Abstract
Baleen whales migrate from productive high-latitude feeding grounds to usually oligotrophic tropical and subtropical reproductive winter grounds, translocating limiting nutrients across ecosystem boundaries in their bodies. Here, we estimate the latitudinal movement of nutrients through carcasses, placentas, and urea for four species of baleen whales that exhibit clear annual migration, relying on spatial data from publicly available databases, present and past populations, and measurements of protein catabolism and other sources of nitrogen from baleen whales and other marine mammals. Migrating gray, humpback, and North Atlantic and southern right whales convey an estimated 3784 tons N yr−1 and 46,512 tons of biomass yr−1 to winter grounds, a flux also known as the “great whale conveyor belt”; these numbers might have been three times higher before commercial whaling. We discuss how species recovery might help restore nutrient movement by whales in global oceans and increase the resilience and adaptative capacity of recipient ecosystems.
Similar content being viewed by others
Introduction
Baleen whales undertake some of the longest mammalian migrations, with many species traveling from high-latitude feeding areas in the summer to low-latitude wintering grounds. Southern Hemisphere humpback whales (Megaptera novaengliae), for example, migrate between foraging areas near Antarctica and tropical and subtropical wintering grounds, where one-way traveling distances of ~8300 km have been recorded1. Gray whales (Eschrichtius robustus) travel more than 11,000 km between feeding grounds off Russia and breeding areas along Baja California2. Specific migration routes vary considerably between species and populations, with many whales displaying natal philopatry to breeding and feeding grounds. Gray, humpback, and right whales (Eubalaena spp.) generally adhere to the accepted model of summer foraging and winter fasts. The migratory patterns of fin (Balaenoptera physalus), blue (B. musculus), and minke whales (B. acutorostrata), however, are less well understood and mostly occur farther offshore3,4.
Migratory baleen whales go through periods of fattening and thinning, typically storing energy on summer foraging grounds to prepare for and recover from energy deficits associated with migration, breeding, and lactation5. With several notable exceptions, such as humpback and blue whales in the Gulf of California, baleen whales typically fast in winter and during migration, with an associated change in body condition. Unsurprisingly, whalers noted that whales killed at the end of the breeding season often weighed significantly less than those killed on the feeding grounds6,7.
Why do whales migrate? A traditional view is that there are physiological and behavioral advantages for giving birth in warm, shallow, sheltered areas. Pregnant females migrate to and give birth in these areas perhaps to reduce the likelihood of their newborn calves being attacked by killer whales (Orcinus orca), which are generally less abundant in these regions8. Warmer waters also represent an advantage for maintaining thermoregulation in calves before they develop their insulative blubber layer, which may allow for faster growth. An emerging concept is that whales travel to molt their skin and shed fouling organisms such as diatoms9.
Most baleen whales are capital breeders, acquiring much of the energy required for migration and reproduction during a relatively short period while on their feeding grounds10. Although their placement on the spectrum between capital and income breeding (when intake and reproduction overlap) varies spatially and temporally, most baleen whale species exhibit some form of traditional seasonal migration4. In general, reproductive females gain the largest energy (lipid) stores, presumably because of their increased energy demands during gestation and lactation11. Pregnant blue, fin, and humpback whales, for example, have energy stores that are ~25% larger than nonpregnant females and 37% more than males12,13. The large body size and energy stores of whales allow them to fast for longer periods and travel farther than other mammals, such as some pinnipeds that also use a capital-breeding strategy14. Many female whales give birth and lactate during this fasting period in tropical and subtropical coastal waters and lagoons, a pattern with ecological consequences that we explore here.
By migrating, cetaceans have the potential to transport elements in their bodies from highly productive, high-latitude feeding areas to low-latitude, usually oligotrophic, calving grounds, as they metabolize stored energy, excreting and releasing nitrogen and other nutrients through urine, feces, and lactation15. Parturient whales also release biomass in the form of placentas and the carcasses of dead neonates. Adult whale carcasses represent large nutrient pulses to these areas, similar to their role in providing subsidies to deep-sea communities16. Collectively, the nutrient and detrital subsidies these migratory whales provide can increase primary and secondary production on the breeding grounds. Their effect on community and ecosystem dynamics, however, is likely to vary according to the physical characteristics and food-web structure of the recipient areas17. Here, we estimate the magnitude of carbon and nutrient flux through this great whale conveyor belt and examine its potential impact on marine ecosystem dynamics (Fig. 1). We consider both the entire populations of four species of baleen whales and a more restricted estimate for mothers and calves. Although in some species, nonreproductive individuals might not migrate, mothers and calves are dependent on these breeding grounds for parturition and nursing. As such, they represent a conservative estimate for nitrogen transport by baleen whales. Considering that many whale populations have been depleted, we examine the global movement of nutrients both in the present and before industrial whaling.
Many baleen whales travel thousands of kilometers from their summer foraging areas to winter grounds, including breeding and calving areas. Nitrogen and other elements can be released in the form of urine, carcasses, placentas, sloughing skin, and feces (primarily from nursing calves). Humpback whales of the Central North Pacific, shown here, primarily feed off the coast of Alaska and spend winters in the shallow waters of the Hawaiian archipelago. (Illustration by A. Boersma).
Results
Nutrient subsidies provided by gray, humpback, and right whales
Our examination of nutrient subsidies provided by baleen whales focused on four relatively well-studied coastal species that exhibit traditional migratory patterns: gray whales, humpback whales, and North Atlantic, North Pacific, and southern right whales (Fig. 2). The entire populations of these species move an estimated 46,512 (38,092; 49,758) tons of biomass, 4874 (3992; 5215) tons C, and 3784 (3193; 4035) tons N yr−1 (Table 1). (Ninety-five percent confidence intervals, or 95% CIs, are in parentheses in tables and text.) Assuming Redfield stoichiometry (106 C:16 N), urea N, which has been observed to increase primary productivity and chlorophyll a concentrations18, could stimulate the production of 18,180 (15,407; 19,386) tons C yr−1 (Table 1). To our knowledge, baleen whales provide the largest long-distance nutrient subsidy on the planet. Although it appears that all mothers with calves migrate, the number of whales that travel to the breeding grounds could vary each year, given that some nonreproductive individuals spend less time on the winter breeding grounds and have lower energetic demands than reproductive females, e.g.,19. A better understanding of migration patterns and the urea production by males and nonreproductive females will help refine these estimates.
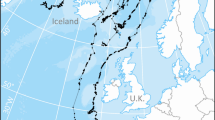
Gray whales are restricted to the North Pacific (A). Humpback whales are found throughout the world's oceans (B). Three species of right whales are found in the North Pacific, North Atlantic, and Southern Oceans (C). The movement of nutrients from high-latitude feeding grounds to lower-latitude winter and calving grounds provides an important resource subsidy. Maps combine nineteenth-century whaling data (whalinghistory.org), with contemporary databases such as OBIS-SEAMAP and Global Biodiversity Information Facility. Basemaps are from www.naturaldata.com. Whale icons are from iStock.com/KBelka.
The nutrient subsidies provided by mother and calf migration represent a conservative estimate for total transport, as reproductive females are obligated to migrate to calving grounds. They stay in more restricted areas, such as shallow protected waters during the months after birth. Migratory pregnant and post-parturition mothers move 12,080 (9894; 12,932) tons of biomass yr−1 (including carcasses and placentas), 1266 (1037; 1355) tons of C, and 2341 (1980; 2497) tons of N yr−1 (SI Appendix, Table S1). The total carbon fixation for the urea N is potentially 12,412 (10,519; 13,233) tons C yr−1.
Funneling effect of migrating whales
Distributional and sighting data allowed us to examine the ratios between foraging and winter areas (Table 2). The breeding areas tend to be smaller because whales aggregate on these grounds, possibly to protect calves from predators, enhance reproductive success, and reduce metabolic costs3. Such aggregations on winter calving grounds mean that released nutrients and biomass will be concentrated in these areas. Though the ratios vary depending on our definition of the calving ground, we believe the feeding-to-calving ratio best represents winter aggregations for the species considered here (SI Appendix, Feeding- and breeding-range distribution polygons). North Pacific gray whales, which tend to aggregate in a few small bays along Baja California, show the highest ratios, with summer feeding grounds being up to 46 times larger than the breeding grounds under the most constrained assumptions (Table 2). Humpback whales breed in slightly more open systems, with ratios that range from 5.1 for the Southern Hemisphere to 2.4 for the North Pacific (Fig. 2). Although the feeding and breeding polygons employed in our study are partly dependent on opportunistic sightings and data from satellite-tracked individuals, we supplemented our understanding of the spatial distribution of mother-calf pairs with designations from the published literature (SI Appendix, Calving-area polygons). Refining these areas will assist in understanding the ecology and conservation of whale migration.
Impact of whale-derived nutrients on recipient ecosystems
Although there is a relatively high degree of uncertainty on the total global nutrient transport by whales, we have data for some populations that allow us to compare whale-derived nutrients to abiotic fluxes. The Hawaiian Islands Humpback Whale National Marine Sanctuary, for example, is one of the few areas where both abiotic nutrient budgets and whale demography have been well studied20,21. The current population of humpback whales is estimated to release 3142 (2619; 3663) kg N day−1 on their calving grounds through urea and 3715 (3103; 4304) kg N day−1 including detrital N. Using estimates from the Hawaii Ocean Time-series site, the nitrogen released by humpbacks would be about 125–175% of the vertical N flux from upwelling and convective mixing (2419 kg N day−1) during the breeding season, based on estimates of 0.048 mmol N m−2 day−1 for the month of April, which coincides with the end of the whale breeding period20 (Fig. 3A).
The movement of N by migrating humpback whales compared to physical processes (e.g., upwelling) in Hawaii (A) and five whale species compared to seabirds in the tropics and subtropics, defined as latitudes between 30° N and 40° S (B). Source data are provided as a Source Data file.
Most of the world’s whale populations remain depleted since the advent of commercial whaling. Before industrial whaling, the global transport of nutrients by humpback, gray, and right whales would have been almost three times higher than it is now. Mothers and calves added 21,959 (18,100; 24,671) tons of biomass, 2301 (1897; 2386) tons C, and 5132 (4393; 5811) tons N yr−1 to breeding-ground ecosystems (Table S1). The increases in C fixation because of urea were expected to be 27,533 (23,619; 31,191) tons C yr−1 for mothers and calves. The total population transported 76,076 (62,669; 85,070) tons of biomass, 7973 (6568; 8915) tons C, and 7822 (6677; 8845) tons N yr−1 (Table 1). The total carbon fixation for the urea N was expected to be 39,044 (33,489; 44,232) tons C yr−1.
Potential importance of other balaenopterid whales
Our understanding of the migration of blue, fin, and other balaenopterid whales is not as well developed as it is for the species discussed above, with fewer sighting records, especially during the winter when many populations are found offshore (SI Appendix, Fig. S1 to S5). Although there is evidence that these whales fast during the winter, they might not have the regular migratory patterns of humpback, gray, and right whales7,18. Nevertheless, due to their immense size, they could have substantial impacts. Our initial estimates for blue and fin whale mothers and calves are that they transport 15,671 (12,864; 16,682) tons of biomass, 1642 (1348; 1748) tons C, and 2775 (2360; 2927) tons N yr−1 (SI Appendix, Table S2). The total carbon fixation for the urea N is expected to be 14,612 (12,453; 15,408) tons C yr−1 at present and 119,801 (105,521; 126,261) tons C yr−1 before commercial whaling. The total population transports 99,537 (81,705; 105,959) tons of biomass, 10,0432 (8563; 11,105) tons C, and 5901 (4995; 6230) tons N yr−1 (SI Appendix, Table S3). Blue and fin whales in the Southern Hemisphere remain depleted, though there are signs of hope that large feeding aggregations are returning to old whaling grounds22.
Discussion
As the largest animals on the planet with the longest mammalian migration, baleen whales move large amounts of biomass and urea N from high-latitude feeding areas to lower-latitude breeding and winter grounds. To our knowledge, these whales transport the highest quantities of carbon and nitrogen ever recorded by animals across thousands of kilometers23,24. Although future studies might reveal similar or higher roles for other animals, our study illustrates the importance of whale migration in linking spatially distant ecosystems across ocean basins and from the surface to the seabed25.
On their feeding grounds, whales transfer nutrients—such as nitrogen, iron, and phosphorous—to and within the euphotic zone in nutrient-rich fecal plumes26,27,28. This vertical movement of nutrients, also known as the whale pump, can move nutrients across the thermocline, similar to physical upwelling, or recycle them within the surface layer. The quantity of fecal nutrients in eutrophic temperate waters tends to reflect patterns of ecosystem productivity29. In contrast, nitrogen and other nutrients transported via the whale conveyor belt will primarily be allochthonous, with a source sometimes thousands of kilometers away, potentially playing an essential role in the carbon cycle and other biogeochemical processes in oligotrophic tropical waters.
Migratory baleen whales feed in spring and summer, and much of their biomass is derived from fish and invertebrate prey in high-latitude areas. As capital breeders, they can metabolize the stored energy during migration and while on winter breeding grounds. Gray, humpback, and right whales, which tend to breed in coastal areas, funnel large quantities of nutrients into low-latitude coastal and reef systems (Figs. 1 and 2). For most species, foraging areas are much larger than breeding areas (Table 2), perhaps because animals feed in multiple places throughout the spring and summer and because there are breeding, calf-rearing, and energetic benefits of staying in smaller, often shallow regions during the winter calving season. North Pacific gray whales particularly exhibit this traditional feeding and breeding pattern, with their summer travels having a much larger range than their winter aggregations (46:1, Table 2). For some species, such as the North Atlantic right whale, mothers and calves show a distinct migratory pattern from the rest of the population30. Pregnant and lactating mothers travel to a narrow area off the US states of Florida and Georgia; nonbreeding adults generally do not migrate to the calving grounds (Fig. 1). It is possible that this pattern is a consequence of whaling and that traditional migratory patterns were disrupted centuries ago, along with the extirpation of the eastern population of this species.
Although many baleen whale species migrate between high-latitude feeding grounds and low-latitude breeding grounds, others display a range of behaviors, including partial migration and nonmigratory populations. The spatial dynamics of several baleen whales are relatively unknown or unexplored4. Acoustic records, for example, show some North Atlantic fin whales stay on their feeding grounds throughout the year31. Even among the traditional migrants, variation occurs: passive acoustic monitoring of North Atlantic right whales shows that some animals remain in higher-latitude feeding areas throughout the year32. In the South Atlantic Ocean, southern right whales also move from offshore to onshore shallow regions of Brazil and Argentina, where their movements can be longitudinal and latitudinal33. A better understanding of the migratory patterns of blue and fin whales, and the degree to which they feed on winter grounds, would enhance our understanding of nutrient transport for the largest baleen whales. If they migrate and fast in a pattern similar to our focal species, the total movement of N by all 6 species would be 3 to 4 times higher (Fig. 3B).
Most of the biomass is supplied by whale carcasses, which support marine scavengers, deep-sea ecosystems, and coastal species. Tiger sharks (Galeocerdo cuvier) and several other large shark species have been observed feeding on whale carcasses in their breeding grounds (Fig. 4). In areas such as Australia and Brazil, sharks reproduce nearshore during the whales’ breeding season, and migrating whales could be an important resource subsidy34.
Humpback mother (top) from camera on a calf in the Hawaiian Islands Humpback Whale National Marine Sanctuary (A). The mother’s urine can be seen on the right. Mackerel scad (Decapterus macarellus) haves been observed feeding on sloughed skin and appear to be attracted to leaked milk. (Courtesy of Marine Mammal Research Program, University of Hawaii. NMFS Permit Number 21476). Tiger shark with a carcass of a humpback whale calf off Ningaloo Reef, Australia (B). The carcasses of neonates and adults provide nutrients to lower-latitude nutrient-limited systems. (Courtesy of J. Leask).
The scale of nitrogen transport by baleen whales is comparable to that of subtropical seabirds (Fig. 3B), a process that has been well studied, with important ecosystem effects, including helping ecosystems adapt to changing environmental conditions35. Yet, the transport distance is far greater for whales, which move nitrogen across thousands of kilometers, generally from highly productive to more oligotrophic areas. Seabirds, in contrast, tend to move nutrients across tens or hundreds of kilometers, with much of this movement occurring in higher latitudes36. Some seabirds, such as sooty shearwaters (Puffinus griseus) and albatrosses do travel across entire ocean basins37, though even these birds must feed their chicks from local prey, rather than milk production, with much of their feces ending up on land. Whales travel to their winter calving grounds only once a year, stay for a few months, and release most of the N in the form of urea, which remains in the ocean (Figs. 3A and 4B). During this migration, whales (including blues and fins) move ~146,049 (119,797; 155,717) tons of biomass and 9686 (8187; 10,265) tons N to the lower latitudes (Fig. 3B), with pregnant and lactating mothers of the best-studied shallow-water species moving about 2341 (1980; 2497) tons N to calving grounds each year. (95% CIs are in parentheses here and throughout the manuscript.) Whales would have played a substantially larger role in the past, when most of the populations we examined were much higher. We did not examine past populations of seabirds, which were not as severely reduced as whales.
How does the migration of whales affect local ecosystems? In the North Pacific, 4130 (3471; 4651) tons of biomass are transported yearly from Alaska to Hawaii via humpback whale migration; 477 (442; 541) tons of N are released during the three months on the calving grounds (Fig. 3A). Using estimates from the Hawaii Ocean Time-series site, this is more than the vertical nitrogen flux of the entire Hawaiian Islands Humpback Whale National Marine Sanctuary during the breeding season.
Even an individual whale can have a substantial impact on the breeding grounds. A single female humpback, for example, could produce 10 calves over the course of her lifetime; this would mean at least 20 return migrations between feeding and breeding grounds (for copulation and parturition). On each calving journey, she would leave 77 kg N—774 kg throughout her life. Based on our calculations above, this could stimulate 4391 kg of C fixation on the calving grounds (at 439 kg C per visit). At the end of her life, her 27-ton body would transport 2782 kg of carbon to the ocean floor, though the ___location of death could be on the breeding grounds, feeding grounds, or in transit. (Factors associated with parturition and mating conflict could enhance mortality on the breeding grounds, though data are sparse16.) Nutrients released on the breeding grounds are assumed to be new nutrients to the system, stimulating new primary production, carbon export, and carbon sequestration38. A successful female could stimulate the production of more than one whale’s worth of carbon on the breeding grounds over her lifetime.
Our study does not quantify the release of feces or skin sloughing. Feces are only produced by feeding whales, which are rarely observed in the winter grounds. It is likely, however, that nursing calves, which spend much of their time in shallow waters39, are excreting and defecating near the surface. Quantifying the release of calf feces and milk leakage by feeding calves would provide a better understanding of the total nutrient flux generated by nursing whales. Whales slough epithelium throughout their lives, though some authors have suggested that cetaceans migrate to warmer waters in part to molt their skin, a feeding-and-molting hypothesis9. Local fishes, birds, and other species consume some of this skin. In Australia, humpback whales have been observed rolling at the sandy bottom of their breeding grounds, perhaps to exfoliate, with fish actively feeding on the skin40. At the surface, silver gulls (Chroicocephalus novaehollandiae) have been observed pecking whales and feeding on sloughed skin41. Although we did not estimate the nutrient contribution of skin sloughing to breeding areas, we recognize that it is likely a food source for some species and is an important area for future research (Fig. 4).
Whaling, loss of ecological function, and conservation
Migrating baleen whales import nutrients to tropical ecosystems, which could increase their productivity and resilience. The amount of nutrients transported would likely have been at least three times higher before commercial whaling. Many populations were reduced by hunting, and some populations, such as the North Atlantic right whale in the Northeast Atlantic, were extirpated from their historical breeding areas before spatial data were recorded42. These nutrient networks were lost before we even knew they existed.
Conservation efforts in place since the 1970s have helped some populations, such as the eastern Australian humpbacks, recover43. As migrating whale populations grow, we expect to see a rise in associated ecosystem services along with conflicts with human activities, such as commercial fisheries and shipping44. Although many populations have increased since the demise of commercial whaling, future recovery is imperiled by human actions such as shipping, fishing, and climate change. Populations of humpback and right whales in the Southern Hemisphere, for example, will likely peak around 2050 and decline along with changes in ocean temperature, sea ice, and primary productivity45. Such changes could disrupt one of the world’s most prominent links between high- and low-latitude marine ecosystems.
Methods
Distribution of baleen whales
Spatial data for feeding and breeding grounds are derived from publicly available databases including Whaling History (www.whalinghistory.org), Ocean Biodiversity Information System-Spatial Ecological Analysis of Megavertebrate Populations (OBIS-SEAMAP, seamap.env.duke.edu), and Global Biodiversity Information Facility (GBIF, www.gbif.org). In some cases, whale sightings from aerial and ship-based surveys were added to supplement missing data (e.g., Antarctic and Brazilian humpback whales). We looked at the sightings for all large whale species in these databases, including Antarctic minke whale, blue whale, bowhead whale, common minke whale, fin whale, gray whale, humpback whale, right whales (North Atlantic, North Pacific, and Southern), Sei whale, and the Bryde’s whale complex (e.g., B. omurai, B. ricei).
High-latitude feeding ranges were modeled using all observations within each ocean basin. Low-latitude breeding ranges were modeled based on all observations within each ocean basin and up to 15° latitude in the opposite hemisphere. This flexibility accounts for whale species and individuals that migrate across the equator during their breeding season.
We chose six species based on evidence of long-distance seasonal migration between high and low latitudes and data availability in public databases with sufficient information for summer foraging and winter breeding grounds. Criteria included catch records and sightings throughout the year and distribution of the calving grounds based on the literature. Species include blue whales, fin whales, gray whales, humpback whales, and North Atlantic and southern right whales. Several species of whales rely on shallow water for shelter in their breeding areas. Mother-calf pairs of gray, humpback, and right whales clearly prefer coastal waters, including bays, lagoons, and reef complexes3,46,47.
We focused on several well-known breeding grounds but acknowledge that our study likely overlooks some breeding areas both in the past and present. Other baleen whales, such as fin, blue, and minke, also exhibit high-to-low latitude migrations. Perhaps because their breeding grounds are often farther from shore, or more dispersed, less is known about these groups. We have included the results for blue and fin whales and recognize that future research will be necessary to better understand nutrient transport by these species (SI Appendix, Tables S2, S3, Figs. S1, S2). Minke whales, which likely have migratory patterns similar to fin and blue whales, were not included in our analysis because of a dearth of knowledge about their seasonal movements48. As abundant, widespread species, minkes likely play an important role in nutrient distribution and warrant further study.
Sightings of North Pacific right whales were rare outside of the summer whaling season, and too few animals were recorded to establish winter grounds for this critically endangered species49. Our spatial analysis did not include them, though numbers appear in our nutrient transport estimates.
Present and historical population sizes
Population sizes are from Smith et al.16. with several updates. Present (2019/20) and past population sizes for the Southern Hemisphere use the baseline model fitted in Tulloch et al. 45. (See also SI Appendix, Population Updates.)
Calculations for nutrient transport by whales from feeding to breeding grounds
Roman et al.15. introduced the idea that migrating whales can transport nitrogen from high-latitude feeding grounds to low-latitude calving grounds. These winter areas are often located in oligotrophic waters, where whale nitrogen can be a source for new production and increase the biomass of animals in the ecosystem. Our original calculations focused on nitrogen excreted in the urine of lactating females. We now propose a more holistic approach to incorporate other sources of nitrogen.
First, we need to determine F, the number of pregnant females on the calving ground. This is computed as
where P is the total population size, qq = the sex ratio, and y = the average calving interval. We assume that the sex ratio is 50:50. Calving intervals, percent mature, and calf and adult survival rates, were estimated for each species based on Taylor et al.50.
For each whale species, we assign a mean adult body mass (m) using the numbers provided by Pershing et al.51. We estimate the mass of a newborn calf (mc) based on the allometric relationship in Webb52:
In our calculations, we envision each female giving birth to one calf. Whale parturitions release placentas that result in a gradual release of nitrogen. We estimated the mass of a placenta (mp) as 10% of the mass of the calf based on observations of killer whale placentas53. We assume that the placenta is 13.43% protein by weight54. Although this estimate is from humans, we do not expect it to vary considerably across species. We used the standard assumption from the FAO that protein is 16% nitrogen by weight. This means that the total nitrogen from all placentas on the calving grounds is
Then, we assume that s proportion of calves survive and (1-s) die on the calving grounds. This means that (1-s) F calf carcasses are produced each year. We followed the assumptions of Jelmert and Oppen-Bensen55 that the dry weight of a whale is 40% of the total and that 20% is protein. This gives the total nitrogen from the calf carcasses:
and adults:
where x is adult survival and P is population size. Future efforts to examine the stoichiometry of great whales, rendered difficult because of their size and the rarity of finding intact and recent strandings, will help refine these assumptions.
The last piece in the calculations is the nitrogen in the urea excreted by both the mother and the calf. We followed our original approach, which used the only measurements available from a fully capital-breeding marine mammal, fasting and lactating northern elephant seals (Mirounga angustirostris), with some modifications. Crocker et al. measured a urea production rate of 84 g day−1 during mid-lactation for a female elephant seal weighing an average of 432 kg56. We assume an allometric relationship:
where A = 0.886. The elephant seal data for late lactation are U = 126.3 g day−1 and m = 331 kg, implying that A = 1.628 (by rearranging Eq. 6). We can express (6) in terms of nitrogen using v = 46.62%, the percentage of urea that is nitrogen:
Using (6) and (7), a 105-ton (105,000 kg) blue whale in mid-lactation would then produce 5171 g urea day−1 and 2411 g N day−1. If they are in late lactation, the numbers increase to 9493 g urea day−1 and 4426 g N day−1. Converting to tons yields
with B = 73.49 and 134.61 for mid- and late-lactation state.
To determine B, Roman et al.15. estimated that a 105-ton lactating female blue whale releases 3300 g N day−1. In our analysis, we opted for a more conservative approach, using lower lactation numbers since whale calves are relatively small in the first few months and consume less milk. We computed n for both mothers and calves, added them together, and then multiplied by the number of days (D) spent on the calving grounds (SI Appendix, Table S4):
We show that Np and Nc are very similar when we input our best information on the sizes of mothers, calves, and placentas. Adults are much larger, and the nitrogen from the few adults who die on the calving rounds is more than 10 times the nitrogen from the calves, even though fewer adults die. Mothers contribute about 90% toward Nu (mothers and calves united), whereas calves contribute only 10%.
Key packages employed for data analysis include R statistical software version 4.3.1, CoordinateCleaner, AdeHabitatHR, Python 3.9.10, SciPy 1.11.2, NumPy 1.22.3, and pandas 1.4.1. The backgrounds for maps are from Natural Earth (www.naturaldata.com). For uncertainty in these input variables see Uncertainty in input data and sensitivity analysis below and Fig. S6.
Migration and time on the winter grounds
Summer and winter residence times for whale populations were set by the seasons in each hemisphere, the calendar days of the whale sightings, and discussions and findings in the available literature (SI Appendix, Calving area polygons and Table S4). In some cases, there are good models or databases for the time that whales spend on the breeding grounds: the migratory patterns and bioenergetics of gray whales, for example, have been well studied19. For other species, there is considerable variation and uncertainty in how much time breeding females and nonbreeding adults spend on the winter grounds. To our knowledge, male and nonreproductive female North Atlantic right whales generally do not migrate to the calving grounds, though we do not know their pattern in the past. A conservative estimate would focus on mothers and calves. Little is known about the migratory patterns of North Pacific right whales in the present or the past. In both cases, present numbers are minimal for these critically endangered species. In many cases, some whales will arrive earlier or stay longer than the average for the population. We estimated that whales spend ~25% of their time (or 90 days, with a range of 60–120 days) on the breeding grounds.
Carcasses: adult mortality
Adult mortality rates used in our calculations are based on survivorship estimates in ref. 50. For our mortality calculations, we assume that adult whales are as likely to die on their winter breeding grounds as anywhere else throughout the year. There are several reasons, however, to suspect that mortality rates could be higher on the breeding grounds or in transit between breeding and feeding grounds, including factors associated with parturition, mating conflict, and decreased body condition during migration15,16,57. Recent high mortality events highlight the potential pulse of nutrients in a single season: in 1999, an unusual mortality event of gray whales was ~7 times higher than the annual mean; many of the strandings occurred in Mexican waters during the breeding season, a pattern that is consistent with most other years57. Although only a few stranded individuals were examined, the emaciated condition of many whales indicated that starvation might have contributed to these mortalities.
Carcasses: calf mortality
Survival rates for calves are typically lower than for adult baleen whales50. Calf mortality can be high in breeding areas. At Península Valdés in Argentina, for example, hundreds of southern right whales (Eubalaena australis) died on their calving ground between 2003 and 2011; 89% of the dead whales observed during this period were calves, with a high number of carcasses observed on the beaches58. In Brazil, the most common cause of stranding or death in humpback calves is neonatal respiratory distress followed by infectious disease59. To maintain a conservative approach, we assumed they were as likely to die in calving areas as elsewhere during their first year of life. Future studies of the causes and locations of calf mortality and stoichiometry, as well as the size and fate of calf carcasses, will improve our understanding of the role of calf mortality in nutrient subsidies.
Urine
Migrating baleen whales rely primarily on energy reserves during the breeding season. The energy required for reproducing females is considerable, and early lactation often occurs during the fasting period of many whales60,61. (Whalers sometimes referred to female whales as “dry-skins” after their breeding-fasting periods because they had little blubber to be rendered into oil62.) Fasting whales catabolize both fat and protein; the concentration of urea, derived from metabolic processes, in the urine of fasting humpback whales harvested off Australia, was calculated as 11 g/L (183 mmol/L), comparable to the concentration in humans and other mammals63. Birukawa et al.64. found levels of urea in baleen whale urine that were higher than that of cattle or sperm whales; the high concentration of urea helps cetaceans maintain fluid homeostasis in the ocean65.
Whereas energy metabolism has been measured in gray whales and has recently been reviewed for marine mammals66, rates of protein catabolism are only available for a few pinnipeds65. We used allometric scaling relationships based on estimates of N excretion rates from fasting and fasting-while-lactating elephant seals to estimate nitrogen transport between whale feeding and breeding grounds15,67. Much of this urine is likely released near the surface: for North Atlantic right whales, Hawaiian humpback whales, and presumably many other baleen whales, resting behaviors dominate the activity budgets for mothers and calves during the months after birth39.
Nitrogen, primary productivity, and the nutricline in oligotrophic waters
In the surface waters of the oligotrophic ocean, nitrogen often occurs at nanomolar concentrations, and ammonium is the dominant form taken up by phytoplankton68,69. We assume that N is limiting in many calving grounds, so any excretion of N will be quickly assimilated by phytoplankton. Assuming a Redfield ratio of 106:16 moles of C:N, every ton of N left on the calving grounds leads to 13.7 tons of new carbon fixed.
To examine the impact of N in calving grounds, we looked at the Hawaiian Islands Humpback Whale National Marine Sanctuary, a well-studied system with more than 10,000 breeding whales, and a nearby long-term oceanographic program, the Hawaii Ocean Time-series (HOT). Letscher et al.20. estimated the vertical flux of N from the HOT site as 0.048 mmol N/m2/day during April, when the whales are present in Hawaii. This implies a total of 0.06 g/m2 over the calving period (90 days). We scaled this flux up to the 3600 km2 of the sanctuary, yielding 218 tons of N over the calving period for the same area.
Uncertainty in input data and sensitivity analysis
Any global analysis of this kind, examining past and present nutrient flows, requires multiple assumptions or simplifications and is subject to considerable uncertainty. Our modeling approach is a necessary simplification of dynamic whale populations, which have important individual and subpopulation-level characteristics. In our study, for example, we consider all adults to have the same body mass, but in reality, some individuals will be larger and smaller than others, and body size can vary from year to year70. In addition, we use parameters (e.g., population size, percentage of lactating females) that have a degree of uncertainty. To ensure that our results encapsulate the impact of these uncertainties, we conducted a Monte Carlo sensitivity analysis that included uncertainty in (1) population size (from literature sources when available and set at ±30% from the best estimate in the absence of CI estimates in the literature), (2) adult mass (set at ±20% from the best estimate, following Smith at al71), (3) time spent on wintering breeding grounds (set at ±30% from the best estimate), (4) percent migrating (set at ±50% from the best estimate), and (5) percent lactating females (set at ±50% from the best estimate). Although there were additional parameter inputs for our model, we focused on these five as they have been identified as the primary drivers of uncertainty in whale nutrient subsidies (see Gilbert et al., Fig. 729), and our preliminary tests established that uncertainty in these parameters overwhelmed the effects of other parameters (e.g., placenta size and N concentration of carcasses). We repeated our model 10,000 times, in each instance randomly selecting a value for each of the five sensitivity parameters from a uniform distribution between the lower and upper confidence intervals as outlined above. See Table S5 for specific lower and upper confidence values used in our sensitivity analysis. We conservatively selected from a uniform distribution as we did not have specific information on distributions and did not want to bias the results72. To provide uncertainty bounds, we then calculated the 2.5th and 97.5th percentiles (i.e., 95% probability of the true result occurring within these bounds) for each of total biomass, biomass carbon, biomass nitrogen, urea nitrogen, carbon fixed by urea, and total nitrogen. Ninety-five percent confidence intervals are reported throughout the manuscript in parentheses.
To find out which sensitivity parameters generated the largest uncertainty within our modeled results, we undertook a Sobol variance-based approach, as has been conducted in similar studies (e.g.,29). We illustrate this sensitivity using the Hawaiian humpback whale population. In general, most of the uncertainty in our estimates was generated by the population parameters (number of whales, percent migrating) rather than in the biological parameters (adult and calf mass and any of the allometric terms). Urea N is most sensitive to population size, days on calving ground, and percentage of lactating females, whereas deadfall N is most sensitive to the percentage of the population migrating (Fig. S6). We think that this is an important finding that leads to actionable recommendations for future work. For example, allometric parameters are difficult to measure, but we have reliable and well-understood methods for estimating population size. Studies that target these population parameters are likely to have the biggest impact on reducing uncertainty in quantifying nutrient transport by baleen whales. However, we note that even our lower estimates are comparable to known important fluxes of abiotic (e.g., upwelling) and biotic (e.g., seabird) inputs.
Future studies would benefit from more direct measurements of the impact of migratory whales on chlorophyll-a concentration, an index of phytoplankton biomass. Nitrogen released by gray seals (Halichoerus grypus) of Sable Island, Canada, for example, may have been responsible for about 20% of the increase of chlorophyll-a standing stock in the past 21 years73. Stable isotope analysis could also identify N sources, particularly the contribution of whale-mediated N vs. other sources. Our understanding of baleen whale metabolism, protein catabolism, and the potential for urea recycling is limited by their large size and a lack of direct studies. A closer examination of the migration of species such as blue and fin whales and their feeding patterns on their winter grounds would enhance our measurement of nutrient transport for the largest baleen whales.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data reported in this study were extracted from published sources cited above and have been deposited in the Figshare database under https://figshare.com/s/69f76e8dcdb0573d9f67. Source data are provided with this paper.
Code availability
The codes to reproduce the complete analysis are available on Figshare: https://figshare.com/s/69f76e8dcdb0573d9f67.
References
Rasmussen, K. et al. Southern Hemisphere humpback whales wintering off Central America: insights from water temperature into the longest mammalian migration. Biol. Lett. 3, 302–305 (2007).
Mate, B. R. et al. Critically endangered western gray whales migrate to the eastern North Pacific. Biol. Lett. 11, 20150071 (2015).
Ford, J. B. K. & Reeves, R. R. Fight or flight: antipredator strategies of baleen whales. Mamm. Rev. 38, 50–86 (2008).
Geijer, C. K. A., Notarbartolo di Sciara, G. & Panigada, S. Mysticete migration revisited: are Mediterranean fin whales an anomaly? Mamm. Rev. 46, 284–296 (2016).
Miller, C. A. et al. Blubber thickness in right whales Eubalaena glacialis and Eubalaena australis related with reproduction, life history status, and prey abundance. Mar. Ecol. Prog. Ser. 438, 267–283 (2011).
Chittleborough, R. G. Dynamics of two populations of the humpback whale, Megaptera novaengliae (Borowski). Aust. J. Mar. Freshw. Res. 16, 33–128 (1965).
Dawbin, W. H. in Whales, Dolphins, and Porpoises (University of California Press, 1966).
Corkeron, P. J. & Connor, R. C. Why do baleen whales migrate? Mar. Mamm. Sci. 15, 1228–1245 (1999).
Pitman, R. L. et al. Skin in the game: epidermal molt as a driver of long‐distance migration in whales. Mar. Mamm. Sci. 36, 565–594 (2019).
Jönsson, K. I. Capital and income breeding as alternative tactics of resource use in reproduction. Oikos 78, 57–66 (1997).
Costa, D. P. & Maresh, J. L. in Encyclopedia of Marine Mammals (eds B. Würsig, J. G. M. Thewissen, & K. M. Kovacs) 329–335 (Academic Press, 2018).
Irvine, L. G., Thums, M., Hanson, C. E., McMahon, C. R. & Hindell, M. A. Quantifying the energy stores of capital breeding humpback whales and income breeding sperm whales using historical whaling records. R. Soc. Open Sci. 4, 160290 (2017).
Lockyer, C. in Mammals in the Sea Vol. 3 379-487 (FAO Fisheries Series, 1981).
Oftedal, O. T. Use of maternal reserves as a lactation strategy in large mammals. Proc. Nutr. Soc. 59, 99–106 (2000).
Roman, J. et al. Whales as marine ecosystem engineers. Front. Ecol. Environ. 12, 377–385 (2014).
Smith, C., Roman, J. & Nation, J. B. A metapopulation model for whale-fall specialists: the largest whales are essential to prevent species extinctions. J. Mar. Res. 77, 283–302 (2019).
Polis, G. A., Anderson, W. B. & Holt, R. D. Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annu. Rev. Ecol. Syst. 28, 289–316 (1997).
Rii, Y. M., Bidigare, R. R. & Church, M. J. Differential responses of eukaryotic phytoplankton to nitrogenous nutrients in the North Pacific Subtropical Gyre. Front. Mar. Sci. 5, 92 (2018).
Villegas-Amtmann, S., Schwarz, L., Sumich, J. & Costa, D. A bioenergetics model to evaluate demographic consequences of disturbance in marine mammals applied to gray whales. Ecosphere 6, 1–19 (2015).
Letscher, R. T., Primeau, F. & Moore, J. K. Nutrient budgets in the subtropical ocean gyres dominated by lateral transport. Nat. Geosci. 9, 815–819 (2016).
Frankel, A. S., Gabriele, C. M., Yin, S. & Rickards, S. H. Humpback whale abundance in Hawai‘i: temporal trends and response to climatic drivers. Mar. Mamm. Sci. 38, 118–138 (2022).
Herr, H. et al. Return of large fin whale feeding aggregations to historical whaling grounds in the Southern Ocean. Sci. Rep. 12, 9458 (2022).
Schmitz, O. J. et al. Animals and the zoogeochemistry of the carbon cycle. Science 362, eaar3213 (2018).
Subalusky, A. L. & Post, D. M. Context dependency of animal resource subsidies. Biol. Rev. 94, 517–538 (2019).
O’Leary, B. C. & Roberts, C. M. Ecological connectivity across ocean depths: Implications for protected area design. Glob. Ecol. Conserv. 15, e00431 (2018).
Lavery, T. J. et al. Iron defecation by sperm whales stimulates carbon export in the Southern Ocean. Proc. R. Soc. B. 277, 3527–3531 (2010).
Roman, J. & McCarthy, J. J. The whale pump: marine mammals enhance primary productivity in a coastal basin. PLoS ONE 5, e13255 (2010).
Roman, J., Nevins, J., Altabet, M., Koopman, H. & McCarthy, J. Endangered right whales enhance primary productivity in the Bay of Fundy. PLoS ONE 11, e0156553 (2016).
Gilbert, L., Jeanniard-du-Dot, T., Authier, M., Chouvelon, T. & Spitz, J. Composition of cetacean communities worldwide shapes their contribution to ocean nutrient cycling. Nat. Commun. 14, 5823 (2023).
Kenney, R. D., Mayo, C. A. & Winn, H. E. Migration and foraging strategies at varying spatial scales in western North Atlantic right whales: a review of hypotheses. J. Cetacean Res. Manage. 2, 251–260 (2020).
Delarue, J., Todd, S., Van Parijs, S. & Di Lorio, L. Geographic variation in Northwest Atlantic fin whale (Balaenoptera physalus) song: implications for stock structure assessment. J. Acoust. Soc. Am. 125, 1774–1782 (2009).
Morano, J. et al. Acoustically detected year-round presence of right whales in an urbanized migration corridor. Conserv. Biol. 26, 698–707 (2012).
Zerbini, A. N. et al. Satellite tracking of Southern right whales (Eubalaena australis) from Golfo San Matias, Rio Negro Province, Argentina. Scientific Committee of the International Whaling Commission SC67b, Bled, Slovenia (2018).
Ferretti, F., Worm, B., Britten, G. L., Heithaus, M. R. & Lotze, H. K. Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 13, 1055–1071 (2010).
Benkwitt, C. E. et al. Seabirds boost coral reef resilience. Sci. Adv. 9, eadj0390 (2023).
Otero, X. L., De La Peña-Lastra, S., Pérez-Alberti, A., Osorio Ferreira, T. & Huerta Diaz, M. A. Seabird colonies as important global drivers in the nitrogen and phosphorus cycles. Nat. Commun. 9, 246 (2018).
Shaffer, S. A. et al. Migratory shearwaters integrate oceanic resources across the Pacific Ocean in an endless summer. PNAS 103, 12799–12802 (2006).
Pearson, H. C. et al. Whales in the carbon cycle: can recovery remove carbon dioxide? Trends Ecol. Evol. 3074, 1–12 (2022).
Lomac-MacNair, K., Zoidis, A. M., Anderson, M. & Blees, M. Humpback whale calf vulnerability to small-vessel collisions; assessment from underwater videography in Hawaiian waters. J. Coast. Sci. 5, 28–36 (2018).
Meynecke, J.-O., Gustafon, J. & Cade, D. E. Exfoliating whales–sandy bottom contact behaviour of humpback whales. J. Mar. Sci. Eng. 11, 600 (2023).
Harkness, P. & Sprogis, K. R. Silver gull harassment of humpback whales in Exmouth Gulf, Western Australia. Mar. Freshw. Res. 72, 584–592 (2020).
Monsarrat, S. et al. A spatially explicit estimate of the pre-whaling abundance of the endangered North Atlantic right whale. Conserv. Biol. 30, 783–791 (2015).
Noad, M. J., Kniest, E. & Dunlop, R. A. Boom to bust? Implications for the continued rapid growth of the eastern Australian humpback whale population despite recovery. Popul. Ecol. 61, 198–209 (2019).
Roman, J., Dunphy-Daly, M. M., Johnston, D. W. & Read, A. J. Lifting baselines to address the consequences of conservation success. TREE 30, 299–302 (2015).
Tulloch, V. J., Plagányi, É. E., Brown, C., Richardson, A. J. & Matear, R. Future recovery of baleen whales is imperiled by climate change. Glob. Change Biol. 25, 1263–1281 (2019).
Ersts, P. J. & Rosenbaum, H. C. Habitat preference reflects social organization of humpback whales (Megaptera novaeangliae) on a wintering ground. J. Zool. 260, 337–345 (2003).
Bruce, E. Distribution patterns of migrating humpback whales (Megaptera novaeangliae) in Jervis Bay, Australia: a spatial analysis using geographical citizen science data. Applied Geography (2014).
Kavanagh, A. S., Kett, G., Richardson, N., Rogan, E. & Jessopp, M. J. High latitude winter sightings of common minke whale calves (Balaenoptera acutorostrata) in the Northeast Atlantic. Mar. Biodivers. Rec. 11, 1–5 (2018).
Clapham, P. J. et al. Distribution of North Pacific right whales (Eubalaena japonica) as shown by 19th and 20th century whaling catch and sighting records. J. Cetac. Res. Manag. 6, 1–6 (2004).
Taylor, B. L., Chivers, S. J., Larese, J. & Perrin, W. F. Generation length and percent mature estimates for IUCN assessments of cetaceans. Administrative Report LJ-07–01 (Southwest Fisheries Science Center, National Marine Fisheries Service, 2007).
Pershing, A. J., Christensen, L. B., Record, N. R., Sherwood, G. D. & Stetson, P. B. The impact of whaling on the ocean carbon cycle: Why bigger was better. PLoS ONE 5, e12444 (2010).
Webb, P. I. Relative body sizes of neonatal marine mammals. Can. J. Zool. 75, 1732–1736 (1997).
Benirschke, K. & Cornell, L. H. The placenta of the killer whale, Orcinus orca. Mar. Mamm. Sci. 3, 82–86 (1987).
Laga, E. M., Driscoll, S. G. & Munro, H. N. Quantitative studies of human placenta II. Neonatology 23, 260–283 (1973).
Jelmert, A. & Oppen-Berntsen, D. O. Whaling and deep-sea biodiversity. Conserv. Biol. 10, 653–654 (1996).
Crocker, D. E., Webb, P. M., Costa, D. P. & Le Boeuf, B. J. Protein catabolism and renal function in lactating northern elephant seals. Physiol. Zool. 71, 485–491 (1998).
Gulland, F. M. D. et al. Eastern North Pacific gray whale (Eschrichtius robustus) unusual mortality event, 1999–2000. NOAA Technical Memo. NMFS-AFSC−150 (Alaska Fisheries Science Center, National Oceanic and Atmospheric Administration, 2005).
Rowntree, V. J. et al. Unexplained recurring high mortality of southern right whale Eubalaena australis calves at Península Valdés, Argentina. Mar. Ecol. Prog. Ser. 493, 275–289 (2013).
Groch, K. R. et al. Pathology and causes of death in stranded humpback whales (Megaptera novaeangliae) from Brazil. PLoS ONE 13, e0194872 (2018).
Hanwell, A. & Peaker, M. Physiological effects of lactation on the mother. Symp. Zool. Soc. Lond. 41, 297–311 (1977).
Villegas-Amtmann, S., Schwarz, L., Gailey, G., Sychenko, O. & Costa, D. East or west: the energetic cost of being a gray whale and the consequence of losing energy to disturbance. Endanger. Species Res. 34, 167–183 (2017).
Roman, J. Whale. (Reaktion, 2006).
Bentley, P. J. Composition of the urine of the fasting humpack whale (Megaptera nodosa). Comp. Biochem. Physiol. 10, 257–259 (1963).
Birukawa, N. et al. Plasma and urine levels of electrolytes, urea, and steroid hormones involved in osmoregulation of cetaceans. Zool. Sci. 22, 1245–1257 (2005).
Costa, D. P. in Encyclopedia of Marine Mammals (eds Würsig, B. Thewissen, J. G. M. & Kovacs, K.) 659–664 (Academic Press, 2018).
Noren, S. & Rosen, D. A. What are the metabolic rates of marine mammals and what factors impact this value: a review. Conserv. Physiol. 11, coad077 (2023).
Cusano, D., Conger, L., Van Parijs, S. & Parks, S. E. Implementing conservation measures for the North Atlantic right whale: considering the behavioral ontogeny of mother‐calf pairs. Anim. Conserv. 22, 228–237 (2019).
Wu, J., Sunda, W., Boyle, E. & Karl, D. Phosphate depletion in the Western North Atlantic Ocean. Science 289, 759–762 (2000).
Villareal, T. A., Pilskaln, C. H., Montoya, J. P. & Dennett, M. Upward nitrate transport by phytoplankton in oceanic waters: balancing nutrient budgets in oligotrophic seas. PeerJ 2, e302 (2014).
Soledade Lemos, L., Burnett, J. D., Chandler, T. E., Sumich, J. L. & Torres, L. G. Intra and inter‐annual variation in gray whale body condition on a foraging ground. Ecosphere 11, e03094 (2020).
Smith, L. A., Link, J. S., Cadrin, S. X. & Palka, D. L. Consumption by marine mammals on the Northeast US continental shelf. Ecol. Appl. 25, 373–389 (2015).
Harrison, R. L. Introduction To Monte Carlo Simulation. AIP Conf. Proc. 1204,17–21 (2010).
Devred, E., Hilborn, A. & den Heyer, C. Enhanced chlorophyll-a concentration in the wake of Sable Island, eastern Canada, revealed by two decades of satellite observations: a response to grey seal population dynamics? Biogeosciences 18, 6115–6132 (2021).
Acknowledgements
J.R. was funded by a Fulbright-National Science Foundation Arctic Research Grant in Iceland, with support from the University of Iceland, and a Whale and Dolphin Conservation grant. A.J.A. was funded by a NASA Biodiversity Grant 18-SLSCVC18-0032 and Horizon Europe Marie Skłodowska-Curie Actions Grant Agreement No. 101062339. Projeto Baleia Jubarte is sponsored by Petroleo Brasileiro S.A. (Petrobras). This is contribution #1802 from the Institute of Environment at Florida International University. We thank Bud Antonelis, Dave Johnston, and David Matilla for early conversations about whale-nutrient transport to Hawaii and Lars Bejder for sharing on-animal videos from the Hawaiian Islands Humpback Whale National Marine Sanctuary. Emma Forsythe helped develop early whale migration maps, and Lola Gilbert provided valuable feedback. Miguel Angel Huerta-Diaz shared seabird data from27, Sophie Monserrat shared historical Atlantic right whale distribution data from34 and Viv Tulloch shared Southern Hemisphere data from37.
Author information
Authors and Affiliations
Contributions
J.R., A.J.A., C.E.D., and AP designed research; J.R., A.J.A., J.J.K., D.C., C.E.D., A.F., L.A.H., M.M., E.W., and A.P. performed research and analyzed data; A.J.A., J.J.K., D.C., C.E.D., and A.P. contributed analytic tools; J.R., A.J.A., and A.P. wrote the paper; and all authors contributed revisions to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Peer review
Peer review information
Nature Communications thanks Fredrik Christiansen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Roman, J., Abraham, A.J., Kiszka, J.J. et al. Migrating baleen whales transport high-latitude nutrients to tropical and subtropical ecosystems. Nat Commun 16, 2125 (2025). https://doi.org/10.1038/s41467-025-56123-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56123-2