Abstract
During cold acclimation in high-latitude and high-altitude regions, japonica rice develops enhanced cold tolerance, but the underlying genetic basis remains unclear. Here, we identify CTB5, a homeodomain-leucine zipper (HD-Zip) transcription factor that confers cold tolerance at the booting stage in japonica rice. Four natural variations in the promoter and coding regions enhance cold response and transcriptional regulatory activity, enabling the favorable CTB5KM allele to improve cold tolerance. CTB5 interacts with OsHox12 and targets gibberellin (GA) metabolism genes to promote GAs accumulation in anthers and facilitate tapetum development under cold stress. Moreover, CTB5 directly regulates PYL9 and improves cold tolerance at the seedling stage by reducing reactive oxygen species (ROS) accumulation. The CTB5KM allele is selected during the cold acclimation of japonica rice to plateau habitats in Yunnan Province. Our findings provide insights into the mechanisms underlying cold adaptation in plateau japonica rice and offer potential targets for breeding cold-tolerant rice varieties.
Similar content being viewed by others
Introduction
Rice (Oryza sativa L.), a staple crop for over half of the world’s population, originates from tropical and subtropical regions and is sensitive to cold stress1. Low temperature is a major environmental challenge that impacts rice production at all stages. In particular, cold stress at the booting stage disrupts anther development, causes pollen abortion, and reduces seed-setting rate, ultimately affecting grain yield and quality2. Globally, more than 15 million hectares of rice fields in 24 countries, including China, South Korea, India, and Japan, are affected by cold stress3. In China, regions such as the Northeastern Plains, the Yunnan Plateau, and the middle and lower reaches of the Yangtze River are frequently affected by cold stress, resulting in an annual loss of 3–5 million tons of rice production4. Therefore, there is an urgent need for genetic resources to develop cold-tolerant rice varieties and mitigate yield loss in cold-prone areas.
Cold tolerance is a complex quantitative trait controlled by multiple loci and influenced by environmental factors, and is likely regulated by distinct genes and mechanisms at different stages. The discovery and cloning of cold-tolerance genes in rice provide essential genetic resources for biological breeding, which greatly improves the efficiency of developing superior varieties. Despite the discovery of several quantitative trait loci (QTL), only a few genes have been functionally characterized, and even fewer regulate cold tolerance at different stages. qLTG3-1, OsSAP16, OsLTPL159, and OsUBC12 confer cold tolerance at the germination stage5,6,7,8, while COLD1, bZIP73, qPSR10, HAN1, COG1, COLD11, SOP10, and COG3 regulate cold tolerance at the seedling stage9,10,11,12,13,14,15,16. Accurate evaluation of cold tolerance at the booting stage is challenging due to factors such as heading date, evaluation conditions, and external climate. To date, only a few related genes have been cloned through forward genetic approaches. Ctb1 confers cold tolerance at the booting stage, but the underlying mechanism remains unclear17. A de novo mutation in the CTB4a promoter increases its expression, resulting in higher ATP levels and improved cold tolerance18. A single standing variation in the CTB2 coding region improves cold tolerance by increasing sterol glycoside levels and maintaining cell membrane permeability19. A point mutation in LTT1 improves cold tolerance by mediating reactive oxygen species (ROS) homeostasis and promoting anther development20.
Plant hormones are essential regulators of plant growth, development, and responses to environmental stresses. Abscisic acid (ABA) and gibberellin (GA) play crucial roles in plant responses to cold stress. Increased ABA accumulation and signaling enhances plant resistance to abiotic stress, particularly at the seedling stage21,22. For instance, overexpression of PYL3 and PYL9 significantly improves both cold and drought tolerance in rice seedlings23. GAs are a class of tetracyclic diterpenoid hormones synthesized from trans-geranylgeranyl diphosphate. Bioactive GAs are produced from GA12/GA53 catalyzed by GA20-oxidases (GA20ox) and GA3-oxidases (GA3ox), while GA deactivation is mainly catalyzed by GA2-oxidase (GA2ox). GA1, GA3, GA4, and GA7 are considered the major bioactive GAs in plants24. Mutants with defects in GA biosynthesis or signaling show abnormal anther development and reduced seed-setting rate, similar to the effects of cold stress at the booting stage25,26. Levels of bioactive GAs are reduced in cold-treated anthers, while exogenous GA application alleviates cold-induced pollen abortion27,28. These results indicate that GAs are essential for anther development and pollen fertility under cold stress. Homeodomain-leucine zipper (HD-Zip) transcription factors (TFs) are plant-specific proteins that contain a homeodomain (HD) closely linked to a leucine zipper (LZ) ___domain. HD-Zip TFs are involved in multiple plant hormone pathways, enhance plant tolerance to abiotic stress, and modulate plant development under unfavorable conditions29,30.
Asian cultivated rice, derived from its wild relatives Oryza rufipogon and O. nivara, comprises two subspecies: indica and japonica31. Divergent natural selection, driven by contrasting temperatures, facilitates the differentiation between these two species. Japonica rice developed greater cold tolerance as it spread to high-altitude and high-latitude areas32. The Yunnan Plateau is one of the few unique rice-growing regions worldwide, with 60% of the rice grown on the plateau at altitudes of 1500–2700 m. Through long-term domestication and selection, a diverse collection of cold-tolerant plateau japonica rice has been developed, such as Kunmingxiaobaigu (KMXBG), Lijiangxiaoheigu (LJXHG), and Chongtui (CT). These cultivars contain abundant favorable alleles that effectively coordinate development with cold tolerance, facilitating growth adaptation to natural cold habitats.
In this work, we identify a critical gene CTB5, with four natural variations in its promoter and coding regions enhancing cold tolerance at the booting stage in japonica rice. CTB5 interacts with OsHox12 and targets the GA catabolism gene OsGA2ox6 and the GA biosynthesis gene OsGA3ox1, promoting GAs accumulation in anthers and facilitating tapetum development under cold stress. By fine-tuning GAs levels, CTB5 enables rice growth adaptation to natural cold habitats. Furthermore, CTB5 directly regulates PYL9 and positively regulates cold tolerance at the seedling stage by improving ABA signaling and reducing ROS accumulation. Our results show that the CTB5KM allele is selected from the standing variation in wild rice and promotes cold acclimation of japonica rice to plateau habitats in Yunnan Province. These findings highlight the importance of CTB5 in cold tolerance and its potential in rice breeding.
Results
Identification of CTB5 for cold tolerance at the booting stage in rice
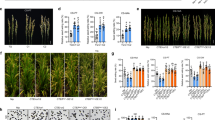
To identify genes for cold tolerance at the booting stage, we previously detected the qCTB7 locus on chromosome 7 in segregating populations from the cross of KMXBG (plateau japonica, cold-tolerant) and Towada (japonica, cold-sensitive). qCTB7 was initially fine-mapped to a 330.2-kb interval between markers RM7237 and RM550833. Here, we identified additional recombinants in the qCTB7 region and narrowed it to a 35-kb interval between markers RM21868 and RM5508 (Fig. 1a). We generated a homozygous near-isogenic line (NIL) with the 35-kb introgressed segment from KMXBG in the Towada background (Supplementary Fig. 1). Using the seed-setting rate as an indicator, cold tolerance at the booting stage was evaluated by three methods: cold stress in a high-altitude area (CS-HAA, altitude 1974 m), in deep water (CS-DW), and in a phytotron (CS-PT) (Supplementary Fig. 2). The NIL showed remarkably higher cold tolerance than Towada at the booting stage (Fig. 1b–d).
a Homozygous recombinants for qCTB7 and their cold tolerance performance. Data represent means ± SD. The number (n) of individuals used for phenotyping were indicated on the bars. b Comparison of field performance between NILCTB5 and Towada under cold stress in a high-altitude area (CS-HAA). c, d Seed-setting rates of NILCTB5 and Towada under normal conditions (NC) and CS-HAA conditions (c), as well as under cold stress in deep water (CS-DW), and in a phytotron (CS-PT) conditions (d). Data represent means ± SD (n = 15 plants for NC and CS-HAA, and n = 11 plants for CS-DW and CS-PT). e Relative expression levels of CTB5 at different time points during cold treatment in NILCTB5 and Towada. Data represent means ± SD (n = 3 biological replicates). f, g Comparison of field performance (f) and panicles (g) between Towada and CTB5 complementation lines under CS-HAA. Scale bar in g = 2 cm. h, i Seed-setting rates of Towada and CTB5 complementation lines under CS-HAA (n = 20/18/18 plants) (h), CS-DW (n = 15/15/10 plants), and CS-PT (n = 15/15/15 plants) (i). j, k Seed-setting rates of Towada and CTB5 overexpression lines under CS-HAA (n = 23/22/19/22/18 plants) (j), CS-DW (n = 13/24/15/10/10 plants), and CS-PT (n = 10/20/12/12/12 plants) (k). l, m Comparison of field performance (l) and panicles (m) between Towada and CTB5 overexpression lines under CS-HAA. Scale bar in m = 2 cm. n, o Comparison of field performance (n) and panicles (o) between Nipponbare and CTB5 knockout lines under CS-HAA. Scale bar in o = 2 cm. p, q Seed-setting rates of Nipponbare and CTB5 knockout lines under CS-HAA (n = 15 plants) (p), CS-DW (n = 10 plants), and CS-PT (n = 10 plants) (q). In (a, c–e), asterisks indicate statistical significance by two-tailed Student’s t-tests (**P < 0.01, ***P < 0.001, data represent means ± SD). In (h–k, p, q), different lowercase letters indicate statistically significant differences at P = 0.05 by one-way ANOVA with Duncan’s multiple-range test, and error bars represent means ± SD. Source data are provided as a Source Data file.
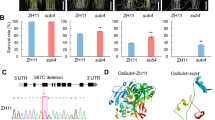
The 35-kb interval contains four open reading frames (ORFs). Genomic sequence comparisons between KMXBG and Towada revealed that the fourth ORF, LOC_Os07g39320, encoding the HD-Zip I transcription factor OsHox14, had 18 single nucleotide polymorphisms (SNPs) in the 2.5-kb promoter region, together with one non-synonymous SNP and one insertion/deletion (InDel) in the coding region. To evaluate whether these variants affect the expression level and transcriptional regulatory activity of LOC_Os07g39320, we performed quantitative real-time PCR (qRT-PCR) and a dual-luciferase reporter assay. LOC_Os07g39320 was significantly induced by cold stress, with higher expression levels and promoter activity (higher LUC/REN ratio) in NIL than in Towada under cold stress (Fig. 1e and Supplementary Fig. 3a). The amino acid variations were located in the conserved HD ___domain and C-terminus, and LOC_Os07g39320KM had stronger transcriptional regulatory activity than LOC_Os07g39320Tow (Supplementary Fig. 3b, c). These results indicated that the variants in the promoter and coding regions enhance the cold response and transcriptional regulatory activity of LOC_Os07g39320 in KMXBG.
We previously conducted a genome-wide association study for cold tolerance at the booting stage using 155 rice germplasms and identified a significant association locus overlapping with qCTB732. Local linkage disequilibrium analysis revealed that this association locus corresponded to a 117-kb interval, with the lead SNP located in the promoter of LOC_Os07g39320 (Supplementary Fig. 3d). Therefore, we proposed that LOC_Os07g39320 is a likely candidate gene for qCTB7 and named it CTB5 (Cold Tolerance at the Booting stage 5).
Phylogenetic analysis showed that CTB5 clustered with known HD-Zip TFs involved in the GA and ABA pathways, such as rice OsHox12 and Arabidopsis HB40, HB53, and HB2134,35,36 (Supplementary Fig. 4a). Sequence alignment of CTB5 homologs from rice, maize, wheat, and sorghum revealed that CTB5 and its homologs share the same HD and LZ domains (Supplementary Fig. 4b). Expression analysis revealed that CTB5 was mainly expressed in the young panicle, anther, sheath, and root, and was significantly induced by both GA and ABA (Supplementary Fig. 5a–h). The CTB5 protein was specifically localized to the nucleus and had a transcriptional activation ___domain at its C-terminus (169–241 aa) (Supplementary Fig. 5i–l).
CTB5 positively regulates cold tolerance at the booting stage
To confirm the candidacy of CTB5, we isolated a 5.1-kb genomic fragment from KMXBG for complementation test in the Towada background. The complementation lines (CP2 and CP5) improved the cold tolerance of Towada (Fig. 1f–i), indicating that CTB5 is the causal gene underlying qCTB7.
To further elucidate the function of CTB5, we used RNA interference (RNAi) to knock down CTB5 expression in the NILCTB5 background (Supplementary Fig. 6a). We also generated CTB5 overexpression (OE) transgenic lines by introducing the full-length KMXBG coding sequence (CDS) into the Towada background (Supplementary Fig. 6b). Moreover, we generated CTB5 homozygous knockout lines using the CRISPR/Cas9 system in the NILCTB5 and Nipponbare backgrounds. The knockout lines carried insertion-deletion mutations at the target sites, resulting in frameshift mutations in the protein (Supplementary Fig. 6c–h). Both the CTB5 RNAi lines (RI2 and RI4) and the knockout lines were sensitive to cold stress compared to the controls (Fig. 1n–q and Supplementary Fig. 7). In contrast, CTB5 OE lines (K-OE3 and K-OE6) showed higher cold tolerance than Towada (Fig. 1j–m). These results demonstrated that CTB5 positively regulates cold tolerance at the booting stage in rice.
Four natural variants facilitate the CTB5 KM allele to increase cold tolerance
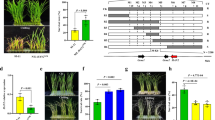
To identify functional variants associated with cold tolerance, we performed an association analysis for CTB5 using variants obtained from the resequencing of 155 rice accessions. Notably, four SNPs in the promoter region and one non-synonymous SNP in the coding region were strongly associated with cold tolerance at the booting stage (Fig. 2a). Site-directed mutagenesis assays using dual-luciferase reporters showed that mutations in proCTB5A-1613G and proCTB5C-1161T increased LUC expression under cold stress (Fig. 2b), suggesting that SNP-1613 and SNP-1161 are functional variants for allelic differences in cold response. Furthermore, mutations in SNP397 and InDel829, located in the HD (+ 94) and transcriptional activation ___domain (+ 205), respectively, resulted in decreased LUC expression (Fig. 2c, d), suggesting that they are functional variants for allelic differences in transcriptional regulatory activity.
a Candidate gene-based association study for CTB5 using 155 rice accessions. Red dots indicate SNPs significantly associated with cold tolerance at the booting stage. b Site-directed mutagenesis of four significant SNPs in the promoter of CTB5 under low-temperature conditions using dual-luciferase reporters. Data are means ± SD (n = 6 biological replicates). c Amino acid variations in CTB5KM and CTB5Tow proteins. d Site-directed mutagenesis of two variants in the coding region of CTB5 using dual-luciferase reporters. EV, empty pGreenII 62-SK vector. Data are means ± SD (n = 5 biological replicates). e Relative expression of CTB5 at different levels of cold treatment in germplasms carrying different CTB5 alleles. Data are means ± SD (n = 6 plants for each allele). f Correlation between cold-induced CTB5 expression and cold tolerance at the booting stage. In (b, d), different lowercase letters indicate statistically significant differences at P = 0.05 by one-way ANOVA with Duncan’s multiple-range test. In (e), asterisks indicate statistical significance by two-tailed Student’s t tests (*P < 0.05, **P < 0.01). Source data are provided as a Source Data file.
To determine whether functional variants in the promoter contribute to the phenotypic differences between the two CTB5 alleles, we quantified CTB5 transcript accumulation at low temperatures in rice germplasms. Germplasms with the CTB5KM allele had higher cold-induced expression than those with the CTB5Tow allele, and we observed a significant positive correlation between the cold-induced expression level of CTB5 and cold tolerance at the booting stage (Fig. 2e, f and Supplementary Fig. 8). Furthermore, CTB5 was significantly induced by low temperatures, with higher induction in NILCTB5 than in Towada (Fig. 1e). In addition, CTB5 overexpression enhanced cold tolerance, whereas knockdown reduced cold tolerance at the booting stage (Fig. 1j–m and Supplementary Fig. 7e–h). These results suggested that functional variants in the CTB5KM promoter correlate with increased induced expression and cold tolerance in rice germplasms.
To clarify the effect of functional variants in the coding region on cold tolerance between the two CTB5 alleles, we overexpressed the CDS of KMXBG and Towada in parallel in the Towada background and selected transgenic lines with similar CTB5 expression levels for cold tolerance evaluation. The CTB5KM OE line (K-OE3) was more cold-tolerant than the CTB5Tow OE lines (T-OE4 and T-OE5) (Fig. 1j–m), indicating that functional variants in the CTB5KM coding region increase cold tolerance at the booting stage. Taken together, these results indicated that the four functional variants (SNP-1613, SNP-1161, SNP397, and InDel829) in the promoter and coding regions enhance the cold response and transcriptional regulatory activity of the CTB5KM allele, facilitating it in increasing cold tolerance at the booting stage.
OsHox12 interacts with CTB5 and regulates cold tolerance at the booting stage
To elucidate the regulatory mechanism of CTB5 in cold tolerance, we identified eight potential CTB5-interacting proteins through yeast library screening and found that OsHox12, another HD-Zip TF, interacts with CTB5 (Supplementary Fig. 9 and Supplementary Table 1). OsHox12 was mainly expressed in the panicle and anther, and was significantly induced by cold (Fig. 3a, b). The OsHox12 protein was specifically localized to the nucleus and had a transcriptional activation ___domain at its C-terminus (152–239 aa) (Supplementary Fig. 10).
a, b Relative expression levels of OsHox12 in different tissues (a) and at different time points during cold treatment (b). Data represent means ± SD (n = 3 biological replicates). c Interactions of OsHox12 with CTB5KM/Tow in yeast. EV, the empty pGADT7 vector. d–f Split-luciferase complementation (d), co-immunoprecipitation (e), and bimolecular fluorescence complementation (f) assays showing the interaction between OsHox12 and CTB5. OsCam1-1 in d and f was used as a negative control. Total proteins from rice protoplasts co-expressing Flag-CTB5 with OsHox12-Myc were immunoprecipitated with Myc beads and detected with anti-Flag antibody in e. Scale bar in f = 5 μm. g Relative expression levels of OsHox12 in OsHox12 overexpression lines. Data represent means ± SD (n = 3 biological replicates). h, i Seed-setting rates of Towada and OsHox12 overexpression lines under cold stress in a high-altitude area (CS-HAA) conditions (n = 17/14/14 plants) (h) and in a phytotron (CS-PT) conditions (n = 12/12/12 plants) (i). j Comparison of panicles between Towada and OsHox12 overexpression lines under CS-HAA. Scale bar = 2 cm. k, l Seed-setting rates of NILCTB5 and OsHox12 knockout lines under CS-HAA (n = 17/14/13 plants) (k) and CS-PT (n = 10/10/10 plants) (l). m Seed-setting rates of NILCTB5 and double mutants of OsHox12 and CTB5 under CS-PT (n = 10 plants). n Comparison of panicles between NILCTB5 and OsHox12 knockout lines under CS-HAA. Scale bar = 2 cm. In (c–f), a representative experiment from at least two independent experiments is shown. In (g–i, k–m), different lowercase letters indicate statistically significant differences at P = 0.05 by one-way ANOVA with Duncan’s multiple-range test, and data represent means ± SD. Source data are provided as a Source Data file.
The interaction between OsHox12 and CTB5 was confirmed by several assays. A yeast two-hybrid (Y2H) assay showed that both CTB5KM and CTB5Tow interacted with OsHox12 in yeast cells (Fig. 3c). A split-luciferase complementation (SLC) assay in Nicotiana benthamiana revealed that co-expression of OsHox12-cLUC and CTB5-nLUC produced a clear luciferase signal (Fig. 3d). A co-immunoprecipitation (Co-IP) assay in rice protoplasts validated that CTB5 was co-immunoprecipitated with OsHox12-Myc (Fig. 3e). Furthermore, a bimolecular fluorescence complementation (BiFC) assay showed that CTB5 interacted with OsHox12 in the nucleus (Fig. 3f). Collectively, these results indicated that CTB5 physically interacts with OsHox12.
To investigate the potential role of OsHox12 in conferring cold tolerance at the booting stage, we generated OsHox12 knockout lines in the NILCTB5 background and OE lines in the Towada background (Fig. 3g and Supplementary Fig. 11). Similar to CTB5, OsHox12 OE lines showed enhanced cold tolerance compared to Towada, whereas knockout lines showed reduced cold tolerance compared to NILCTB5 (Fig. 3h–l, n), indicating that OsHox12 positively regulates cold tolerance at the booting stage. Furthermore, double-knockout mutants of CTB5 and OsHox12 had significantly lower cold tolerance than single knockouts (Fig. 3m), suggesting that they may synergistically regulate cold tolerance at the booting stage.
CTB5 modulates GAs levels in anthers by targeting OsGA2ox6 and OsGA3ox1
To identify downstream genes regulated by CTB5, we compared the transcriptomes of the CTB5 OE line (K-OE3) and Towada under normal and cold stress conditions. We identified 1367 and 1250 differentially expressed genes (DEGs) in K-OE3 compared to Towada under normal and cold stress conditions, respectively (Supplementary Fig. 12a, b). Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis showed that CTB5 was mainly involved in the photosynthesis pathway under normal conditions, but in the diterpenoid biosynthesis pathway under cold stress (Supplementary Fig. 12c, d). Diterpenoid biosynthesis regulates GA homeostasis and has been reported to enhance cold tolerance at the booting stage by influencing tapetum development genes28,37,38. An exogenous application assay showed that GA application increased the seed-setting rates of both CTB5 knockout and OE plants at low temperatures (Fig. 4a, b).
a, b Seed-setting rates of CTB5 knockout (a) and overexpression (b) plants exposed to cold stress with and without gibberellin (GA) application. Data represent means ± SD (n = 11/9, 10/9 plants for a and n = 10/10, 10/10, 7/7 plants for b). c–h The contents of GAs in developing anthers of CTB5 knockout and overexpression plants under normal conditions (NC) and cold stress (CS) conditions (16 °C, 7 days). Data represent means ± SD (n = 3 biological replicates). i, m Yeast one-hybrid assays testing the binding of CTB5 to the promoters of OsGA2ox6 (i) and OsGA3ox1 (m). EV, empty vector. j, n EMSA assays demonstrating that CTB5 binds to the TAATTA motifs of the OsGA2ox6 (j) and OsGA3ox1 (n) promoters. k, o ChIP-qPCR assays revealing that CTB5 binds to the OsGA2ox6 (k) and OsGA3ox1 (o) promoters in vivo. Data represent means ± SD (n = 3 biological replicates). l, p Dual-luciferase reporter assays confirming the binding of CTB5 to promoters of OsGA2ox6 (l) and OsGA3ox1 (p) in rice protoplasts. EV, empty pGreenII 62-SK vector. Data represent means ± SD (n = 5 biological replicates for l and n = 4 biological replicates for p). q–t Dual-luciferase reporter assays showing that OsHox12 enhances the regulatory effect of CTB5 on OsGA2ox6 and OsGA3ox1 in rice protoplasts (q, s) and tobacco leaves (r, t). EV, empty pGreenII 62-SK vector. Data represent means ± SD (n = 4 biological replicates for q and s, and n = 5 biological replicates for r and t). In (i–t), a representative experiment from at least two independent experiments is shown. In (a, b, k, o), asterisks indicate statistical significance by two-tailed Student’s t tests (*P < 0.05, **P < 0.01, ***P < 0.001). In (c–h, l, p–t), different lowercase letters indicate statistically significant differences at P = 0.05 by one-way ANOVA with Duncan’s multiple-range test. Source data are provided as a Source Data file.
To investigate whether CTB5 is involved in the GA pathway, we measured the levels of various GA components in anthers of CTB5 transgenic and wild-type plants under normal and cold stress conditions using HPLC-MS/MS. GA1, GA3, GA4, and GA7 are the major bioactive GAs in rice with distinct tissue distributions: GA1 and GA3 mainly in vegetative organs, whereas GA4 and GA7 mainly in reproductive organs38. In our study, we also observed significantly higher levels of GA4 and GA7 in anthers than GA1 and GA3 (Fig. 4c, d). Under normal conditions, no significant differences in the levels of bioactive GAs were found between CTB5 knockout, OE, and wild-type plants (Fig. 4c, d). However, after 7 days of cold treatment at 16 °C, the levels of GA4 and GA7 were significantly reduced in all plant types, with knockout plants showing lower levels than Nipponbare and OE plants showing higher levels than Towada (Fig. 4c, d). Similarly, the levels of the intermediate precursors GA9 and GA15 and the product GA34 were lower in CTB5 knockout plants than in Nipponbare and higher in OE plants than in Towada (Fig. 4e–h). In contrast, the levels of GA1, GA3, their intermediate precursors GA19 and GA20, and the product GA8 were consistently low under both conditions, with similar levels between CTB5 knockout, OE, and wild-type plants (Fig. 4c–h). These results indicated that CTB5 mediates GA homeostasis and promotes the accumulation of bioactive GAs in anthers under cold stress.
To elucidate the mechanism by which CTB5 regulates GA-mediated cold tolerance at the booting stage, we analyzed the expression changes of 18 GA metabolism genes in CTB5 transgenic and control plants using transcriptome data and qRT-PCR. CTB5 affected the expression of both catabolism and biosynthesis genes under normal and cold stress conditions, with their dynamic balance maintaining GA homeostasis (Supplementary Figs. 12e, 13, 14, and Supplementary Table 2). In particular, the expression of the catabolism genes OsGA2ox4 and OsGA2ox6 and the biosynthesis genes OsGA20ox3 and OsGA3ox1 was significantly regulated by CTB5 (Supplementary Figs. 13d, f and 14f, g). Under cold stress, OsGA2ox6 expression decreased in CTB5 OE lines but increased in knockout lines, while OsGA3ox1 showed the opposite pattern (Supplementary Figs. 13f, 14g). Tissue expression analysis showed that OsGA2ox6 and OsGA3ox1 were mainly expressed in the anther during the reproductive stage (Supplementary Fig. 15a, b, and Supplementary Table 2). Cold-induced expression analysis revealed that both genes responded to low temperatures, with lower OsGA2ox6 and higher OsGA3ox1 expression in NILCTB5 than in Towada (Supplementary Fig. 15c, d).
HD-Zip I TFs bind to the AH1 motif (CAAT(A/T)ATTG) and the conserved site (TAATTA) in target promoters34,39. To test whether CTB5 directly regulates OsGA2ox6 and OsGA3ox1, we analyzed the promoters of both genes and identified the TAATTA motifs. Yeast one-hybrid (Y1H) assays showed that CTB5 directly interacted with the promoter segments of OsGA2ox6 and OsGA3ox1 (Fig. 4i, m). DNA electrophoretic mobility shift assays (EMSA) showed that the CTB5-MBP fusion protein could bind to the probes containing the TAATTA motif but not to the mutated ones (Fig. 4j, n). Chromatin immunoprecipitation (ChIP) analyses confirmed that only the fragments containing the TAATTA motif were significantly enriched (Fig. 4k, o). Dual-luciferase reporter assays revealed that CTB5 repressed OsGA2ox6 and activated OsGA3ox1, with CTB5KM exhibiting stronger regulatory effects than CTB5Tow (Fig. 4l, p). Furthermore, co-expression of OsHox12 and CTB5 significantly suppressed the LUC expression of OsGA2ox6 and enhanced that of OsGA3ox1, suggesting that OsHox12 promoted the regulatory effects of CTB5 on both genes (Fig. 4q–t). Taken together, these results indicated that CTB5 directly regulates OsGA2ox6 and OsGA3ox1 and confers cold tolerance at the booting stage by facilitating the accumulation of bioactive GAs in anthers under cold stress.
CTB5 regulates tapetum development and pollen fertility under cold stress
Tapetum cells supply necessary nutrients for the developing microspores, and premature or delayed tapetum degradation often causes pollen abortion40. Cold stress at the booting stage has been reported to delay tapetum degradation27. To clarify the histological mechanisms by which CTB5 regulates cold tolerance at the booting stage, we examined the expression of key genes involved in tapetum development, including EAT1, LAP6, and DPW3. Under cold stress, the expression of these genes was significantly lower in Nip-ctb5-cr2 than in Nipponbare, and significantly higher in K-OE3 than in Towada. However, no significant differences in the expression levels of these genes were found between Nip-ctb5-cr2 and Nipponbare, or between K-OE3 and Towada under normal conditions (Fig. 5a).
a Relative expression levels of EAT1, LAP6, and DPW3 in CTB5 knockout and overexpression lines under normal and cold stress conditions. Data represent means ± SD (n = 3 biological replicates). b, c Histological analysis of anther development in NILCTB5 and Towada (b), as well as Nipponbare and CTB5 knockout (c) plants under cold stress in a high-altitude area (CS-HAA) conditions. Scale bar = 10 μm. Ep, epidermal cell layer; En, endothelial cell layer; YM, microspore; VP, vacuole pollen; BP, bicellular pollen; MP, mature pollen; T, tapetum. d Pollen staining of NILCTB5, Towada, Nipponbare, and CTB5 knockout plants under normal conditions (NC) and CS-HAA. Scale bar = 100 μm. e, f Stained pollen rates of NILCTB5 and Towada (e), as well as Nipponbare and CTB5 knockout (f) plants under NC and CS-HAA. Data represent means ± SD (n = 10 biological replicates). In (a, e, f), asterisks indicate statistical significance by two-tailed Student’s t-tests (*P < 0.05, **P < 0.01, ***P < 0.001). Source data are provided as a Source Data file.
By examining the dynamic development of the tapetum and microsporocytes, we found that under normal conditions, there were no significant differences in tapetum degradation and microspore development between NILCTB5 and Towada or between Nipponbare and Nip-ctb5-cr2 (Supplementary Fig. 16). Under CS-HAA, at stage 9, little difference in tapetum and microspores was observed between NILCTB5 and Towada or between Nipponbare and Nip-ctb5-cr2. However, at stage 10, the tapetums of Towada and Nip-ctb5-cr2 plants became thicker than those of NILCTB5 and Nipponbare. At stage 11 and late stage 11, the tapetums of Towada and Nip-ctb5-cr2 didn’t degenerate in time, while those of NILCTB5 and Nipponbare underwent normal degradation, suggesting that the tapetums of NILCTB5 and Nipponbare were not damaged by low temperatures compared to Towada and Nip-ctb5-cr2. At stage 12, the pollen grains of Towada and Nip-ctb5-cr2 aborted, whereas those of NILCTB5 and Nipponbare matured normally and were filled with reserve substances (Fig. 5b, c). Further examination of pollen fertility showed that the changes in stained pollen rates of NILCTB5, Towada, Nipponbare, and CTB5 transgenic plants were consistent with their cold tolerance at the booting stage (Fig. 5d–f and Supplementary Fig. 17). Taken together, these results indicated that CTB5 enhances cold tolerance at the booting stage by promoting tapetum development and pollen fertility under cold stress.
CTB5 mediates rice growth adaptation to natural cold habitats
HD-Zip TFs regulate plant growth plasticity and development in response to environmental stimuli30. To investigate the role of CTB5 in regulating rice growth and development, we examined the morphological characteristics of CTB5 OE lines under normal and CS-HAA conditions. Under normal conditions, both K-OE3 and T-OE4 exhibited typical GA-deficient phenotypes characterized by semi-dwarfism, delayed heading date, and reduced panicle exertion compared to Towada (Supplementary Fig. 18a–e). In addition, K-OE3 and T-OE4 had shorter panicle lengths and fewer primary and secondary branches, grains per panicle, and yield per plant than Towada (Supplementary Fig. 18f–j). The changes in the morphological traits of the CTB5 OE lines were consistent with their decreased GAs levels under normal conditions (Supplementary Fig. 19).
In contrast to the phenotypic changes observed under normal conditions, K-OE3 and T-OE4 showed attenuated changes in plant height, heading date, panicle exsertion, and other panicle-related traits under CS-HAA compared with Towada (Supplementary Fig. 18a–h). Notably, K-OE3 had a higher grain number per panicle and yield per plant than Towada under CS-HAA (Supplementary Fig. 18i, j). These results suggested that CTB5 is involved in the growth adaptation of rice to natural cold stress.
CTB5 directly regulates PYL9 and confers cold tolerance at the seedling stage
Tissue expression analysis showed that CTB5 was highly expressed not only in the panicle and anther, but also in vegetative organs such as the root, sheath, and leaf (Supplementary Fig. 5a–d). To investigate the potential role of CTB5 in cold tolerance at the seedling stage, we evaluated the cold tolerance of NILCTB5, Towada, Nipponbare, and CTB5 transgenic plants at this developmental stage. After 4 days of cold treatment at 4 °C followed by 7 days of recovery, the CTB5 knockout lines showed significantly lower survival rates than Nipponbare, whereas NILCTB5 and CTB5 OE lines showed significantly higher survival rates than Towada, suggesting that CTB5 positively regulates cold tolerance at the seedling stage in rice (Fig. 6a–c and Supplementary Fig. 20a, b).
a Performance of seedlings of CTB5 knockout and overexpression lines before and after recovery from cold treatment at 4 °C for 4 days. b, c Survival rates of CTB5 knockout (b) and overexpression (c) lines after recovery from cold treatment. Data represent means ± SD (n = 3/3, 4/4 biological replicates for b, and n = 4/4, 4/4 biological replicates for c). d, e Relative expression levels of PYL9 in CTB5 knockout (d) and overexpression (e) lines under normal conditions (NC) and cold stress (CS) conditions (4 °C, 8 h). Data represent means ± SD (n = 3 biological replicates). f–i Yeast one-hybrid (f), EMSA (g), ChIP-qPCR (h), and dual-luciferase reporter (i) assays demonstrating that CTB5 binds to the AH1 motif of the PYL9 promoter. A representative experiment from at least two independent experiments is shown. EV in f and i indicates the empty pB42AD and pGreenII 62-SK vector, respectively. Data represent means ± SD (n = 3 biological replicates for h, and n = 5 biological replicates for i). j Performance of seedlings of Towada and PYL9 overexpression lines before and after recovery from cold treatment at 4 °C for 4 days. k Relative expression levels of PYL9 in PYL9 overexpression lines. Data represent means ± SD (n = 3 biological replicates). l Survival rates of Towada and PYL9 overexpression lines after recovery from cold treatment. Data represent means ± SD (n = 3 biological replicates). m, n H2O2 (m) and MDA (n) content of CTB5 and PYL9 overexpression lines under different low-temperature conditions. Data represent means ± SD (n = 3 biological replicates). In (b–e, h, k–n), asterisks indicate statistical significance by two-tailed Student’s t tests (*P < 0.05, **P < 0.01, ***P < 0.001). In (i), different lowercase letters indicate statistically significant differences at P = 0.05 by one-way ANOVA with Duncan’s multiple-range test. Source data are provided as a Source Data file.
OsHox12, a paralog and interacting protein of CTB5, has been reported to be involved in the ABA signaling pathway34. Expression analysis showed that ABA significantly induced CTB5 expression, and CTB5 regulated key ABA signaling and response genes such as SAPK6, SAPK9, bZIP23, bZIP62, and LEA3 (Supplementary Figs. 5h and 20c, d), suggesting that CTB5 may be involved in ABA signaling. By analyzing the promoters of ABA signaling genes, we identified PYL9, an ABA receptor gene with an AH1 motif in its promoter, which positively regulates cold tolerance in rice seedling23. PYL9 was mainly expressed in vegetative organs such as root, sheath, and leaf, and could be induced by ABA (Supplementary Fig. 21a, b). Moreover, low temperatures significantly induced PLY9 expression, with stronger induction in NILCTB5 than in Towada (Supplementary Fig. 21c). In addition, PYL9 showed significantly lower expression in CTB5 knockout lines than in Nipponbare and higher expression in CTB5 OE lines than in Towada (Fig. 6d, e). These results suggested that PYL9 may be a target of CTB5 in regulating cold tolerance at the seedling stage.
Using Y1H, EMSA, ChIP-qPCR, and dual-luciferase reporter assays, we demonstrated that CTB5 directly bound to the AH1 motif in the PYL9 promoter to promote its expression, with CTB5KM exhibiting a stronger effect than CTB5Tow, while OsHox12 enhanced the regulatory effect of CTB5 on PYL9 (Fig. 6f–i). Overexpression of PYL9 significantly increased the survival rate compared to Towada after 4 days of treatment at 4 °C, indicating that PYL9 positively regulates cold tolerance at the seedling stage (Fig. 6j–l). Given that ABA generally modulates abiotic stress tolerance by affecting ROS homeostasis, we further examined H2O2 and MDA levels in CTB5 and PYL9 OE lines and found that the levels of H2O2 and MDA in these lines were significantly lower than those in Towada under cold stress (Fig. 6m, n). These results suggested that CTB5 directly regulates PYL9 and confers cold tolerance at the seedling stage by improving ABA signaling and reducing ROS accumulation.
The CTB5 KM allele contributes to cold acclimation in plateau japonica rice
To elucidate the natural variation and identify the favorable allele of CTB5 in germplasm resources, we performed haplotype analysis using 155 rice samples (Supplementary Data 1). Two major haplotypes were identified in the japonica subspecies: the KMXBG-type haplotype Hap1-KM and the Towada-type haplotype Hap2-Tow, with Hap2-Tow being the most common. Two haplotypes were identified in the indica subspecies, with Hap3 being the most common (Fig. 7a). Hap1-KM showed greater cold tolerance than the other haplotypes at the booting stage (Fig. 7b).
a Haplotype analysis of CTB5 in 155 rice germplasms. Jap, japonica. Ind, indica. TemJ, temperate japonica. TroJ, tropical japonica. No., the number of samples used. SNPs from KMXBG and Towada are highlighted in blue and orange shading, respectively. b Seed-setting rates of main haplotypes in japonica and indica subspecies under cold stress. The middle, upper, and lower lines in the violin plot represent the median and two quartiles of values in each group (n = 14/56, 82/3 plants). c, d Haplotype network (c) and phylogenetic tree (d) of CTB5 using 275 wild and 709 cultivated accessions. e Distribution of CTB5 haplotypes in wild rice from different sources. SEA, Southeast Asia. SA, South Asia. SC, Southern China. f Global distribution of wild and cultivated accessions used. g Distribution of cultivated accessions with different CTB5 haplotypes in China. I, South China.II, Central China. III, Southwest China. IV, North China. V, Northeast China. h, i Distribution of CTB5 haplotypes in landraces (LAN) and improved varieties (IMP) from Yunnan Province (h) and Northeast China (i). j Distribution of Hap1-KM-containing varieties in Yunnan Province. Source data are provided as a Source Data file.
To investigate the allelic origin and spread of CTB5, we constructed a haplotype network and phylogenetic tree using 275 wild and 709 cultivated rice samples (Supplementary Data 2). The haplotype network showed that CTB5 underwent differentiation in wild rice, with Hap1-KM and Hap2-Tow originating from Ruf1 and Hap3 derived from Niv2 (Fig. 7c). Phylogenetic analysis revealed that Hap1-KM and Hap2-Tow were predominant in temperate japonica, with Hap1-KM clustering closely with Ruf1 (Fig. 7d). Moreover, the haplotype distribution in wild rice suggested that Hap1-KM and Hap2-Tow originated from southern China, while other haplotypes originated from southeast Asia or south Asia (Fig. 7e and Supplementary Data 2).
Geographical distribution analysis showed that Hap2-Tow was widely distributed in Northeast, North, and Central China, Japan, and North Korea, whereas Hap1-KM was mainly found in Yunnan Province (Fig. 7f, g). Further examination of allele proportions in landraces and improved varieties revealed a high prevalence of Hap2-Tow in both categories of temperate japonica in Northeast and North China. In contrast, Hap1-KM was concentrated in temperate japonica within Yunnan Province, accounting for 61.1% of landraces and 37.6% of improved varieties (Fig. 7h, i, Supplementary Fig. 22a–c, and Supplementary Data 2). The geographical distribution of improved varieties with Hap1-KM in Yunnan Province showed that these varieties were mainly distributed in cold plateau regions above 1600 m (Fig. 7j).
To evaluate whether CTB5 was selected during domestication, we analyzed nucleotide diversity in 396 wild and 2449 cultivated rice samples (Supplementary Data 3). Compared with indica (π = 0.0658) and tropical japonica (π = 0.1027), the nucleotide diversity of CTB5 was much lower in temperate japonica (π = 0.0080) than in wild rice (π = 0.0925) (Supplementary Fig. 22d and Supplementary Table 3). To determine whether the reduction in nucleotide diversity was due to selection, we analyzed the nucleotide diversity of the 100-kb flanking region, as selection typically induces genomic changes. We observed a significant decrease in nucleotide diversity within this region for temperate japonica compared to wild rice, suggesting that CTB5 may have undergone selection in temperate japonica (Supplementary Fig. 22e and Supplementary Table 3). In addition, the neutral test indicated that Tajima’s D for CTB5 was significantly negative in temperate japonica (Supplementary Table 3), indicating that CTB5 has undergone directional selection in this subspecies, resulting in a decrease in nucleotide diversity. These results suggested that Hap1-KM and Hap2-Tow originated from standing variations in wild rice from southern China and underwent selection during domestication. During cold acclimation of japonica rice, Hap1-KM facilitates the expansion of temperate japonica into the cold plateau regions of Yunnan Province (Supplementary Fig. 22f).
The CTB5 KM allele enhances rice yield under natural cold habitats
To evaluate the breeding potential of CTB5, we examined the yield components of NILCTB5 and Towada under normal and CS-HAA conditions. Under normal conditions, no significant differences were observed between NILCTB5 and Towada in terms of effective panicle number, grain number per panicle, 1000-grain weight, seed-setting rate, and yield per plant. However, under CS-HAA conditions, NILCTB5 exhibited a significantly higher seed-setting rate and yield per plant compared to Towada (Fig. 8a–g), indicating that the CTB5KM allele can effectively mitigate yield losses under natural cold habitats by improving cold tolerance at the booting stage.
a, b Comparison of whole plants (a) and panicles (b) between NILCTB5 and Towada under normal conditions (NC) and cold stress in high-altitude area (CS-HAA) conditions. Scale bar = 5 cm. c–g Comparison of effective panicle number (c), grain number per panicle (d), 1000-grain weight (e), seed-setting rate (f), and yield per plant (g) between NILCTB5 and Towada under NC and CS-HAA. Data represent means ± SD (n = 15/15, 30/30 plants for c, d and f–h, and n = 15/15, 15/15 plants for e). Asterisks indicate statistical significance by two-tailed Student’s t tests (*P < 0.05, ***P < 0.001). h Model of how CTB5 determines rice cold tolerance in cold plateau areas. Source data are provided as a Source Data file.
To evaluate the utilization of CTB5 in breeding, we analyzed the presence of the CTB5KM allele in 158 varieties from major japonica-producing provinces in China, including Heilongjiang, Yunnan, and Jiangsu. About 36.2% of the main varieties in Yunnan, including Fengdao 29, Lijing 2, Lijing 9, and Lijing 14, have utilized the CTB5KM allele to ensure grain yield in cold plateau regions above 1850 m. For instance, the average yield of Lijing 9 reached 8582 kg/hm2 when grown at altitudes of 2190–2400 m in Yunnan Province41. However, the main varieties in Heilongjiang and Jiangsu have not yet utilized the CTB5KM allele (Supplementary Data 2). These results suggested that CTB5 holds promising potential for cold-tolerance breeding in rice, particularly in regions where the CTB5KM allele remains underutilized.
Discussion
Cold tolerance at the booting stage is a complex quantitative trait influenced by multiple factors. To date, only a few genes have been cloned through forward genetics approaches18,19,20,42. HD-Zip I TFs are crucial for plant growth and stress resistance. In this study, we cloned a gene CTB5, which encodes an HD-Zip I TF that regulates cold tolerance at the booting stage in japonica rice (Fig. 1). Four natural variants in the promoter and coding regions enhanced the cold response and transcriptional regulatory activity of the CTB5KM allele, enabling it to confer increased cold tolerance at the booting stage (Fig. 2). Due to the differential regulatory activity conferred by the functional variants in the coding region, CTB5KM exhibited a stronger effect on target genes such as the GA catabolism gene OsGA2ox6 and the GA biosynthesis gene OsGA3ox1, resulting in improved cold tolerance at the booting stage (Fig. 4). As the differential cold response of CTB5 alleles contributed to the variation in cold tolerance, further efforts are underway to identify the upstream regulators that interact with functional variants in the CTB5 promoter to influence cold response and tolerance.
HD-Zip TFs typically form homodimers or heterodimers with other members of the same subfamily, and dimerization is a prerequisite for their DNA-binding capability43. In this study, we identified OsHox12, another HD-Zip I TF that interacts with CTB5 (Fig. 3c–f). Both CTB5 and OsHox12 were highly expressed in the anther and synergistically regulated cold tolerance at the booting stage (Fig. 3a, b, g–n). Previous studies have shown that when HD-Zip TFs bind to DNA as dimers, the heterodimeric form exhibits greater stability than the homodimeric counterpart44. Co-expression of CTB5 and OsHox12 resulted in significant changes in LUC expression compared to CTB5 alone (Figs. 4q–t, 6i), suggesting that OsHox12 interacts with CTB5 to form heterodimers that enhance the regulatory effect of CTB5 on target genes.
The phytohormone GA is a critical regulator of cold tolerance at the booting stage28. Cold stress significantly reduces GA levels in anthers, resulting in reduced pollen fertility and seed-setting rate38. In this study, we demonstrated that CTB5 bound directly to the promoters of the GA catabolism gene OsGA2ox6 and the GA biosynthesis gene OsGA3ox1, repressing OsGA2ox6 and activating OsGA3ox1. This regulation results in increased accumulation of bioactive GAs in anthers, thereby alleviating cold-induced pollen abortion (Fig. 4c–t). Notably, OsGA2ox6 was highly expressed in the panicle, and its over-accumulation significantly reduced bioactive GA levels, resulting in severe dwarfism and significantly reduced seed-setting rate45,46. OsGA3ox1 was mainly expressed in the tapetum and is critical for maintaining bioactive GA levels in anthers25. Our results revealed that both OsGA2ox6 and OsGA3ox1 were mainly expressed in anthers and responsive to cold stress (Supplementary Fig. 15), which is consistent with previous studies and supports their role in cold tolerance at the booting stage.
HD-Zip I TFs contribute to the adaptation of plant growth to external stimuli47. CTB5 OE plants exhibited GA-deficient phenotypes under normal conditions, but these defects were significantly alleviated under CS-HAA conditions (Supplementary Fig. 18). Through expression analysis, Y1H, EMSA, ChIP-qPCR, and dual-luciferase reporter assays, we demonstrated that CTB5 could bind to the promoter of the GA biosynthesis gene OsGA20ox3 and repress its expression (Supplementary Figs. 14f, 23). OsGA20ox3 was expressed in roots, leaves, and sheaths during the early vegetative stage and in anthers during the reproductive stage, and its knockdown caused a GA-deficient phenotype with semi-dwarfism and reduced seed-setting rate48. This tissue expression pattern is consistent with our observations (Supplementary Fig. 23a), suggesting that OsGA20ox3 is critical for both vegetative growth and anther development in rice.
Based on the distinct spatio-temporal expression patterns of OsGA2ox6, OsGA3ox1, and OsGA20ox3, it was proposed that CTB5 primarily repressed OsGA20ox3 during the early vegetative stage (Supplementary Figs. 15a, b, 23 and Supplementary Table 2). Over-accumulation of CTB5 resulted in reduced levels of bioactive GAs in vegetative organs and a GA-deficient phenotype (Supplementary Fig. 18a–e), consistent with phenotypes in plants overexpressing CTB5 paralogs such as OsHox12 and HB4034,35. During the reproductive stage, CTB5 regulated OsGA2ox6 and OsGA3ox1 to promote the accumulation of bioactive GAs in anthers (Fig. 4c–t). In addition, increased CTB5 expression up-regulated OsGA20ox2 expression (Supplementary Fig. 14e), potentially compensating for the decreased bioactive GA levels caused by OsGA20ox3 repression in anthers due to the functional redundancy in GA20ox proteins25. As a result, CTB5 accumulation maintained bioactive GA levels in anthers, ensuring normal pollen fertility and seed-setting rate (Supplementary Fig. 17a, b, i). However, under CS-HAA conditions, the enhanced regulation of CTB5 on GA metabolism genes, such as OsGA2ox6, OsGA2ox7, and OsGA20ox2, enabled CTB5 OE plants to maintain bioactive GA levels and alleviate the phenotype deficiency (Supplementary Figs. 13, 14 and 18). In anthers under cold stress, cold-induced CTB5 expression further enhanced its regulation on OsGA2ox6 and OsGA3ox1, which promoted the accumulation of bioactive GAs and reduced cold-induced pollen abortion, thereby improving seed-setting rate (Fig. 4c–t). The phenotypic changes in CTB5 OE plants are similar to those in wheat overexpressing TaHDZipI-5, which exhibited a GA-deficient phenotype under well-watered conditions and had minimal differences under drought stress44. These results suggested that CTB5 is involved in the adaptation of rice growth to cold plateau habitats.
Bifunctional transcription factors capable of both activating and repressing transcription have been identified in several plant species, including SlERF.H5 and SlERF.H7 in tomato49, AtNLP7, WUSCHEL, and APETALA2 in Arabidopsis50,51,52, ZmCCT in maize53, and MtNLP1 in M. truncatula54. For instance, AtNLP7 regulated the early nitrate response in Arabidopsis by both activating and repressing different target genes, probably by interacting with different partners50. In this study, CTB5 activated OsGA3ox1 but repressed OsGA2ox6 and OsGA20ox3 (Fig. 4i–p and Supplementary Fig. 23b–f), indicating that CTB5 functions as a bifunctional transcription factor. The dual-luciferase reporter assay showed that different transcription factors interacting with CTB5 modulated its regulatory activity. OsHox12 and OsbZIP24 enhanced CTB5 regulation on OsGA3ox1 and OsGA20ox3, while OsWRKY76 and OsIAA19 specifically enhanced CTB5 activation on OsGA3ox1 without affecting its repression on OsGA20ox355,56 (Supplementary Fig. 24). These results suggested that the bifunctionality of CTB5 may depend on the recruitment of different interacting partners, which is crucial for maintaining GA homeostasis under different conditions and coordinating growth adaptation and stress resistance in rice.
The discovery of genes that confer cold tolerance at multiple stages is of great importance for rice breeding. CTB5 regulates cold tolerance at both the seedling and booting stages (Figs. 1f–q and 6a–c) and increases rice yield under cold stress (Fig. 8a–g), suggesting that CTB5 is an important target for cold-tolerance breeding in rice. Besides the GA metabolism genes, CTB5 also targeted the ABA receptor gene PYL9 to regulate cold tolerance at the seedling stage (Fig. 6), suggesting that CTB5 may confer cold tolerance at the seedling and booting stages through different mechanisms (Fig. 8h). ABA signaling mediates ROS production in anthers, which are critical signals for initiating programmed cell death (PCD) in the tapetum57. A loss-of-function mutation in SAPK2, a component of the ABA signaling pathway, impaired ABA-induced ROS production in anthers, resulting in abnormal tapetal PCD and reduced pollen fertility58. Our results revealed that the ABA signaling and response genes PYL9, SAPK6, SAPK9, bZIP23, bZIP62, and LEA3 were significantly up-regulated in anthers of CTB5 OE lines compared to Towada, but decreased in knockout lines compared to Nipponbare under cold stress (Supplementary Fig. 25a–f). Moreover, PYL9 OE lines showed a higher seed-setting rate than Towada under three cold stress conditions (Supplementary Fig. 25g–j). These results suggested that CTB5 regulates PYL9 and enhances ABA signaling in anthers, which may also contribute to pollen development under cold stress.
To adapt to diverse ecological environments, rice has evolved cold-tolerance genes with different origins and spread routes. We observed that CTB5 has undergone differentiation in wild rice, with the CTB5KM and CTB5Tow alleles in japonica originating from Ruf1 and the allele in indica originating from Niv2 (Fig. 7c–e). Although the CTB5KM and CTB5Tow alleles have similar origins, they are distributed in different regions. The CTB5Tow allele was widely distributed in japonica-growing regions, whereas the CTB5KM allele was concentrated in the Yunnan Plateau (Fig. 7f–j), suggesting that the CTB5KM allele may contribute to the cold acclimation of japonica to Yunnan Plateau habitats (Supplementary Fig. 22f). The origin and dispersal of CTB5 are similar to those of the reported cold-tolerance genes CTB4a, Ctb1, OsLEA9, and HAN1, which have facilitated cold acclimation of temperate japonica in high-latitude and high-altitude regions12,17,18,59. The distribution of rice germplasms is determined by multiple genes related to cold tolerance or other domestication traits. In addition to CTB5, other cold-tolerance genes may also contribute to the distribution observed in this study. Our results elucidated the origin and spread of the cold-tolerance gene in plateau japonica, offering insights into the mechanisms underlying cold acclimation in japonica rice.
Methods
Plant materials and growth conditions
In our previous study, the qCTB7 locus was identified and initially fine-mapped using a backcross population derived from the Yunnan cold-tolerant plateau japonica landrace KMXBG and the Japanese cold-sensitive japonica cultivar Towada33. Here, we constructed BC8F2:4 populations to further fine-map qCTB7. A near-isogenic line (NILCTB5, BC8F5) was developed from recombinant HYD30-42 containing an introgressed segment from KMXBG within the newly delineated 35-kb interval in the Towada background.
KMXBG, Towada, and Nipponbare were used for genetic transformation. CTB5 GUS lines, complementation lines, OE transgenic lines, and OsHox12 and PYL9 OE lines were generated in the Towada background. CTB5 RNAi transgenic lines, OsHox12 knockout lines, and double knockout mutants of CTB5 and OsHox12 were generated in the NILCTB5 background. CTB5 knockout lines were generated in both Nipponbare and NILCTB5 backgrounds.
All rice plants were grown at experimental farms in Beijing or Hainan under natural paddy conditions, or in Yunnan under natural low-temperature conditions. Homozygous transgenic plants of the T1 generation or later, confirmed by PCR or sequencing, were used for phenotypic evaluation.
Evaluation of cold tolerance
Three methods, including cold stress in a high altitude area (CS-HAA), in deep water (CS-DW), and in a phytotron (CS-PT), were used to evaluate cold tolerance at the booting stage. For CS-HAA, rice plants were planted in summer in the experimental field of Yunnan Academy of Agricultural Sciences in Songming County, Yunnan Province, where the altitude is above 1974 m, and the minimum daily temperature is below 20 °C. The average seed-setting rate of the main panicles was measured after harvest. For CD-DW and CS-PT, rice plants were planted in summer in the experimental field of China Agricultural University in Beijing. Rice plants at the booting stage were transferred to a pool irrigated with cold water (16–18 °C) or a phytotron (15–17 °C) for 7 days and then transplanted back to the field. The relative seed-setting rate was assessed after harvest.
To evaluate cold tolerance at the seedling stage, germinated seeds were placed on PCR plates and grown in Hoagland’s solution in a plant growth chamber (28 °C, 14 h day/10 h night photoperiod and 80% relative humidity). Two-week-old seedlings were treated at 4 °C for 4 days, followed by recovery at 28 °C for 7 days. Cold tolerance was assessed by survival rate, with a minimum of 45 plants evaluated for each line.
Fine-mapping of qCTB7
A BC8F2 population of 8075 individuals, constructed from a cross between KMXBG and Towada, was grown in the winter of 2014 in Sanya City, Hainan Province, and 44 recombinants were identified between markers RM7237 and RM5508. The BC8F3 families, with 40 plants per recombinant, were planted in the summer of 2015 in Kunming City, Yunnan Province, to identify homozygous recombinants. Homozygous recombinants and their corresponding controls within the same family were planted in the summer of 2016 in Kunming to evaluate cold tolerance at the booting stage. Open reading frames were predicted in RGAP (http://rice.uga.edu/). Primer sequences for fine mapping are provided in Supplementary Data 4.
Vector construction and genetic transformation
To construct the complementation vector, a 5.1-kb genomic fragment of CTB5 was amplified from NILCTB5 and cloned into the pMDC163 vector. The RNAi vector was constructed by amplifying two 265-bp reverse complementary sequences at the 3’ end of CTB5 from young panicle cDNA of NILCTB5 and cloning them into the PTCK303 vector. The OE vector was constructed by amplifying the full-length CDSs of CTB5 from young panicle cDNA of NILCTB5 and Towada and cloning them into the PCM1307 vector with the CaMV35S promoter and Flag tag. The knockout vector was constructed by ligating target sequences from the first two exons of CTB5 to the SK-gRNA vector and then to the pC1300-Cas9 vector. To construct the GUS vector, a 2640-bp sequence upstream of the start codon of CTB5 was amplified from Towada and cloned into the pMDC162 vector. The green fluorescent protein (GFP) vector was generated by amplifying the full-length CDS of CTB5 from the young panicle cDNA of NILCTB5 and cloning it into the PS1300GFP vector with the CaMV35S promoter. OsHox12 and PYL9 OE vectors were constructed by amplifying full-length CDSs from young panicle cDNA of NILCTB5 and cloning them into the PS1300Myc vector with the Super promoter and Myc tag. OsHox12 knockout or double knockout vectors for OsHox12 and CTB5 were constructed by cloning target sequences in the first exon into the pBUE411 vector. Primer sequences for vector construction are provided in Supplementary Data 4.
All plasmids verified by sequencing were transformed into Agrobacterium tumefaciens strain EHA105 and transferred into the calli of recipient materials using Agrobacterium-mediated methods.
Gene expression analysis
For cold-induced expression analysis, NILCTB5, Towada, Nipponbare, and CTB5 transgenic plants at the booting stage were subjected to cold treatment at 16 °C in a phytotron. Panicle and anther samples were collected at different time points during chilling treatment. Different tissues of NILCTB5 were sampled at both seedling and heading stages for tissue expression analysis. For the analysis of induced expression at the seedling stage, two-week-old seedlings of these plants were treated at 4 °C in a plant growth chamber or with 100 μM GA and 100 μM ABA. Seedlings were collected at different time points during treatment.
Total RNA was extracted using RNAiso Plus (Takara, Japan) and cDNA was prepared using M-MLV reverse transcriptase (Takara, Japan). qRT-PCR was performed using an ABI QuantStudio 6 Flex system (Applied Bio-Systems). OsActin1 (LOC_Os03g50885) was used as an internal reference. Three biological samples and three technical replicates per sample were analyzed for each experiment. Primers for qRT-PCR are provided in Supplementary Data 4.
Linkage disequilibrium and candidate-gene association analysis
For local linkage disequilibrium analysis of the associated locus, the r2 value between the lead SNP and the flanking 500-kb was calculated using Tassel 5.060. The continuous region closely associated with the lead SNP (r2 ≥ 0.6, P < 0.05) was considered the local LD interval61.
A total of 3,373,254 high-quality SNPs (missing rate < 20%, MAF ≥ 5%) from 155 rice germplasms were used for kinship estimation using GEMMA 0.94162. SNPs in linkage equilibrium were filtered using PLINK 2.0 with the argument ‘indep-pairwise 50 5 0.2′63. Eighteen SNPs and four InDels polymorphic between KMXBG and Towada were obtained from the raw resequenced variants of the 155 rice germplasms and used for association analysis using a mixed linear model in GEMMA. The significance threshold was determined using 1/N, where N is the total number of SNPs in linkage equilibrium. The LD block was plotted using the LD heatmap R package (R v4.3.3). Information on the 155 rice accessions is provided in Supplementary Data 1.
Transactivation activity and yeast two-hybrid assays
To determine the transactivation ___domain, the truncated CTB5 proteins were cloned into the pGBKT7 vector and co-transfected with an empty pGADT7 vector into the yeast strain Y2H Gold. Positive yeast clones were screened on a synthetic defined (SD) medium lacking Trp and Leu (SD-Trp-Leu). Positive clones containing different truncated proteins were then spotted onto SD-Trp-Leu-His-Ade plates, and growth was observed after 3 days at 30 °C. To screen for interacting proteins, the truncated CTB5 protein without the transactivation ___domain was cloned into the pGBKT7 vector as bait to screen a cDNA library constructed using panicles of KMXBG after 3 days of cold stress at 16 °C. To verify protein interaction, the truncated CTB5 protein without the transactivation ___domain was cloned into the pGBKT7 vector, and the full-length CDSs of the interacting proteins were cloned into the pGADT7 vector.
Split-luciferase complementation assay
Full-length CDSs of CTB5, OsHox12, and OsCam1-1 were amplified from NILCTB5 to construct fusion constructs CTB5-nLUC, OsHox12-cLUC, and OsCam1-1-cLUC, and transformed into Agrobacterium tumefaciens strain EHA105. Agrobacterium containing different combinations of constructs was co-infiltrated into N. benthamiana leaves. The infiltrated leaves were harvested after 2 days and incubated with 1 mM luciferin. Luminescence activity was recorded using a Fusion X7 chemiluminescence detector.
Co-immunoprecipitation assay
To confirm the interaction between CTB5 and OsHox12 in vivo, the 35S:Flag-CTB5KM and Super:OsHox12-Myc plasmids were co-transformed into rice protoplasts and incubated overnight. Total protein was extracted with 10 ml IP buffer (150 mM NaCl, 50 mM Tris-HCl (pH 7.5), 0.1% (v/v) NP-40, 10 mM MgCl2, 3 mM DTT, and 1 × protease inhibitor cocktail) and the lysate was centrifuged at 18,514 g for 15 min at 4 °C. Supernatants of 100 µl were taken as input and the remaining supernatants were incubated with Myc agarose beads (LABLEAD, MNA-25-500) for 2 h at 4 °C. Western blotting was performed using anti-FLAG (Sigma, F1804, dilution 1:5000) and anti-Myc (Sigma, M4439, dilution 1:5000).
Subcellular localization and bimolecular fluorescence complementation assay
To observe subcellular localization, the 35S:CTB5KM-GFP plasmid was transfected into Agrobacterium strain EHA105, and the Agrobacterium harboring fusion plasmid and p19 were co-transfected into tobacco leaves using a suspension (10 mM MES, 10 mM MgCl2, and 150 μM acetosyringone). The leaves were harvested 3 days later. In addition, 35S:CTB5KM-GFP was transfected into rice protoplasts and incubated in the dark at 28 °C for 16 h. DAPI and RGN1-mcherry were used to observe fluorescence signals in the nucleus.
To verify protein interaction, the full-length CDSs of CTB5, OsHox12, and OsCam1-1 were amplified and cloned into the N- or C-terminal half of YFP to generate CTB5-nYFG, OsHox12-cYFG, and OsCam1-1-cYFP constructs. Different combinations of constructs were co-transfected into rice protoplasts and incubated in the dark at 28 °C for 16 h. Fluorescent signals were observed using a confocal microscope (Olympus FV1000).
GA application and quantification of GAs, H2O2 and MDA
At the booting stage, Towada, Nipponbare, and CTB5 transgenic plants were subjected to cold treatments at 16 °C for one week in a phytotron. During the treatment, the plants were irrigated with water containing 5 µM GA3, and their leaves were also sprayed with 5 µM GA3 every other day28. After harvest, seed-setting rates of cold-treated plants with and without GA treatment were assessed.
Anther samples (1 g) from Towada, Nipponbare, and CTB5 transgenic plants were collected at the booting stage under both normal and CS-PT (16 °C for 7 days) conditions. Stem and sheath samples (1 g) from the same plants were collected at the heading stage under normal conditions. Endogenous GAs, including GA1, GA3, GA4, GA7, GA8, GA9, GA15, GA19, GA20, and GA34, were quantified using HPLC-MS/MS analysis with three biological replicates by Nanjing Webiolotech Testing Technology Co., Ltd. (https://www.webiolotech.com/).
Two-week-old seedlings of Towada, K-OE3, and PYL9-OE8 plants treated with cold treatment at 4 °C were sampled for measurement of H2O2 and MDA levels. H2O2 and MDA levels were quantified according to the instructions of the Hydrogen Peroxide Assay Kit (Beyotime, S0038) and Lipid Peroxidation MDA Assay Kit (Beyotime, S0131S), respectively.
RNA-sequencing analysis
The transcriptomes were analyzed using three biological replicates of RNA samples extracted from panicles of Towada and K-OE3 plants at the booting stage under normal and cold stress conditions (16 °C for 5 days in a phytotron). Libraries were prepared using the NEBNext® Ultra™ RNA Library Prep Kit for Illumina (Illumina, San Diego, CA, USA) and sequenced using the Illumina NovaSeq 6000 system (Illumina, USA). High-quality clean reads were obtained using fastp 0.19.7 and then aligned to the reference genome using HISAT2. Gene expression abundance (FPKM) was calculated using Kallisto. P-value and fold-change values were calculated using DESeq2. DEGs were defined as absolute log2 values (fold change) > 1 and P < 0.01. KEGG categories enriched for DEGs were identified using the ClusterProfile R package (R v4.3.3).
Yeast one-hybrid assay
The full-length CDS of CTB5 was amplified from the cDNA of NILCTB5 and cloned into the pB42AD vector. The promoters of OsGA2ox6, OsGA3ox1, OsGA20ox3, and PYL9 were amplified from NILCTB5 and cloned into the pLacZi2μ vector. The resulting plasmids were co-transformed into yeast strain EGY48 and the transformants were grown on SD-Trp-Ura plates containing 20 mg/mL X-gal.
Electrophoretic mobility-shift assay
The full-length CDS of CTB5 was amplified, cloned into the pMAL-c5x vector, and transformed into E. coli BL21. Prokaryotic proteins were induced by adding 0.75 mM IPTG to LB medium and incubated at 16 °C with shaking for 24 h. CTB5-MBP protein was purified using amylose resin (NEB, E8021V). DNA probes were designed and synthesized for the CTB5 binding motif in the promoters of OsGA2ox6, OsGA3ox1, OsGA20ox3, and PYL9, with biotin-labeled at the 5’ end. Probes with mutations in the binding motifs were used as negative controls, while unlabeled probes were used as competitors. Purified proteins were incubated with DNA probes for 20 min at room temperature (24–28 °C). DNA gel shift assays were conducted according to the instructions of the EMSA Gel-Shift Kit (Beyotime, GS009). Photographs were taken using a Fusion X7 chemiluminescence detector.
Chromatin immunoprecipitation-quantitative PCR
Panicles and leaves of the CTB5 OE line K-OE3 containing the 35S:CTB5KM-Flag were collected for ChIP analysis. Briefly, 2 g samples of panicles and leaves at the booting stage were cross-linked with a cross-linking solution containing 1% (w/v) formaldehyde, and the reaction was terminated with 0.125 M glycine for 5 min. Samples were thoroughly ground to powder in liquid nitrogen to isolate chromatin and sonicated to obtain 200–700 bp fragments. The fragmented chromatin solution was pre-incubated with Protein A/G beads for 6 h and then immunoprecipitated with Flag M2 affinity gel (Sigma, A2220) for 12 h at 4 °C. Chromatin immunoprecipitation without antibody was used as a control. Protein-DNA complexes were eluted and the precipitated DNA was purified and quantified by qRT-PCR.
Transient dual-luciferase reporter assay
To assess promoter activity, the CTB5 promoter (2.5-kb upstream of ATG) from NILCTB5 and Towada was amplified and cloned into the pGreenII 0800-LUC vector to generate reporters. To evaluate the transcriptional regulatory activity, the full-length CDS of CTB5 was amplified from NILCTB5 and Towada and cloned into the GAL4BD vector as an effector, while empty LUC and REN vectors were used as reporters. To investigate the transcriptional regulation of CTB5 on target genes, the full-length CDSs of CTB5 and interacting genes from NILCTB5 and Towada were amplified and cloned into the pGreenII 62-SK vector as effectors; while the promoters of OsGA2ox6, OsGA3ox1, OsGA20ox3, and PYL9 (2-kb upstream of ATG) were amplified from NILCTB5 and cloned into the pGreenII 0800-LUC vector as reporters. Analysis of transcriptional activity was conducted in rice protoplasts or tobacco leaves. Transfected protoplasts were incubated in the dark at 28 °C for 16 h, and the infiltrated leaves were harvested after 2 days. The protoplasts or leaves were then lysed, and LUC and REN activities were detected using the dual LUC reporter assay kit with a Promega Luminometer (GloMax® Explorer System). The REN gene driven by the 35S promoter was used as an internal control. At least three independent transformations were performed for each plasmid combination.
Microscopy
Spike samples at different developmental stages were collected from NILCTB5, Towada, Nipponbare, and CTB5 transgenic plants under normal conditions in Beijing and CS-HAA in Yunnan Province, and fixed in FAA solution (Servicebio, G1108). Semi-thin sections were stained with 0.5% (w/v) toluidine blue and observed under a light microscope (Olympus CX23). The developmental stages of anthers were classified based on anther length and toluidine blue staining results as follows64: Stage 9, 1.1–1.5 mm; Stage 10, 1.5–2.0 mm; Stage 11, 2.0–2.2 mm; Stage 12, 2.2 mm.
For pollen grain staining, spike samples were collected 1 day before flowering and fixed in FAA solution. Anthers were crushed to release pollen grains, stained with 1% (w/v) I2-KI solution, and observed under a light microscope (Olympus CX23). Pollen fertility was assessed by the stained pollen rate.
Evolution and selection sweep analysis
The protein sequences of CTB5 homologs in monocots and dicots were obtained from the NCBI website (https://blast.ncbi.nlm.nih.gov/Blast.cgi) using BLASTP, and sequence alignment was performed using ClustalX 1.83. High-density SNPs for evolutionary analysis were obtained from 275 wild and 709 cultivated rice65,66. Quality control (missing rate < 20%, MAF ≥ 5%) and format conversion were performed using Tassel 5.060. Phylogenetic trees of CTB5 homologs and rice germplasm were constructed with MEGA 11 using the neighbor-joining method with a bootstrap value of 100067. The tree was edited using EvolView (https://evolgenius.info//evolview-v2). For haplotype network construction, high-quality SNPs (missing rate < 20%, MAF ≥ 5%) from the 275 wild and 709 cultivated rice were imputed using Beagle 5.2 and then used for haplotype analysis using DnaSP 5.1068. The haplotype network was constructed using PopART 1.7 with the minimum spanning method69. The geographical distribution annotated on a world map was analyzed using the Maps R package (R v4.3.3) with a GPL-2 license. The altitude map of Yunnan Province was created using ARCGIS 10.8.1 software from the Environmental Systems Research Institute, Inc. Information on the accessions used in the evolutionary analysis is provided in Supplementary Data 2.
To assess nucleotide diversity, high-density SNPs in the coding region and 100-kb flanking regions of CTB5 were obtained from 396 wild and 2449 cultivated rice65,70. π values were calculated using a custom Perl script. Tajima’s D values were calculated using DnaSP 5.1068. Information on the accessions used in the nucleotide diversity analysis is provided in Supplementary Data 3.
Statistical analyses
Data are presented as mean ± standard deviation with error bars. A two-tailed Student’s t test was used to determine significant differences between the two groups, with P < 0.05 considered statistically significant. Three or more groups were analyzed by one-way analysis of variance (ANOVA) with Duncan (D)’s multiple comparison test in IBM SPSS Statistics 21.0.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data supporting the findings of this work are available within this paper and its Supplementary Information files. The genetic materials used in this study are available from the corresponding authors upon request. The RNA-Seq data generated in this study have been deposited in the NCBI/Sequence Read Archive (SRA) database under accession PRJNA1106432. Gene sequence and annotation information is available from the Rice Genome Annotation Project (https://rice.uga.edu/). Source data are provided in this paper.
Code availability
The custom Perl scripts for calculating nucleotide diversity (π values) are available at Zenodo [https://doi.org/10.5281/zenodo.14587313].
References
Gross, B. L. & Zhao, Z. J. Archaeological and genetic insights into the origins of domesticated rice. Proc. Natl. Acad. Sci. USA 111, 6190–6197 (2014).
Sharma, K. D. & Nayyar, H. Regulatory networks in pollen development under cold stress. Front. Plant Sci. 7, 402 (2016).
Pradhan, S. K., Pandit, E., Nayak, D. K., Behera, L. & Mohapatra, T. Genes, pathways and transcription factors involved in seedling stage chilling stress tolerance in indica rice through RNA-Seq analysis. BMC Plant Biol. 19, 352 (2019).
Zhu, Y. J. et al. Identification and fine mapping of a stably expressed QTL for cold tolerance at the booting stage using an interconnected breeding population in rice. PLoS ONE 10, e0145704 (2015).
Fujino, K. et al. Molecular identification of a major quantitative trait locus, qLTG3-1, controlling low-temperature germinability in rice. Proc. Natl. Acad. Sci. USA 105, 12623–12628 (2008).
Wang, X. et al. Natural variation reveals that OsSAP16 controls low-temperature germination in rice. J. Exp. Bot. 69, 413–421 (2018).
Zhao, J., Wang, S. S., Qin, J. J., Sun, C. Q. & Liu, F. X. The lipid transfer protein OsLTPL159 is involved in cold tolerance at the early seedling stage in rice. Plant Biotechnol. J. 18, 756–769 (2020).
Zhang, C. Z. et al. A transposon insertion in the promoter of OsUBC12 enhances cold tolerance during japonica rice germination. Nat. Commun. 15, 2211 (2024).
Ma, Y. et al. COLD1 confers chilling tolerance in rice. Cell 160, 1209–1221 (2015).
Liu, C. T. et al. Early selection of bZIP73 facilitated adaptation of japonica rice to cold climates. Nat. Commun. 9, 3302 (2018).
Xiao, N. et al. Identification of genes related to cold tolerance and a functional allele that confers cold tolerance. Plant Physiol 177, 1108–1123 (2018).
Mao, D. H. et al. Natural variation in the HAN1 gene confers chilling tolerance in rice and allowed adaptation to a temperate climate. Proc. Natl. Acad. Sci. USA 116, 3494–3501 (2019).
Xia, C. X., Liang, G. H., Chong, K. & Xu, Y. Y. The COG1-OsSERL2 complex senses cold to trigger signaling network for chilling tolerance in japonica rice. Nat. Commun. 14, 3104 (2023).
Li, Z. T. et al. Natural variation of codon repeats in COLD11 endows rice with chilling resilience. Sci. Adv. 9, eabq5506 (2023).
Zu, X. F. et al. A mitochondrial pentatricopeptide repeat protein enhances cold tolerance by modulating mitochondrial superoxide in rice. Nat. Commun. 14, 6789 (2023).
Liu, D. F. et al. COG3 confers the chilling tolerance to mediate OsFtsH2-D1 module in rice. New Phytol 241, 2143–2157 (2024).
Saito, K., Hayano-Saito, Y., Kuroki, M. & Sato, Y. Map-based cloning of the rice cold tolerance gene Ctb1. Plant Sci 179, 97–102 (2010).
Zhang, Z. Y. et al. Natural variation in CTB4a enhances rice adaptation to cold habitats. Nat. Commun. 8, 14788 (2017).
Li, J. L. et al. Stepwise selection of natural variations at CTB2 and CTB4a improves cold adaptation during domestication of japonica rice. New Phytol. 231, 1056–1072 (2021).
Xu, Y. F., Wang, R. C., Wang, Y. M., Zhang, L. & Yao, S. G. A point mutation in LTT1 enhances cold tolerance at the booting stage in rice. Plant Cell Environ. 43, 992–1007 (2020).
Jia, M. R. et al. Chilling-induced phosphorylation of IPA1 by OsSAPK6 activates chilling tolerance responses in rice. Cell Discov. 8, 71 (2022).
Wang, J. C. et al. Transcriptional activation and phosphorylation of OsCNGC9 confer enhanced chilling tolerance in rice. Mol. Plant 14, 315–329 (2021).
Tian, X. J. et al. Characterization and functional analysis of pyrabactin resistance-like abscisic acid receptor family in rice. Rice 8, 28 (2015).
He, J. et al. CYP72A enzymes catalyse 13-hydrolyzation of gibberellins. Nat. Plants 5, 1057–1065 (2019).
Sakamoto, T. et al. An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol. 134, 1642–1645 (2004).
Chen, X. et al. CRISPR-Based assessment of gene specialization in the gibberellin metabolic pathway in rice. Plant Physiol. 180, 2091–2105 (2019).
Oda, S. et al. Morphological and gene expression analysis under cool temperature conditions in rice anther development. Genes Genet. Syst. 85, 107–120 (2010).
Tang, J. Q. et al. WRKY53 negatively regulates rice cold tolerance at the booting stage by fine-tuning anther gibberellin levels. Plant Cell 34, 4495–4515 (2022).
Harris, J. C., Hrmova, M., Lopato, S. & Langridge, P. Modulation of plant growth by HD-Zip class I and II transcription factors in response to environmental stimuli. New Phytol. 190, 823–837 (2011).
Sharif, R. et al. HD-ZIP Gene family: Potential roles in improving plant growth and regulating stress-responsive mechanisms in plants. Genes 12, 1256 (2021).
Kovach, M. J., Sweeney, M. T. & McCouch, S. R. New insights into the history of rice domestication. Trends Genet. 23, 578–587 (2007).
Guo, H. F. et al. Differentiation, evolution and utilization of natural alleles for cold adaptability at the reproductive stage in rice. Plant Biotechnol. J. 18, 2491–250 (2020).
Zhou, L. et al. Fine mapping a QTL qCTB7 for cold tolerance at the booting stage on rice chromosome 7 using a near-isogenic line. Theor. Appl. Genet. 121, 895–905 (2010).
Gao, S. P., Fang, J., Xu, F., Wang, W. & Chu, C. C. Rice HOX12 Regulates panicle exsertion by directly modulating the expression of ELONGATED UPPERMOST INTERNODE1. Plant Cell 28, 680–695 (2016).
Dong, S. C. et al. The HB40-JUB1 transcriptional regulatory network controls gibberellin homeostasis in Arabidopsis. Mol. Plant 15, 322–339 (2022).
González-Grandío, E. et al. Abscisic acid signaling is controlled by a BRANCHED1/HD-ZIP I cascade in Arabidopsis axillary buds. Proc. Natl. Acad. Sci. USA 114, E245–E254 (2017).
Aya, K. et al. Gibberellin modulates anther development in rice via the transcriptional regulation of GAMYB. Plant Cell 21, 1453–1472 (2009).
Sakata, T. et al. Reduction of gibberellin by low temperature disrupts pollen development in rice. Plant Physiol. 164, 2011–2019 (2014).
Johannesson, H., Wang, Y. & Engström, P. DNA-binding and dimerization preferences of Arabidopsis homeodomain-leucine zipper transcription factors in vitro. Plant Mol. Biol. 45, 63–73 (2001).
Li, N. et al. The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. Plant Cell 18, 2999–3014 (2006).
Yang, H. et al. Selection and cultivation technology of the new alpine Japonica rice variety ‘Lijing 9’ (in Chinese). Vol 1, 56–58 (Yunnan Agricultural Science and Technology, 2014).
Yang, L. M. et al. qCTB7 positively regulates cold tolerance at booting stage in rice. Theor. Appl.Genet. 136, 135 (2023).
Meijer, A. H., de Kam, R. J., d’Erfurth, I., Shen, W. & Hoge, J. H. HD-Zip proteins of families I and II from rice: interactions and functional properties. Mol. Gen. Genet. 263, 12–21 (2000).
Yang, Y. F. et al. Overexpression of the class I homeodomain transcription factor TaHDZipI-5 increases drought and frost tolerance in transgenic wheat. Plant Biotechnol. J. 16, 1227–1240 (2018).
Huang, J. et al. Activation of gibberellin 2-oxidase 6 decreases active gibberellin levels and creates a dominant semi-dwarf phenotype in rice (Oryza sativa L.). J. Genet. Genomics 37, 23–36 (2010).
Lo, S. F. et al. A novel class of gibberellin 2-oxidases control semidwarfism, tillering, and root development in rice. Plant Cell 20, 2603–2618 (2008).
Kovalchuk, N. et al. The homeodomain transcription factor TaHDZipI-2 from wheat regulates frost tolerance, flowering time and spike development in transgenic barley. New Phytol. 211, 671–687 (2016).
Qin, X. et al. Gibberellin 20-oxidase gene OsGA20ox3 regulates plant stature and disease development in rice. Mol. Plant Microbe Interact. 26, 227–239 (2013).
Pei, Y. G. et al. Bifunctional transcription factors SlERF.H5 and H7 activate cell wall and repress gibberellin biosynthesis genes in tomato via a conserved motif. Dev. Cell 59, 1345–1359 (2024).
Marchive, C. et al. Nuclear retention of the transcription factor NLP7 orchestrates the early response to nitrate in plants. Nat. Commun. 4, 1713 (2013).
Ikeda, M., Mitsuda, N. & Ohme-Takagi, M. Arabidopsis WUSCHEL is a bifunctional transcription factor that acts as a repressor in stem cell regulation and as an activator in floral patterning. Plant Cell 21, 3493–3505 (2009).
Yant, L. V. et al. Orchestration of the floral transition and floral development in Arabidopsis by the bifunctional transcription factor APETALA2. Plant Cell 22, 2156–2170 (2010).
Su, H. H. et al. ZmCCT regulates photoperiod-dependent flowering and response to stresses in maize. BMC Plant Biol. 21, 453 (2021).
Luo, Z. P., Moreau, C., Wang, J., Frugier, F. & Xie, F. NLP1 binds the CEP1 signalling peptide promoter to repress its expression in response to nitrate. New Phytol. 234, 1547–1552 (2022).
Abe, M. et al. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309, 1052–1056 (2005).
Yokotani, N. et al. WRKY76 is a rice transcriptional repressor playing opposite roles in blast disease resistance and cold stress tolerance. J. Exp. Bot. 64, 5085–5097 (2013).
Asad, M. A. U. et al. Abiotic stresses intervene with ABA signaling to induce destructive metabolic pathways leading to death: Premature leaf senescence in plants. Int. J. Mol. Sci. 20, 256 (2019).
Zhao, Q. et al. ABA-triggered ROS burst in rice developing anthers is critical for tapetal programmed cell death induction and heat stress-induced pollen abortion. Plant Cell Environ. 46, 1453–1471 (2023).
Lou, Q. et al. Cold-adaptive evolution at the reproductive stage in Geng/japonica subspecies reveals the role of OsMAPK3 and OsLEA9. Plant J. 111, 1032–1051 (2022).
Bradbury, P. J. et al. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23, 2633–2635 (2007).
Yano, K. et al. Genome-wide association study using whole-genome sequencing rapidly identifies new genes influencing agronomic traits in rice. Nat. Genet. 48, 927–934 (2016).
Zhou, X. & Stephens, M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 44, 821–824 (2012).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Zhang, D. B., Luo, X. & Zhu, L. Cytological analysis and genetic control of rice anther development. J. Genet. Genomics 38, 379–390 (2011).
Jing, C. Y. et al. Multiple domestications of Asian rice. Nat. Plants 9, 1221–1235 (2023).
Zhao, Y. et al. Loci and natural alleles underlying robust roots and adaptive domestication of upland ecotype rice in aerobic conditions. PLoS Genet. 14, e1007521 (2018).
Tamura, K., Stecher, G. & Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 38, 3022–3027 (2021).
Librado, P. & Rozas, J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452 (2009).
Leigh, J. W. & Bryant, D. PopART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 6, 1110–1116 (2015).
Wang, W. S. et al. Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature 557, 43–49 (2018).
Acknowledgements
This work was supported by grants from the Yunnan Fundamental Research Project (202201AS070071, Recipient: P.Y.), STI 2030-Major Projects (2023ZD0407104, Recipient: X.S.), National Natural Science Foundation of China (32372080, Recipient: Jinjie L.), and China Postdoctoral Science Foundation (2021M693438, Recipient: H.G.).
Author information
Authors and Affiliations
Contributions
Jinjie L. and Z.L. supervised the project. H.G. performed fine-mapping and genetic transformation. S.G. performed SLC, BiFC, Co-IP, and ROS quantification. H.G. performed yeast assay, RNA-sequencing, EMSA, Chip-qPCR, dual-luciferase reporter assay, GA measurement, microscopy observation, haplotype analysis, and evolutionary analysis. Jin L., Y.G., Q.L., R.S., W.Y., and A.Z. assisted in sample collection, expression analysis, and phenotypic evaluation. Y.W. conducted breeding utilization analysis. H.L., J.Y., Y.Z., and P.Y. performed the cold-tolerance evaluation in Yunnan Province. X.S., Z.Z., H.Z., and Y.P. contributed to the project discussion. H.G., Jinjie L., and Z.L. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Michael Schläppi and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guo, H., Gao, S., Li, H. et al. Natural variation of CTB5 confers cold adaptation in plateau japonica rice. Nat Commun 16, 1032 (2025). https://doi.org/10.1038/s41467-025-56174-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56174-5
This article is cited by
-
Suppressing an auxin efflux transporter enhances rice adaptation to temperate habitats
Nature Communications (2025)
-
Cell-cell communication-mediated cell-type-specific parent-of-origin effects in mammals
Nature Communications (2025)