant of PDDA when interacting with various ROS is proved to be succinic acid (SA)36, a pivotal proliferative molecule in energy metabolism for tissue repair and regeneration42,43,44. Therefore, PDDA has the potential to tailor diabetic wound treatment by promoting tissue proliferation while modulating the unpredictable inflammatory microenvironment. The supporting layer of the dressing is made of chitosan (CS), a natural polysaccharide known for its hemostatic and antibacterial features45,46,47 (Fig. 1a). PDDA-CS double layer dressing (PCD) exhibits good hydrophilicity and porosity, which is important for hemostasis. Through its degradation, PCD can clear ROS, modulate the wound microenvironment on demand, and simultaneously generate succinic acid to foster tissue regeneration (Fig. 1b). We have validated the excellent performance of PCD on both diabetic mouse and porcine models, in which PCD significantly speeds up the wound healing process, underscoring the significant translational potential of this straightforward material design.
Results
PDDA eliminates ROS and generates proliferative degradants
We first evaluated whether PDDA could be used to eliminate the high-level ROS in the wound area, and generate degradants that promote angiogenesis and tissue regeneration in diabetic wounds (Fig. 2a). The main ROS generated in various types of cells include superoxide radical (·O2−), hydroxyl radical (·OH), and hypochlorite ion (ClO−)10, and we examined the clearance of these ROS by PDDA via an assay kit (for ·O2−) or using 3,3′,5,5′-tetramethyl benzidine (TMB) as an indicator (for ·OH and ClO−). PDDA could eliminate all these ROS in a dose-dependent manner (Fig. 2b–d). Further analysis of PDDA degradants through 1H NMR (Fig. 2e), 13C NMR (Fig. 2f), and high-resolution mass spectroscopy (Fig. 2g) confirmed that SA was the primary product of the reaction between PDDA and these ROS36.
a Schematic illustration of the simultaneous ROS elimination and proliferative SA generation by PDDA. b–d Concentration-dependent elimination of ·O2− (b), ·OH (c), and ClO− (d) by PDDA (n = 3 independent experiments). e–g 1H NMR (e), 13C NMR spectra (f), and HR-MS spectra (g) of crude mixture of PDDA degradants by ·OH. SA was used as a reference. h Representative images of HUVECs migration following 24 h of incubation with SA and PDDA degradants (n = 5). Scale bars: 100 μm. i Relative mRNA expression levels of growth factor VEGFA and angiogenesis marker Angpt2 in SA and PDDA degradants-treated HUVECs (n = 3 independent experiments). j Representative images and quantitative analysis of transwell migration assay in PDDA degradants-treated HUVECs (n = 5 independent experiments). Scale bars: 100 μm. k Representative images and quantification of tube formation in PDDA degradants- treated HUVECs (n = 3 independent experiments). Scale bars: 100 μm. All data were presented as the mean ± SEM. Statistical significance was determined using unpaired two-sided Student’s t-tests. Parts of a were created with BioRender.com.
To assess the role of PDDA degradants, succinic acid in the majority, in energy metabolism for tissue repair and regeneration, we conducted scratch wound healing assay on human umbilical vein endothelial cells (HUVECs) that are essential for wound healing and vascularization48. The PDDA degradants as well as succinic acid significantly promoted the migration of HUVECs and induced faster closure of scratch gap compared with the control (Fig. 2h and Supplementary Fig. 1), suggesting that they could enhance the mobility of HUVECs. We further studied the effect of PDDA degradants on angiogenesis by measuring the expression of relevant genes by real-time quantitative polymerase chain reaction (RT-qPCR). The results demonstrated that treating the cells with either PDDA degradants or succinic acid could significantly upregulate the expression of VEGFA and Angpt2 (Fig. 2i), which were important to regulate vascular development and maintenance, implying that PDDA degradants had similar effects on cell migration and angiogenesis as succinic acid. Following this, we conducted additional assessments of the effects of PDDA degradants on cell mobility and vessel-forming capability using transwell migration and Matrigel tube formation assays. Our data revealed that the degradants could significantly enhance HUVECs’ migration and tube length when compared to the control (Fig. 2j, k). These results collectively evidenced that the degradants of PDDA upon scavenging ROS are effective in promoting cell migration and angiogenesis.
Fabrication and characterization of PCD
The efficient ROS elimination effect of PDDA and its proliferative degradants inspired us to fabricate a PDDA-based dressing for the healing of diabetic wounds. In addition to the PDDA layer, we prepared a supporting layer of CS to increase the mechanical property of PDDA as well as to endow the dressing with additional properties of hemostasis and antibacterial. The dual layers of the dressing well adhered to each other through electrostatic and hydrogen bonding interactions, and the PDDA layer was exposed to the tissue when applying the dressing for diabetic wounds. As shown in Fig. 3a, a film of CS appeared colorless and transparent, whereas the PCD exhibited an overall red and opaque appearance, given the large π-conjugated backbone in PDDA. Interestingly, the CS film was fragile and could easily break when bent. As a comparison, PCD was more flexible with a better mechanical property. Since CS was commonly used as bandages after being treated with alkaline, we employed alkaline-soaked CS (CN) as a control in the study. The CN film exhibited a much better folding endurance (Fig. 3b), tensile strength (Fig. 3c), and maximum load (Fig. 3d) than both the CS film and PCD (Supplementary Fig. 2), but demonstrated a similar Young’s modulus (Fig. 3e) to the CS film.
a Optical images of pristine and folded CS film, CN film, and PCD. Red rectangle: broken film area. Scale bar: 1 cm. b Folding endurance of CS films, CN films, and PCD (n = 3 independent samples). c Tensile strength of CS films, CN films, and PCD (n = 3 independent samples). d Maximum load of CS films, CN films, and PCD (n = 3 independent samples). e Young’s modulus load of CS films, CN films, and PCD (n = 3 independent samples). f Scanning electron microscope images of the cross-section and surface of CS film, CN film, and PCD (images are representative of 3 independent samples). Scale bar: 5 μm. g, h Representative images (g) and quantification (h) of water contact angles of CS films, CN films, and PCD (n = 3 independent samples). i, j Swelling rate (i) and porosity (j) of CS films, CN films, and PCD (n = 3 independent samples). All data were presented as the mean ± SEM. Statistical significance was determined using a one-way ANOVA test followed by Tukey’s multiple comparison analysis.
The mechanical properties of the CN film were consistent with its densely compact cross-section as revealed by the scanning electron microscopy images (Fig. 3f). As a comparison, the cross-section of the CS film was relatively loose with spreading cracks, indicating its weaker mechanical strength. Interestingly, the cross-section of PCD demonstrated two distinct layers, one CS layer that displayed no crack and a more compact structure than the CS film and another PDDA layer that was loose with a rough surface (Fig. 3f and Supplementary Fig. 3). The abundant hydrogen bonds and electrostatic interactions between the carboxyl groups on PDDA and the amine groups on CS (Supplementary Fig. 4) made the two layers closely attached to each other. Moreover, PCD exhibited a lower contact angle (47.83° ± 0.62°) than CS (72.67° ± 1.25°) and CN (91.33° ± 0.47°) films in the contact angle measurement, suggesting a significantly enhanced hydrophilicity (Fig. 3g, h). PCD also presented a higher swelling ratio (Fig. 3i) and porosity (Fig. 3j) than CS and CN films, which were essential for moisture control, gas exchange, and hemostasis in the wound healing process. As expected, PCD also inherits the ROS response degradation property of PDDA, and the degradation of the PCD accelerated when the ROS level increased (Supplementary Fig. 5).
Hemostasis, antibacterial, and ROS scavenging properties of PCD
Hemostasis represents the initial and crucial phase of wound healing. In vivo, the hemostatic performance of CS films, CN films, and PCD was evaluated by the amount of bleeding and hemostatic time in the mouse liver injury model. All groups displayed significant hemostatic effects, resulting in a reduction in bleeding compared to the control (Fig. 4a). However, the CN films exhibited higher blood loss (Fig. 4b) and required a longer hemostatic time (Fig. 4c) than the CS films and PCD. Similar results were observed in a mouse tail injury model (Supplementary Fig. 6). The relatively poor hemostatic properties of CN films are positively correlated with their reduced hydrophilicity and diminished swelling capacity.
a Representative image of in vivo hemostasis of dressings in mice liver bleeding. b, c Corresponding blood loss (b) and hemostasis time (c) in mice (n = 3). d Antibacterial effect of the extract solution of various dressings against S.aureus (n = 3 independent experiments). e Degradation profiles for CS films, CN films, and PCD in PBS (n = 3 independent samples). f Dilution-dependent antibacterial effect of the extract solution of various dressings against S.aureus (n = 3 independent experiments). g Cell viability of NIH/3T3 cells after being incubated with the extract solution of various dressings (n = 5 independent experiments). h Images and quantitative analysis of hemolysis of the extract solution of various dressings (n = 3 independent experiments). i Images (inset) and the absorption time course curve of TMB at 652 nm with CS film, CN film, and PCD in the presence of H2O2 (300 μM) and Fe2+ (300 nM). j Time-dependent 1H NMR spectroscopic change of PDDA degradants in the presence of H2O2 (10 mM) and Fe2+ (10 μM). k Time-dependent 1H NMR spectroscopic change of PDDA degradants without ROS. l Quantification of succinic acid with different concentrations of ROS. Low ROS: H2O2 (10 mM) and Fe2+ (2 μM); Medium ROS: H2O2 (10 mM) and Fe2+ (5 μM); High ROS: H2O2 (10 mM) and Fe2+ (10 μM). All data were presented as the mean ± SEM. Statistical significance was determined using a one-way ANOVA test followed by Tukey’s multiple comparison analysis.
Due to the broad-spectrum antimicrobial properties of CS molecules, we tested the antimicrobial characteristics of the films. Through co-culturing of the films with Staphylococcus aureus (S.aureus) and Escherichia coli (E.coli), the bacterial colony counts exhibited a noticeable reduction (Fig. 4d and Supplementary Fig. 7), which indicates the antibacterial capacity of the wound dressings originates from the releasing of CS molecule. Therefore, CN films, with significantly slower hydrolytic degradation properties compared to CS films and PCD (Fig. 4e), the extraction solution displayed a weaker inhibitory effect on both S.aureus (Fig. 4f) and E.coli (Supplementary Fig. 8). Cell biocompatibility and hemolysis are crucial parameters for evaluating wound dressings. The cell biocompatibility of the film extract solutions was assessed using normal mouse fibroblasts NIH3T3 cells, and no cell toxicity was observed (Fig. 4g). Additionally, all the extract solutions exhibited hemolysis ratios of less than 3.0%, and the color of all film groups was transparent (Fig. 4h). Furthermore, we also assessed the biocompatibility of wound dressings in vivo, the H&E staining results showed no pathological changes in these major organs (Supplementary Fig. 9). Collectively, this suggests excellent biocompatibility of the films, making them a safe choice for wound dressings.
The elimination of ROS and the responsive degradation into proliferative SA form the basis of PCD. The clearance of ROS by PCD was quantified by TMB, using its time-dependent absorbance change at 652 nm in the presence of H2O2 (300 μM) and Fe2+ (300 nM). PCD could completely eliminate ROS (Fig. 4i), whereas CS film and CN film exhibited no clearance of ROS. To confirm the degradation of PCD by the microenvironmental ROS and the subsequent release of SA, we further analyzed the kinetics of the degradation of a PDDA membrane and concurrent SA generation in response to a simulated microenvironment ROS level. In 1H NMR analysis, the relative area of the proton signal at 2.41 ppm for various masses of succinic acid was determined, utilizing the proton peak of DMSO (0.25 mg) as an internal reference (Supplementary Fig. 10a). DMSO was selected as the internal reference due to its common use as a solvent in NMR, its miscibility with water, non-volatility, pH insensitivity, clear NMR signals, and absence of overlap with target peaks. The standard curve of integrated area relative to the mass of succinic acid was calculated accordingly (Supplementary Fig. 10b). SA was released in the presence of ROS, but no SA was generated in the absence of ROS (Fig. 4j, k). The release rate of SA was accelerated in the presence of ROS and slowed down during ROS-free phases (Supplementary Fig. 11). Upon stimulation with different concentrations of ROS, the quantity of succinic acid is positively correlated with the concentration of ROS, while its release ceased in the absence of ROS (Fig. 4l). These findings collectively suggested that PCD, with antimicrobial and hemostatic properties, could effectively eliminate ROS in the wound microenvironment and simultaneously release SA that was favorable for tissue regeneration, which was ideal to deliver treatment tailored to the specific conditions in the localized microenvironment.
Wound healing efficacy in diabetic mouse model
To test the efficacy of PCD in healing diabetic wounds, a diabetic mouse model was established by injecting streptozotocin (STZ) intraperitoneally at two weeks prior to the study (Fig. 5a). The mice were considered diabetic when their fasting plasma glucose levels were higher than 11.1 mM49 (Fig. 5b), and an 8-mm-diameter full-thickness skin wound was made on the dorsum of each mouse. As shown in Fig. 5c, the ROS levels at the wound site of the diabetic mice were significantly higher than that of the healthy mice, assessed using a red fluorescent ROS probe dihydroethidium (DHE). The diabetic mouse wounds were treated with commercially available alginate-based dressings that are used in clinical settings (Clinical), CS films, CS membranes loaded with succinic acid (CA films), CN films, and PCD respectively, while the mice receiving no treatment served as controls (Ctrl). The mice treated by PCD exhibited a significantly faster healing rate than those receiving other treatments (Fig. 5d). Specifically, PCD achieved around 80% wound closure on Day 12, in contrast to ~30% for the control, clinical, and CN films, and only 20% for CS films and CA films (Fig. 5e). Notably, the recovery process of the CN-treated group appeared to be even slower than the control group during the first 6 days, possibly because that the strong mechanical strength and poor degradation capability of CN films could impede the progress of wound healing.
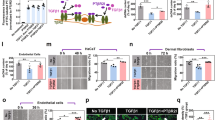
a Schematic illustration of the study design. b Blood glucose of normal and diabetic mice before operation (n = 6). c Confocal laser scanning microscope (CLSM) images of wound regions of normal and diabetic mice stained with DHE (images are representative of 3 biologically independent samples). Scale bar: 200 μm. d Representative images of the wound area after the mice received different treatments on Day 0, 3, 7, 9, and 12 (n = 5 biologically independent samples). Scale bar: 5 mm. e Wound area changes in diabetic mice by time (n = 5 biologically independent samples). f CLSM images of wound regions in different treatments stained with DHE (n = 3 biologically independent samples). Scale bar: 200 μm. g The relative mRNA expression levels of proinflammatory cytokine in the wound homogenates of mice (n = 3 biologically independent samples). h Masson staining of wound tissue with different treatments on Day 12 (images are representative of 3 biologically independent samples). Scale bar: 100 μm. i Representative images of the blood vessel CD31-positive endothelial cells (black triangle) analyzed by immunohistochemistry (n = 3 biologically independent samples). Scale bar: 100 μm. j, k The relative mRNA expression levels of VEGFA (j) and collagen deposition-related genes α-SMA (k) in the wound homogenates of mice (CS group: n = 3 biologically independent samples; CA film and CN film groups: n = 3 biologically independent samples; Ctrl, Clinical, and PCD groups: n = 5 biologically independent samples). All data were presented as the mean ± SEM. Statistical significance was determined using a one-way ANOVA test followed by Tukey’s multiple comparison analysis. Parts of a were created with BioRender.com.
To assess the ability of PCD to relieve oxidative stress in vivo, we measured the ROS levels in the regenerated tissues by DHE staining. In the granulation tissues of the mice in the Control, Clinical, CS film, CA film, and CN film groups, bright red fluorescence of DHE was observed, indicating the existence of large quantities of ROS. In sharp contrast, the mice treated by PCD showed almost no red fluorescence in the regenerated tissues (Fig. 5f), both CLSM images and fluorescence quantification demonstrating a robust mitigation of oxidative stress in vivo. Since the ROS level was closely associated with the inflammation in the wound microenvironment, we examined the mRNA expression of key proinflammatory cytokine IL-1β and oxidative damage marker 8-hydroxy-2′-deoxyguanosine (8-OHdG) in the granulation tissues, and PCD significantly downregulated the expression of IL-1β (Fig. 5g) and 8-OHdG (Supplementary Fig. 12). Additionally, PCD significantly increased the ratio of M2/M1 macrophages (Supplementary Fig. 13), further confirming its ability to modulate the microenvironment. Moreover, the hematoxylin and eosin (H&E) staining and Masson’s trichome staining demonstrated that the wounds of the mice treated by PCD formed complete and uniform dermal and epidermal structures on Day 12, whereas the mice in other groups showed loose and incomplete structure of the epidermis and dermis (Supplementary Fig. 14). Interestingly, the Masson staining and picrosirius red staining image of the PCD group showed a distinctly visible fibrous structure, indicating high collagen expression and suggesting tissue repair (Fig. 5h and Supplementary Fig. 15). Furthermore, PCD-treated wounds were populated by CD31+ vascular endothelial cells (Fig. 5i), which was essential to promote angiogenesis during the healing process. In addition, the mRNA expression of vascularization-related VEGFA (Fig. 5j) and collagen deposition-related α-SMA (Fig. 5k) was also the highest in the PCD group. Since the major degradant of PCD after interacting with ROS was succinic acid, the above results firmly proved that PCD could effectively annihilate the high level of ROS at the diabetic wound site while simultaneously releasing proliferative molecules to promote tissue regeneration and vascularization.
Wound healing efficacy in a diabetic porcine model
To further validate the efficacy and translational potential of PCD in diabetic wound healing, we employed a diabetic porcine model, the skin structure and wound healing process of which closely resembled that of human50 (Fig. 6). Diabetes was induced in 2 Bama pigs through intravenous STZ injection, as monitored by the fasting blood glucose level. After being in a diabetic state for 2 weeks, an array of square full-thickness wounds (1.5 cm in side length) was created on both sides of the dorsum on each pig (Fig. 6a) and treated with Clinical, CS films, CA films, CN films, and PCD, respectively. During the whole healing procedure, the wounds treated by PCD demonstrated expedited wound area shrinkage compared to those by other treatments (Fig. 6b). Since the change in wound volume was a more accurate evaluation for the healing process, we measured the wound depths and assessed the change in each wound volume (Fig. 6c). Markedly, PCD showed a more pronounced volume reduction compared to the other groups on Day 3 and Day 6 (Fig. 6d).
a Schematic illustration of the study design and the blood glucose levels of the Bama pigs. b Representative images of the wound area by different treatments after the operation (n = 6 biologically independent samples). Scale bar: 1 cm. c Quantification of relative wound areas on Day 3 and Day 18 after receiving different treatments (n = 6 biologically independent samples). d Quantification of relative wound volumes on Day 3 and Day 6 after receiving different treatments (n = 6 biologically independent samples). e H&E staining of wound tissues with different treatments on Day 18 (images are representative of 6 biologically independent samples). Blue arrow: inflammation; yellow arrow: necrobiosis; green arrow: panniculitis; white arrow: hemorrhage. Scale bar: 1 mm. f Semiquantitative analysis of wound healing through treatment-blinded pathohistological scoring of H&E and Masson images for granulation tissue, collagen deposition, epithelialization, and inflammation, yielding a cumulative wound healing score for each scaffold type tested (n = 6 biologically independent samples). All data were presented as the mean ± SEM. Statistical significance was determined using a one-way ANOVA test followed by Tukey’s multiple comparison analysis. Parts of a were created with BioRender.com.
The histological analysis of the porcine wounds at the endpoint of the study (Day 18) revealed the difference in tissue integration and pathological conditions among different treatment groups. From the H&E staining images (Fig. 6e), in the granulation tissues of the wound sites treated by Control, Clinical, CS, CA, and CN groups, there existed dense clusters of inflammatory infiltrates. In contrast, in the PCD-treated wounds, the granulation tissues were uniformly distributed and exhibited much fewer inflammatory cells. Phenotypes such as peritonitis and necrobiosis were observed in the Control, CS, CA, and CN groups, and hemorrhage occurred in the Clinical group, whereas they were absent in the PCD group, further proving a lower immune response in the wounds treated by PCD. To further explore the effect of PCD on various aspects of skin repair and restoration, H&E and Masson images were subjected to a blinded semiquantitative evaluation (Fig. 6f, scoring criteria are provided in Supplementary Table 1). PCD-treated wounds obtained the highest scores in granulation tissue quality, collagen formation, and epithelialization, as well as the lowest score in inflammation (Fig. 6f and Supplementary Fig. 16). Therefore, the total wound healing score of PCD-treated wounds was significantly higher than wounds treated by other methods, evidencing the reconstruction of dermal architecture and wound healing quality by PCD.
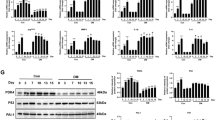
The oxidative stress in the wound sites was further examined by DHE staining. In the diabetic porcine wounds treated by PCD, the oxidized DHE fluorescence was significantly weaker compared to other groups, confirming the effective clearance of ROS by PCD in the wound microenvironment (Fig. 7a and Supplementary Fig. 17). Additionally, PCD was effective in downregulating the mRNA expression of TLR-4, a major receptor involved in inflammation (Fig. 7b). PCD also led to a significant reduction in the expression of the proinflammatory transcription factor NFκB in diabetic porcine wounds (Supplementary Fig. 18), further reaffirming the role of PCD in alleviating inflammation through ROS elimination. To validate the effect of succinic acid-containing degradants of PCD upon eliminating ROS, we assessed Mason’s staining and picrosirius red staining image of each wound. As anticipated, the PCD-treated wounds also exhibited higher collagen deposition with tightly arranged fibers (Fig. 7c and Supplementary Fig. 19). We also evaluated the impact of PCD on the gene expression that associated with granulation tissue proliferation and the remodeling processes of wound healing (Fig. 7d). PCD treatment significantly upregulated the expression of FGF2, thereby promoting cell proliferation and differentiation to facilitate skin wound granulation and re-epithelialization. The expression of VEGFA and Col-1 were also upregulated in PCD-treated wounds, confirming that PCD degradants could aid vascularization and collagen production during wound healing.
a CLSM images of wound regions in different treatments stained with DHE (images are representative of 6 biologically independent samples). Scale bar: 200 μm. b The relative mRNA expression levels of inflammatory-related genes in the wound homogenates of porcine (n = 6 biologically independent samples). c Masson staining of wound tissues receiving different treatments on Day 18 (images are representative of 6 biologically independent samples). Scale bar: 1 mm. d The relative mRNA expression levels of proliferative, angiogenesis, and collagen production-related genes in the wound homogenates of porcine (n = 6 biologically independent samples). All data were presented as the mean ± SEM. Statistical significance was determined using a one-way ANOVA test followed by Tukey’s multiple comparison analysis.
Discussion
Deviating from the sequential recovery observed in acute wound healing, the healing process of diabetic wounds is usually entangled in a disorganized and self-perpetuating inflammatory phase6. The heightened inflammation in diabetic wounds creates a harsh microenvironment that hinders the healing process, accompanied by a prolonged delay in tissue repair5. Consequently, effective diabetic wound management involves both the sustained control of the unpredictable local inflammatory microenvironment over an extended period and the precise orchestration of tissue growth. Compared with contemporary strategies that integrate multiple functional elements15,32,33, it is of great interest that the coordination of inflammation alleviation and tissue regeneration can be achieved by PDDA, a conjugated polymer that can eliminate various types of ROS and simultaneously generate proliferative succinic acid as a product of its oxidation reaction. On this basis, we have developed a rational dressing design PCD incorporating both PDDA and CS to meet the requirements of diabetic wound healing. With excellent biocompatibility, PCD inherits the capability of PDDA to eliminate ROS, downregulate gene expression of innate immune receptors, and responsive release of succinic acid. As a result, it contributes to homeostasis, provides on-demand microenvironment modulation, and accurately promotes tissue regeneration, ultimately expediting the healing of diabetic wounds in both diabetic mouse and porcine.
Up to now, the commercial products for treating diabetic foot ulcers mainly incorporate different agents, such as growth factors (Becaplermin), fibroblast (Dermagraft), keratinocytes and fibroblasts (Apligraf), or extracellular matrix (Integra Dermal Regeneration Template) to modulate the diabetic environment11,51. However, the proportion of non-responding patients remains significant11,51,52,53,54,55. Combination dressing therapy brings pivotal opportunities for diabetic wound healing, in that it enables simultaneous modulation of the intricate microenvironment and promotion of delayed tissue regeneration. However, challenges persist in determining the optimal timing for the delivery of each component in combinational dressings. Our streamlined approach avoids the incorporation of multiple components as well as costly biology-derived elements6, offering a straightforward yet effective strategy for diabetic wound care with significant translational advantages.
Methods
Ethics statement
The mice experiments were approved by the Institutional Animal Care and Ethic Committee of Huazhong University of Science and Technology ([2022] IACUC Number: 3568). The porcine experiments were approved by the Institutional Animal Care and Ethic Committee of Hubei Yizhicheng Biotechnology Co., LTD (IACUC Issue No. WDRM 202312002), and conducted according to the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) guidelines.
Materials
Poly (deca-4,6-diynedioic acid) (PDDA) was synthesized following previously reported protocols35. Chitosan (average MW of 100,000, 85% deacetylation degree) was purchased from Golden-shell Pharmaceutical (Zhejiang, China). 3,3′,5,5′-Tetramethyl benzidine (TMB) Substrate was purchased from BOSTER Biological Technology (Wuhan, China). A superoxide anion scavenging capacity assay kit, crystal violet was purchased from Solarbio Science & Technology (Beijing, China). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Energy Chemical (Shanghai, China). Matrix gel was purchased from Corning Inc. (NY, USA) and 24-well transwell chambers were purchased from Labselect (Beijing, China). The glucometer and glucose test strips were purchased from Sinocare Co., Ltd. (Changsha, China).
Cell lines
The murine fibroblast NIH3T3 cells were purchased from the American Type Culture Collection, the HUVECs were kindly provided by Dr. Jianglin Wang. Cells were cultured in DMEM supplemented with 10% FBS, and 1% penicillin/streptomycin at 37 °C in a 5% CO2 atmosphere.
Animals
BALB/c mice (female, 6–8 weeks, 18–20 g) were purchased from the Liaoning Changsheng Biotechnology Co., Ltd. (Benxi, China). Mice were housed on a 12/12 light/ dark cycle with constant temperature (22 °C) and humidity (50–60%) and allowed to feed food and water ad libitum. Two Bama pigs (male, 6–8 months, 18–20 kg) were obtained from Hubei Yizhicheng Biotechnology Co., Ltd (Hubei, China). The pigs were fed with food and water ad libitum and housed at a temperature of 22 ± 2 °C and relative humidity of 45–55% with a 12-h light/12-h dark cycle.
Synthesis of PDDA
Poly (deca-4,6-diynedioic acid) (PDDA) was synthesized using a host-guest topochemical polymerization strategy. The monomer guest deca-4,5-diynedioic acid (DDA) and the host bis(pyridyl)oxalamide host were synthesized following the literature procedure35. The monomer guest (194 mg) and host (270 mg) were dissolved in 300 mL of ethanol, followed by co-crystallization to form monomer-host complexes. Heating these co-crystals at 120 °C for 12 h led to the topochemical polymerization of the monomers. The obtained PDDA was characterized using 1H NMR and single crystal X-ray crystallography, which were consistent with the literature results35.

Radicals scavenging activity
Superoxide radical-scavenging assay
The scavenging capability of PDDA against superoxide radicals (·O2−) was assessed by measuring the amount of generated red azo compounds using a superoxide anion scavenging capacity assay kit. After mixing the reagents and PDDA (0, 1, 2, 5 mg mL−1) for 30 min, the absorbance value at 530 nm of the mixed solutions was recorded and the scavenging efficiency of ·O2− was calculated as follows:
Hydroxyl radical-scavenging assay
The elimination capability of PDDA against ·OH was assessed by TMB chromogenic assay. PDDA (0, 20, 50, 100 µg mL−1) were mixed with 100 µM H2O2, 100 nM Fe2+, and 500 µg mL−1 TMB in 0.2 M sodium acetate-acetic acid buffer (pH 3.6), and the absorbance at 652 nm was recorded every 10 min for a total of 60 min on the microplate reader (Varioskan LUX, Thermo Scientific).
Hypochlorous acid-scavenging assay
Hypochlorous acid (HClO) was prepared immediately before each use by adjusting the pH of a 10% (v/v) NaClO solution to 6.2 with 0.2 M sodium acetate-acetic acid buffer (pH 3.6). The concentration of HClO was determined by TMB using the absorbance at 652 nm. The scavenging efficiency of ClO− was calculated as follows:
∆Con: concentration change of HClO compared to initial concentration.
Characterization of PDDA degradation products of Fenton reaction
40 µM of FeSO4·7H2O was added to an aqueous PDDA dispersion (10 mL, 1 mg mL−1), and diluted hydrochloric acid was added to adjust the pH value to 4. After stirring and dispersing, 200 µL of 30% (w/w) H2O2 was added every 12 h and stirred at room temperature for 48 h. The reaction was terminated after another 72 h. The reaction mixture was added with NaOH until the pH value reached 12, then filtered to remove FeSO4·7H2O through a 0.22 µm nylon filter film and lyophilized into degradation products. Lyophilized degradation product was dissolved in 450 µL of deuterium water for 1H and 13C NMR (400 MHz) measurement (Agilent 400-MR 400 MHz). NMR spectra were processed using MestReNova software (v.6.1.0-6224). High-resolution mass spectroscopy (HR-MS) was conducted on an Orbitrap LC/MS (Q Exactive) (Thermo Fisher Scientific, USA) with an electrospray interface, a Q Exactive mass analyzer, and an ion detector operating in negative ion mode (M−), coupled with an Ultimate 3000 UPLC system. Data processing for HR-MS was performed using Thermo Scientific Xcalibur (v.4.1).
Quantitative analysis of PDDA degradation products over time
Diluted hydrochloric acid was added to water to adjust the pH to 4 and prepared to use. A PDDA film (10 mg) was placed in a covered vial containing 10 mL of deionized water (pH 4), followed by the addition of 2 µM, 5 µM, and 10 µM of FeSO4·7H2O respectively, and 100 µL of 30% (w/w) H2O2 as low, medium, and high-level ROS stimulations. The vial was kept at room temperature and steady, and the supernatant was collected every 24 h. Simultaneously, the remaining PDDA film was reintroduced into the 10 µM of FeSO4·7H2O and 100 µL of 30% (w/w) H2O2 for further degradation, repeating this cycle for 120 h. The reaction mixture collected was filtered with a 0.22 µm membrane and then lyophilized to obtain the degradation products. Lyophilized degradation products were dissolved in 450 µL of deuterium water and 0.25 mg of DMSO was added as an internal standard for 1H NMR (400 MHz) measurement (Agilent 400-MR 400 MHz).
Scratch wound healing assay
For the scratch wound healing assay, HUVECs were seeded at a density of 1 × 105 cells per well in 24-well plates. A sterile 10 µL pipette tip was used to create a scratch, and unattached cells were washed away with PBS. The remaining adherent cells were then treated with or without PDDA degradants. Images of the HUVECs were captured at 24 h after the initial wounding. The migration rate was determined by calculating the ratio of the closed area to the initial wound area.
Real-time quantitative PCR (RT-qPCR) analysis
Total RNA was isolated from the cells or tissues using RNA isolater Total RNA Extraction Reagent (Vazyme) following the manufacturer’s instructions. For each sample, 1 µg mRNA was reverse-transcribed into cDNA using the Hifair® III 1st Strand cDNA Synthesis SuperMix (Yeasen). The resulting cDNA was then subjected to qPCR using Hieff UNICON® Universal Blue qPCR SYBR Green Master Mix (Yeasen). All primers were synthesized by Huayu Gene Biological Technology (Wuhan, China). The sequences of the primers for cell experiments were as follows:
VEGFA-forward primer | 5′-TATGCGGATCAAACCTCACCA-3′ |
VEGFA-reverse primer | 5′-CACAGGGATTTTTCTTGTCTTGCT-3′ |
Angpt2-forward primer | 5′-TTATCACAGCACCAGCAAGC-3′ |
Angpt2-reverse primer | 5′-TTCGCGAGAACAAATGTGAG-3′ |
GAPDH-forward primer | 5′-GAGTCAACGGATTTGGTCGT-3′ |
GAPDH-reverse primer | 5′-TTGATTTTGGAGGGATCTCG-3′ |
The sequences of the primers for diabetic mouse were as follows:
IL-1β-forward primer | 5′-TGGACCTTCCAGGATGAGGACA-3′ |
IL-1β-reverse primer | 5′-GTTCATCTCGGAGCCTGTAGTG-3′ |
VEGFA-forward primer | 5′-CAGGCTGCTCTAACGATGAA-3′ |
VEGFA-reverse primer | 5′-CAGGAATCCCAGAAACAACC-3′ |
α-SMA-forward primer | 5′-AGGGAGTGATGGTTGGAATG-3′ |
α-SMA -reverse primer | 5′-AGGGAGTGATGGTTGGAATG-3′ |
GAPDH-forward primer | 5′-TCAACGGCACAGTCAAGG-3′ |
GAPDH-reverse primer | 5′-ACTCCACGACATACTCAGC-3′ |
The sequences of the primers for diabetic pig were as follows:
TLR-4-forward primer | 5′-TGGTGTCCCAGCACTTCATA-3′ |
TLR-4-reverse primer | 5′-CGGCATGACTCCTCAGAAAC-3′ |
NFκB-forward primer | 5′-TGTGAAGACCACCTCTCAGG-3′ |
NFκB -reverse primer | 5′-CTGTCACAGATGCTGTCATCC-3′ |
VEGFA-forward primer | 5′-TATGCGGATCAAACCTCACCA-3′ |
VEGFA-reverse primer | 5′-CACAGGGATTTTTCTTGTCTTGCT-3′ |
ColA1-forward primer | 5′-AGTGTGAGGCCACGCATGAGC-3′ |
ColA1-reverse primer | 5′-GGTTTCCTGGTCGGTGGGTGA-3′ |
FGF2-forward primer | 5′-TCAAAGGAGTGTGTGCGAAC-3′ |
FGF2-reverse primer | 5′-CAGGGCCACATACCAACTG-3′ |
GAPDH-forward primer | 5′-GGTCACCAGGGCTGCTTTTA-3′ |
GAPDH-reverse primer | 5′-CCTTGACTGTGCCGTGGAAC-3′ |
The RT-qPCR was run with Bio-Rad CFX96 Real-Time PCR System, each qPCR reaction contained 30–45 ng of cDNA. Quantification of gene expression was carried out using the 2−ΔΔCt method, with gene expression levels normalized to the housekeeping gene GAPDH.
Transwell migration assay
HUVECs were introduced into the upper chamber at a density of 1 × 104 cells per well in a serum-free medium, while the lower chamber was filled with medium either containing or lacking PDDA degradation products. After 24 h, the cells remaining on the upper surface of the membrane were carefully wiped off with a cotton swab. Cells that had migrated across the membrane were then stained with 1% crystal violet solution for 15 min. The migrated cells were photographed and quantified using an optical microscope (Mingmei, Guangdong, China).
Tube formation assay
For tube formation assay, 50 µL of thawed Matrigel was added to each well of a pre-cooled 96-well plate and incubated at 37 °C for 1 h. HUVECs were then seeded at a density of 1 × 104 cells per well into the Matrigel-coated chamber, incubated with or without PDDA degradants. To assess the tube formation, HUVECs were imaged under a microscope and quantified using ImageJ software (Version: 2.1.0).
Synthesis of CS films, CN films, and PCD
1.0% CS water solution was added to a thorough cleaning and completely dried the glass plate. The solution was degassed and the solvent was evaporated at 60 °C to obtain CS films. After obtaining the CS film, it was soaked in a 0.1 M NaOH solution for 15 min to ensure thorough wetting of the entire film. CN films were generated after the film was rinsed with water and dried completely. For PCD, 1.67 mg mL−1 PDDA water solution was added to a 1:10 mass ratio of PDDA:CS. Leave the mixed solution for 15 min to allow sufficient interaction between PDDA and the CS film, and then dry to obtain PCD. Peel the films from the glass plate, rinse them three times with ethanol to eliminate excess salt, and then subject them to vacuum drying to obtain the final CS films, CN films, and PCD. For the mouse study, 100 µL of 10 mg mL−1 CS solution was used in CS film, CN film, and the CS layer of PCD, and 60 µL of 1.67 mg mL−1 PDDA solution was used in PCD. A 1 mm diameter round glass plate was used. The mass of the CS film and CN film was approximately 1 mg, and PCD was approximately 1.1 mg. For the porcine study, 1 mL of 10 mg mL−1 CS was used in CS film, CN film, and the CS layer of PCD, and 600 µL of 1.67 mg mL−1 PDDA solution was used in PCD. A square glass plate with a side length of 1 cm was used. The mass of the CS films and CN films was approximately 10 mg, and PCD was approximately 11 mg. The thicknesses of the CS film, CN film, and PCD were 9.4 µm, 6.8 µm, and 13.2 µm, respectively.
Mechanical strength measurement
CS films, CN films, and PCD were prepared in a rectangular shape with 2 cm × 1 cm. The mechanical properties, including tensile strength, maximum load, and Young’s modulus, were evaluated using a universal testing machine under ambient temperature and normal humidity conditions, at a stretching speed of 20 mm min−1.
Scanning electron microscopy (SEM)
Surface and cross-sectional morphologies were analyzed by SEM (GeminiSEM300, Carl Zeiss, Germany). Prior to the experiment, all the samples were sputter-coated with a thin layer of platinum. Surface imaging was taken at 10 kV, while cross-section imaging was taken at 15 kV.
Water contact angle assay
The contact angles were measured using a sessile-drop method at room temperature. Before the assessment, the untreated CS films, CN films, and PCD were prepared on glass slides. During the measurement, 1 μL of ultrapure water was dropped onto the film surface, and the pictures of the contact angle were taken promptly.
Swelling rate
The mass of each lyophilized CS film, CN film, and PCD was weighed and denoted as m0. Subsequently, the samples were then put into 0.9% NaCl solution immediately under room temperature and taken out after being soaked in 0.9% NaCl solution for 30 min. The weights of swollen CS films, CN films, and PCD were measured and denoted as mt. The swelling rate was calculated as follows:
Porosity measurement
The porosity of the films was determined using a solvent exchange method. Three sets of CS films, CN films, and PCD were prepared, and the mass of each film was measured and recorded as W1. The films were placed into 5 mL of ethanol, and the total mass of the films and ethanol was recorded as W2. The films were then immersed in ethanol for 48 h to ensure thorough solvent exchange. After solvent exchange, the films were removed, and the remaining ethanol mass was measured and recorded as W3. The calculation formula for film porosity is as follows:
In vivo hemostatic assay
In vivo mouse tail hemostatic ability
A mouse tail model (female, BALB/c mice, 16–20 g) was used to investigate the in vivo hemostatic ability of CS films, CN films, and PCD. Pre-weighed filter paper was placed under the tails of the mice, the tails were cut approximately 2 cm from the tip after by surgical scissors. The tails were left undisturbed for 15 s to ensure normal bleeding. After 15 s, CS films, CN films, and PCD were applied to the bleeding wound, and the nontreatment wound served as the negative control. The bleeding time for each group of mice was recorded, once the tail bleeding was completely stopped, the total mass of the blood-soaked filter paper was measured again. The bleeding volume from the mouse tails was determined by subtracting the initial filter paper mass from the final mass. Photographs of the filter paper were taken for each group after hemostasis.
In vivo mouse liver hemostatic ability
After extracting the liver from mice (female, BALB/c mice, 16–20 g), pre-weighed filter paper was placed under the mouse liver. Subsequently, a wound was created at the site of the mouse liver using a needle from a syringe. The liver was left undisturbed for 15 s to ensure normal bleeding. After 15 s, the nontreatment wound served as the negative control, while each experimental group applied the corresponding film to the bleeding wound. The bleeding time for each group of mice liver was recorded. After liver bleeding was completely stopped, the total mass of the blood-soaked filter paper was measured again. The bleeding volume from the mouse liver was determined by subtracting the initial filter paper mass from the final mass. Photographs of the filter paper were taken for each group after hemostasis.
Antibacterial effect of dressings
Gram-positive Staphylococcus aureus and Gram-negative Escherichia coli were chosen to verify the antibacterial activity of wound dressings. Two kinds of bacterial were cultured until the turbidity of the bacterial suspension to OD600 = 0.1. Subsequently, the bacterial was collected and resuspended using PBS buffer. Circular-shaped CS films, CN films, and PCD were prepared with 1 mg of chitosan and 0.1 mg of PDDA in around 1 cm diameter. The CS films, CN films, and PCD were sterilized in 75% alcohol and dried to remove any remaining alcohol. The resuspended bacteria were then incubated with sterile and dry CS film, CN film, and PCD, respectively, in 12-well plates. After 2 h, the Colony-forming unit (CFU) of each group was measured through the drop plate method. The inhibition rate (%) was calculated as below:
Antibacterial effect of the extract solution of dressings
CS films, CN films, and PCD were prepared with the same properties for the antibacterial effect assay. Three pieces of sterilized and dried CS films, CN films, and PCD were each immersed in 300 μL of sterile 10 mM PBS at 37 °C for 2 h to obtain the original extract solutions of each film group. The original extract solutions were then diluted twofold 11 times to obtain 12 concentrations of film extract solutions. 100 µL of film extract solutions were added to 400 µL of Staphylococcus aureus or Escherichia coli at a concentration of 1.0 × 104 CFU mL−1 and incubated for 16 h. The OD600 of each group was measured. The OD600 of the PBS group at the endpoint was recorded as OD600neg, while the OD600 of different film extract solutions was recorded as OD600sample. The absorption of PBS itself was recorded as OD600blank. The Relative OD600 (%) was calculated as below:
In vitro biocompatibility
CS films, CN films, and PCD were fully immersed in a DMEM culture medium at room temperature for 7 days to obtain the extraction solution. NIH/3T3 cells were then diluted with either DMEM culture medium or the extraction solution and seeded into a 96-well plate at a density of 3000 cells per well. The cells were then incubated at 37 °C in a 5% CO2 atmosphere for 24 and 48 h. After the respective time points, 0.5 mg mL−1 MTT was added to each well and cultured for 4 h. Following incubation, the MTT was removed and replaced with 150 µL of DMSO per well to dissolve the formazan salt. The absorbance at 570 nm was measured using a microplate reader.
Hemolysis assay
The red blood cells (RBCs) were obtained by centrifuging blood at 210 × g for 10 min, they were PBS-rinsed thrice and diluted to the concentration of 5% v/v. The films were incubated in 0.9% NaCl solution at 37 °C for 30 min, 150 µL of the extract solution and 150 µL of the RBC suspension were mixed and incubated at 37 °C for 3 h, then it was centrifuged at 210 × g for 10 min. The obtained supernatants were reading absorption at 540 nm with a microplate reader, 0.1% Triton X-100 and 0.9% NaCl solution were used as positive and negative controls, respectively. The hemolysis rate (%) was calculated as below:
In vivo diabetic mouse skin wound healing study
The mice were administered with streptozotocin (STZ, 50 mg/kg; Yeasen, Shanghai) dissolved in sodium citrate buffer daily for five days. After two weeks of the initial STZ injection, the diabetic mouse model was established when the plasma glucose level was ≥16.7 mM for two consecutive days. The animals remained in a diabetic state for the duration of the wound healing experiment. Under sterile conditions, the mice were anesthetized with isoflurane, and an 8-mm biopsy punch was used to create a full-thickness wound on the dorsal center, by removing both the epidermis and dermis. The animals were then randomly allocated into six groups: control, Clinical, CS films, CA films, CN films, and PCD. The pictures of the wounds were taken on the day of surgery and every two days thereafter. Tissue samples for DHE staining and immunofluorescence analysis were collected on Day 5, and those for H&E staining, Masson’s trichrome staining, picrosirius red staining, and CD31 analysis were collected on Day 12. Harvested tissues were either fixed in 4% paraformaldehyde (PFA) in PBS or flash-frozen in liquid nitrogen for further evaluations, including H&E, DHE, Masson’s trichrome, immunohistochemical staining, and mRNA expression analysis. Fluorescence images were captured using a confocal laser scanning microscope (CLSM, Olympus FV3000, Japan), while bright-field images were obtained with an optical microscope (Mingmei, Guangdong, China).
In vivo diabetic porcine skin wound healing study
A diabetic male Bama miniature pig model was established by a single intravenous injection of STZ (150 mg/kg) dissolved in sodium citrate buffer. The high blood glucose level was maintained for more than two weeks before wounds were created. Under sterile conditions, the diabetic pigs were anesthetized with an intramuscular injection of Zoletil®50 (Zolazepam and Tiletamine in a 1:1 mass ratio, 10 mg kg−1). Six full-thickness wounds (1.5 cm in length) were created on each side of the dorsal region, with at least 2 cm of spacing between each wound to prevent collateral effects. Each wound site was marked with a sterile surgical marker. Full-thickness dermal wounds approximately 0.5 cm deep were carefully made by removing the skin through precise dissection with skin forceps and double-bladed scissors. Various treatments were then applied to the wounds. The wounds were covered with a cotton dressing and secured with an elastic net to protect the area. Antibiotics were administered during the first three days to prevent bacterial infections. Digital images of the wounds were taken on the day of surgery and every two days thereafter. On the 18th day post-surgery, the wound tissues were harvested. The harvested tissues were either fixed in 4% paraformaldehyde (PFA) in PBS or flash-frozen in liquid nitrogen for further analysis, including H&E staining, DHE staining, Masson’s trichrome staining, picrosirius red staining, and mRNA expression analysis. Blinded evaluations were performed to score wound histology based on established standards (Table S1)18. Fluorescence images were captured using a confocal laser scanning microscope (CLSM; Olympus FV3000, Japan), and bright-field images were taken with an optical microscope (Mingmei, Guangdong, China).
Immunohistochemical and immunofluorescence analysis
After the tissue harvesting, and their skins were harvested and then sliced into 4 µm-thick sections. The sections were stained with anti-CD31 antibody (ab182981, Abcam) to detect endothelial cells, stained with antibody-8-OHdG (bs-1278R, Bioss) for oxidative damage, stained with anti-CD86 (CY5238, Abways) for M1 macrophages as well as anti-CD206 (A02285-2, Boster Bio) for M2 macrophages.
In vivo biosafety
The healthy mice were anesthetized with isoflurane under sterile conditions, and then an 8 mm full-thickness wound was made on the dorsum center of each mouse. The animals were randomly treated with CS films, CN films, and PCD. After 7 days, the mice were sacrificed, the major organs (heart, liver, spleen, lung, and kidney) were collected for H&E staining.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data generated in this study are provided in the Article, the Supplementary Information, and the Source Data file. The full image dataset is available from the corresponding author upon request. Source data are provided with this paper.
References
Armstrong, D. G., Boulton, A. J. M. & Bus, S. A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 376, 2367–2375 (2017).
Armstrong, D. G., Tan, T. W., Boulton, A. J. M. & Bus, S. A. Diabetic foot ulcers: a review. JAMA 330, 62–75 (2023).
McDermott, K., Fang, M., Boulton, A. J. M., Selvin, E. & Hicks, C. W. Etiology, epidemiology, and disparities in the burden of diabetic foot ulcers. Diabetes Care 46, 209–221 (2023).
Chen, L., Sun, S., Gao, Y. & Ran, X. Global mortality of diabetic foot ulcer: a systematic review and meta-analysis of observational studies. Diabetes Obes. Metab. 25, 36–45 (2023).
Falanga, V. et al. Chronic wounds. Nat. Rev. Dis. Prim. 8, 50 (2022).
Eming, S. A., Martin, P. & Tomic-Canic, M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci. Transl. Med. 6, 265sr266 (2014).
Dupré-Crochet, S., Erard, M. & Nüβe, O. ROS production in phagocytes: why, when, and where? J. Leukoc. Biol. 94, 657–670 (2013).
Mittal, M., Siddiqui, M. R., Tran, K., Reddy, S. P. & Malik, A. B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 20, 1126–1167 (2014).
Davis, F. M., Kimball, A., Boniakowski, A. & Gallagher, K. Dysfunctional wound healing in diabetic foot ulcers: new crossroads. Curr. Diabetes Rep. 18, 2 (2018).
Schreml, S. et al. Oxygen in acute and chronic wound healing. Br. J. Dermatol. 163, 257–268 (2010).
Jeffcoate, W. J., Vileikyte, L., Boyko, E. J., Armstrong, D. G. & Boulton, A. J. M. Current challenges and opportunities in the prevention and management of diabetic foot ulcers. Diabetes Care 41, 645–652 (2018).
Frykberg, R. G. & Banks, J. Challenges in the treatment of chronic wounds. Adv. Wound Care 4, 560–582 (2015).
Dong, R. et al. An in situ dressing material containing a multi‐armed antibiotic for healing irregular wounds. Aggregate https://doi.org/10.1002/agt2.493 (2024).
Yi, K. et al. Gold nanoclusters encapsulated microneedle patches with antibacterial and self‐monitoring capacities for wound management. Aggregate https://doi.org/10.1002/agt2.509 (2024).
Chen, Y. et al. Research advances in smart responsive-hydrogel dressings with potential clinical diabetic wound healing properties. Mil. Med. Res. 10, 37 (2023).
He, L. et al. Photothermal antibacterial materials to promote wound healing. J. Control. Release 363, 180–200 (2023).
He, X. et al. Recent antioxidative nanomaterials toward wound dressing and disease treatment via ROS scavenging. Mater. Today Nano https://doi.org/10.1016/j.mtnano.2021.100149 (2022).
Patil, P. et al. Reactive oxygen species–degradable polythioketal urethane foam dressings to promote porcine skin wound repair. Sci. Transl. Med. 14, eabm6586 (2022).
Xin, P. et al. Natural okra-based hydrogel for chronic diabetic wound healing. Chin. Chem. Lett. https://doi.org/10.1016/j.cclet.2022.108125 (2023).
Luo, M. et al. Solid-state atomic hydrogen as a broad-spectrum RONS scavenger for accelerated diabetic wound healing. Natl Sci. Rev. 11, nwad269 (2024).
Yang, L. et al. Biofilm microenvironment triggered self-enhancing photodynamic immunomodulatory microneedle for diabetic wound therapy. Nat. Commun. 14, 7658 (2023).
Lohmann, N. et al. Glycosaminoglycan-based hydrogels capture inflammatory chemokines and rescue defective wound healing in mice. Sci. Transl. Med. 9, eaai9044 (2017).
Puthia, M. et al. A dual-action peptide-containing hydrogel targets wound infection and inflammation. Sci. Transl. Med. 12, eaax6601 (2020).
Powell, L. C. et al. Topical, immunomodulatory epoxy-tiglianes induce biofilm disruption and healing in acute and chronic skin wounds. Sci. Transl. Med. 14, eabn3758 (2022).
Dong, R. & Guo, B. Smart wound dressings for wound healing. Nano Today 41, 101290 (2021).
Xu, J., Zhu, X., Zhao, J., Ling, G. & Zhang, P. Biomedical applications of supramolecular hydrogels with enhanced mechanical properties. Adv. Colloid Interface Sci. 321, 103000 (2023).
Yu, C., Hu, Z.-Q. & Peng, R.-Y. Effects and mechanisms of a microcurrent dressing on skin wound healing: a review. Mil. Med. Res. 1, 1–8 (2014).
Mochizuki, M. et al. Growth factors with enhanced syndecan binding generate tonic signalling and promote tissue healing. Nat. Biomed. Eng. 4, 463–475 (2020).
Ishihara, J. et al. Laminin heparin-binding peptides bind to several growth factors and enhance diabetic wound healing. Nat. Commun. 9, 2163 (2018).
Theocharidis, G. et al. A strain-programmed patch for the healing of diabetic wounds. Nat. Biomed. Eng. 6, 1118–1133 (2022).
Jiang, Y. et al. Wireless, closed-loop, smart bandage with integrated sensors and stimulators for advanced wound care and accelerated healing. Nat. Biotechnol. 41, 652–662 (2023).
Guo, Y. et al. Multifunctional PtCuTe nanosheets with strong ROS scavenging and ROS-independent antibacterial properties promote diabetic wound healing. Adv. Mater. 36, 2306292 (2023).
Li, S. et al. Microneedle patches with antimicrobial and immunomodulating properties for infected wound healing. Adv. Sci. 10, 2300576 (2023).
Liu, G. et al. Janus hydrogel with dual antibacterial and angiogenesis functions for enhanced diabetic wound healing. Chin. Chem. Lett. https://doi.org/10.1016/j.cclet.2022.07.048 (2023).
Tian, S. et al. Polydiacetylene-based ultrastrong bioorthogonal Raman probes for targeted live-cell Raman imaging. Nat. Commun. 11, 81 (2020).
Tian, S. et al. Complete degradation of a conjugated polymer into green upcycling products by sunlight in air. J. Am. Chem. Soc. 143, 10054–10058 (2021).
Zhang, Y. et al. Reactive oxygen species-responsive and Raman-traceable hydrogel combining photodynamic and immune therapy for postsurgical cancer treatment. Nat. Commun. 13, 4553 (2022).
Xin, Y. et al. Microenvironment modulating nanogels by Shiitake-derived lentinan and a reactive oxygen species scavenging conjugated polymer for the treatment of Alzheimer’s disease. Nano Today https://doi.org/10.1016/j.nantod.2024.102178 (2024).
Zhang, D. et al. Near infrared-activatable biomimetic nanogels enabling deep tumor drug penetration inhibit orthotopic glioblastoma. Nat. Commun. 13, 6835 (2022).
Zhao, L. et al. Multivalent nanobody conjugate with rigid, reactive oxygen species scavenging scaffold for multi-target therapy of Alzheimer’s disease. Adv. Mater. 35, e2210879 (2023).
Tian, S. et al. Polydiacetylene-based poly-ion complex enabling aggregation-induced emission and photodynamic therapy dual turn-on for on-demand pathogenic bacteria elimination. Sci. China-Chem. 65, 1782–1790 (2022).
Chouchani, E. T. et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515, 431–435 (2014).
Murphy, M. P. & Chouchani, E. T. Why succinate? Physiological regulation by a mitochondrial coenzyme Q sentinel. Nat. Chem. Biol. 18, 461–469 (2022).
Zhang, W. & Lang, R. Succinate metabolism: a promising therapeutic target for inflammation, ischemia/reperfusion injury and cancer. Front. Cell. Dev. Biol. 11, 1266973 (2023).
Ji, M. et al. Advances in chitosan-based wound dressings: modifications, fabrications, applications and prospects. Carbohydr. Polym. 297, 120058 (2022).
Moeini, A., Pedram, P., Makvandi, P., Malinconico, M. & d’Ayala, G. G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: a review. Carbohydr. Polym. 233, 115839 (2020).
Dong, R., Zhang, H. & Guo, B. Emerging hemostatic materials for non-compressible hemorrhage control. Natl Sci. Rev. 9, nwac162 (2022).
Gurtner, G. C., Werner, S., Barrandon, Y. & Longaker, M. T. Wound repair and regeneration. Nature 453, 314–321 (2008).
Hu, J. et al. Mechanically active adhesive and immune regulative dressings for wound closure. Matter 4, 2985–3000 (2021).
Sullivan, T. P., Eaglstein, W. H., Davis, S. C. & Mertz, P. The pig as a model for human wound healing. Wound Repair Regen. 9, 66–76 (2001).
Driver, V. R. et al. A clinical trial of Integra Template for diabetic foot ulcer treatment. Wound Repair Regen. 23, 891–900 (2015).
Marston, W. A., Hanft, J., Norwood, P., Pollak, R. & Group, D. D. F. U. S. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care 26, 1701–1705 (2003).
Zaulyanov, L. & Kirsner, R. S. A review of a bi-layered living cell treatment (Apligraf®) in the treatment of venous leg ulcers and diabetic foot ulcers. Clin. Interv. Aging 2, 93–98 (2007).
Ziyadeh, N., Fife, D., Walker, A. M., Wilkinson, G. S. & Seeger, J. D. A matched cohort study of the risk of cancer in users of becaplermin. Adv. Ski. Wound Care 24, 31–39 (2011).
Matoori, S., Veves, A. & Mooney, D. J. Advanced bandages for diabetic wound healing. Sci. Transl. Med. 13, eabe4839 (2021).
Acknowledgements
We thank Dr. Jianglin Wang for kindly providing HUVECs. We also thank the Research Core Facilities for Life Science (HUST) and the Analytical and Testing Center of Huazhong University of Science and Technology for the related analyses. This work is supported by the National Natural Science Foundation of China (52325304 and 21877042, 22077038 L.L.) and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Contributions
L.C. and Z.Z. contributed equally. L.C. and Z.Z. designed and performed the experiments. L.C., Z.Z., and Y.L. performed the in vitro sample characterization. S.L. and M.Z. participated in the synthesis. Y.L., Z.H., and L.Z. assisted with the in vivo mouse experiments. M.Y. assisted with the in vivo pig experiments. F.M., S.T., and L.L. designed and supervised the experiments. S.T. and L.L. conceived and obtained funding for the project. L.C., S.T., and L.L. wrote the paper. All authors discussed the results and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The Authors declare no competing interest.
Peer review
Peer review information
Nature Communications thanks Hongzhong Chen, Craig Duvall, and Jianwen Hou for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cheng, L., Zhuang, Z., Yin, M. et al. A microenvironment-modulating dressing with proliferative degradants for the healing of diabetic wounds. Nat Commun 15, 9786 (2024). https://doi.org/10.1038/s41467-024-54075-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-54075-7
This article is cited by
-
Diabetic wound healing via a co-delivery system for bone marrow derived stem cells and euphorbia hirta extract: an in vitro and in vivo study
Journal of Materials Science: Materials in Medicine (2025)
-
Helping diabetic wounds heal
Nature Reviews Materials (2024)