Abstract
Sphingolipid, ceramide for example, plays an essential role in regulating cancer cell death. Defects in the generation and metabolism of ceramide in cancer cells contribute to tumor cell survival and resistance to chemotherapy. Ceramide Transfer Protein (CERT) determines the ratio of ceramide and sphingomyelin in cells. Targeting CERT sensitizes solid cancer cells to chemotherapy. However, whether targeting CERT to induce ceramide accumulation thereby improving AML therapy efficiency remains elusive. Here, we show that knocking down CERT inhibits the growth and promotes the apoptosis of AML cells carrying FLT3-ITD mutation. Combining CERT inhibitor with FLT3 inhibitor exhibits synergistic effects on FLT3-ITD mutated acute myeloid leukemia (AML) cells. Additionally, co-treatment of HPA-12 and Crenolanib is effective in FLT3-ITD+ and FLT3-TKD+ AML patients. The synergistic effects are found to be mediated by the endoplasmic reticulum stress-GRP78/ATF6/CHOP axis and mitophagy. Our data provide an effective strategy to enhance the efficacy of FLT3 inhibitors in AML.
Similar content being viewed by others
Introduction
Cancer cells exhibit alterations in lipid profiles and metabolism that enable them to meet the various energy requirements of cancer initiation, progression, and metastasis1,2. Sphingolipids are a type of key lipid in cancer: sphingomyelin (SM), ceramide (Cer), and glycosphingolipids serve as the structural lipids in cell membranes, forming microdomains rich in sphingolipids in the cell membrane and regulating cancer cell signaling3,4,5. Notably, Cer is considered a tumor suppressor in most cancer types6,7. Defects in Cer generation and clearance in cancer cells result in their survival and resistance to chemotherapy. In contrast, SM is critical for cancer cell proliferation, migration, and immune evasion8,9. Therefore, the key players involved in the turnover of Cer to SM represent the metabolic vulnerabilities of cancer cells, which can be targeted through ceramide-based therapeutics.
Ceramide transfer protein (CERT), a lipid transfer protein, specifically transports Cer from the endoplasmic reticulum (ER) to the Golgi apparatus10. CERT is important for the clearance of Cer and determines the ratio of Cer to SM in cells. Inactivation of CERT has been shown to induce apoptotic responses in several cell lines, including colon cancer, breast cancer and lung carcinoma cells11,12. Studies have shown that CERT is highly expressed in drug-resistant cell lines13. CERT was identified as a potential target to sensitize cancers to multiple chemotherapy drugs in a RNA interference screening. Interestingly, CERT knockdown augmented ER stress and activated the PERK arm of the UPR in response to paclitaxel treatment13. A follow-up study reported that the induction of autophagic flux is responsible for CERT-specific multidrug sensitization in colorectal and breast cancers14,15. Downregulation of CERT was also proven to participate in the therapeutic inhibition of pancreatic cancer cells by γ-tocotrienol15. Thus, pharmacological inhibition of CERT could represent an effective anticancer strategy for overcoming drug resistance. Ceramide-mimetic and nonmimetic inhibitors have been developed to facilitate precise inhibition of CERT16. Among them, HPA-12, an HPA that has a C12 alkyl chain, has been found to be a mimetic inhibitor of CERT17. HPA-12 and its derivatives have been used in various biological studies, including those involving its use in the brain and in keratinocytes and as an anticancer agent13,18,19,20,21.
Inhibition of Cer generation has been reported in acute myeloid leukemia (AML) patients carrying Fms-like tyrosine kinase 3 (FLT3)/internal tandem duplication (ITD) mutations. Signaling pathways activated by FLT3/ITD suppress Cer synthase 1 (CerS1)/Cer metabolism22. As a result, targeting FLT3-ITD with the FLT3 inhibitor crenolanib (Creno) induced Cer accumulation, which eventually led to the death of AML cells. Moreover, targeting sphingosine kinase 1 (SPHK1) with its inhibitor MP-A08 induced the accumulation of ceramide, which activated the downstream apoptotic integrated stress response and sensitized AML cells to venetoclax23. However, whether targeting key players involved in the clearance pathway of Cer can sensitize AML cells to FLT3 inhibitors remains unknown.
In this study, we show that targeting CERT by genetic or pharmacological approaches inhibits growth and leads to cell death in AML cells. The CERT inhibitor HPA-12 alone or in combination with the FLT3 inhibitor Creno inhibits growth and triggers apoptosis in FLT3-ITD+ AML cells in vitro and in vivo. HPA-12 synergizes with the FLT3 inhibitor Creno by inducing pro-cell death ceramide accumulation. Mechanistically, this synergism is achieved by activating the GRP78/ATF6/CHOP axis and mitophagy. These findings suggest the use of agents that promote Cer accumulation to rejuvenate FLT3 inhibitors for the treatment of AML.
Results
Suppression of CERT inhibits the growth and promotes the apoptosis of FLT3-ITD+ AML cell lines
CERT has been implicated as an important target of sphingolipid metabolism to induce cell death in cancer cells14. First, we determined the expression level of CERT in various AML cell lines. Interestingly, western blot analysis demonstrated that CERT protein expression was increased in MV4-11/Molm13 (FLT3-ITD) cells compared with other AML cells (FLT3-WT) and healthy donors (Figs. 1a and S1a). We next explored whether inhibition of CERT by its inhibitor HPA-12 affected AML cell growth. The AML cells lines were treated with different concentrations of HPA-12 for 48 h, and a CCK-8 assay was used to assess cell viability. Notably, HPA-12 significantly inhibited the viability of FLT3-ITD mutant cell lines (MV4-11, Molm13) in a dose-dependent manner. In contrast, HPA-12 at the same concentration had no obvious effect on the FLT3/WT cell lines (HL-60, THP-1, OCI-AML3, Kasumi-1, and KG-1α) (Fig. 1b). To verify the effect of inhibiting CERT in these two FLT3/ITD cell lines, we used a shRNA interference lentiviral vector system to construct stable CERT-knockdown cell lines (hereinafter referred to as WT and CERT-KD). CERT-KD2 MV4-11 and Molm13 cells presented the best knockdown efficiency and were selected for further cell viability tests for 5 consecutive days (Fig. S1b, c). As expected, the viability of these two FLT3-mutant AML cell lines in which CERT was knocked down was significantly decreased (Fig. 1c, d). To test whether the inhibition of CERT inhibited the proliferation of these two FLT3-mutant AML cell lines, an EdU assay was performed with HPA-12. EdU assays also demonstrated a dose-dependent decrease in cell proliferation (Figs. 1e, f and S1d, e). Moreover, we tested whether the inhibition of CERT induced cell death. HPA-12 also promoted cell death, as demonstrated by an increase in the number of annexin V+ cells, indicating the induction of apoptosis (Figs. 1g, h, and S1f, g). Taken together, these data reveal that CERT was preferentially overexpressed in AML cell lines with a FLT3-ITD mutation. Genetic or pharmacological inhibition of CERT effectively decreased cell viability in FLT3-mutant AML cell lines by inhibiting cell growth and inducing cell apoptosis.
a Western blot of the CERT protein in healthy donors and AML cell lines (HL-60, THP-1, KG-1α, MV4-11, and Molm13). HD, bone marrow nucleated cells from healthy donors. b Viability of OCI-AML3, HL-60, THP-1, Kasumi-1, KG-1α, MV4-11, and Molm13 cells treated with HPA-12 at the indicated concentrations for 48 h. Effects of CERT knockdown (KD) on the proliferation of MV4-11 (c) and Molm13 (d) cells. n = 5 independent experiments (c), and n = 3 independent experiments (d). KD, knockdown. e–h Molm13 cells were treated with increasing concentrations of HPA-12 for 48 h. Cells treated with the indicated concentrations of HPA-12 were subjected to an EdU assay (e, f) and Annexin V/PI staining (g, h). EdU-positive cells were identified by ImageJ. Scale bar, 50 μM. n = 3 independent experiments. The data are presented as the means ± SDs, and differences were compared by 2-tailed Student’s t tests. Multiple groups were analyzed with one-way ANOVA. ns: not significant, *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Source data are provided as a source data file.
CERT inhibitors synergize with FLT3 inhibitors to inhibit the growth of AML cells
Through analysis, we found that patients who were insensitive to FLT3 inhibitors presented high CERT expression levels (Fig. 2a, b). Therefore, inhibiting CERT might increase the sensitivity of AML cells to FLT3 inhibitors. To test this hypothesis, we treated AML cells with HPA-12 and Creno, a FLT3 inhibitor. Combined treatment with HPA-12 and Creno reduced cell viability more effectively than either HPA-12 or Creno alone (Fig. 2c, d). To assess whether the combined effects were synergistic or additive, we calculated the combination index (CI) with Compusyn software. The CI values ranged from 0.113 to 0.179 in Molm13 cells and 0.134 to 0.779 in MV4-11 cells, indicating a strong synergistic anti-proliferative effect between HPA-12 and Creno (Figs. 2e and S2a, b). Interestingly, in the KG1-α (FLT3-WT) cell line, the CI value ranged from 1.266 to 1.405 (Fig. S2c), indicating an antagonistic effect. Moreover, synergism was further verified with the Bliss models for both the MV4-11 and Molm13 cells (Fig. 2f). EdU assays demonstrated that the proportion of proliferating cells was dramatically lower in the Combo group than in either the HPA-12 or Creno group (Figs. 2g, h and S2d, e). In addition, we also observed a prominent increase in apoptosis in the Combo group, as indicated by annexin V/PI staining (Fig. 2i, j and S2f–g). To rule out the possibility that the synergism between the inhibition of CERT and the FLT3 inhibitor was due to off-target effects of HPA-12, we knocked down CERT in FLT3/ITD AML cells and examined whether HPA-12 still demonstrated any synergistic activity with Creno in CERT-KD cells. Notably, after CERT was knocked down, the synergistic activity between HPA-12 and Creno weakened, as indicated by the increase in the CI index. The predictive model index also changed from a synergistic effect (value greater than 10), as shown in Fig. 2, to an antagonistic effect (value less than 0) (Fig. S3). Collectively, these results illustrate that inhibiting CERT sensitized FLT3-mutant AML cell lines to FLT3 inhibitors and that the CERT inhibitor HPA-12 synergized with the FLT3 inhibitor to inhibit proliferation and induce apoptosis in AML cells.
a, b Correlation of the CERT expression level with the sensitivity of AML cells to gilteritinib and sorafenib (TCGA). RPKM, per kilobase per million mapped reads. c, d Viability of Molm13 and MV4-11 cells treated with the CERT inhibitor HPA-12 or/and Creno. n = 3 independent experiments. e Combination index (CI) for Molm13 and MV4-11 cells treated with HPA-12 and Creno. The CI was calculated by Compusyn software. CI < 1, CI = 1, and CI > 1 indicate synergistic, additive, and antagonistic effects, respectively. Bliss synergy model displaying the viability of MOLM13 cells and MV4-11 cells (f) treated with HPA-12 and Creno. The cells were treated with the indicated concentrations of HPA-12 (0–100 µM) and Creno (0–8 µM), and cell viability was determined by the CCK-8 assay after 48 h. Treatment synergy was determined with the Synergy Finder website, and the Bliss synergy index is denoted as the red regions in the graphs. g, h Molm13 cells were treated with HPA-12 (80 µM) and Creno (6 µM), alone or in combination, for 48 h. The cells were subjected to an EdU assay (g, h) and annexin V/PI staining (i, j). g EdU-positive cells were identified by ImageJ. Scale bar, 50 μM. n = 3 independent experiments for (h) and (j). The data are presented as the means ± SDs, and differences were compared by 2-tailed Student’s t tests. Multiple groups were analyzed with one-way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. The source data are provided as a source data file.
HPA-12 combined with Creno prolongs the survival of AML xenograft mice
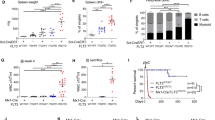
To further examine whether HPA-12 monotherapy alone or in combination with Creno was effective at eradicating leukemic cells in vivo, we generated a xenograft model using MV4-11-luc+ cells (Fig. 3a). The mice were randomly divided into four groups and treated with vehicle, Creno (15 mg/kg), HPA-12 (4 mg/kg), or Creno plus HPA-12 (referred to as the Combo group) for 2 weeks. Compared with mice in the other groups, the mice in the Combo group presented dramatically lower bioluminescence signals on Day 27 (Fig. 3b). Compared with single agent or control treatment, combined treatment led to prolonged survival of diseased mice (Fig. 3c). Spleen enlargement was reduced in all three groups treated with monotherapy or the combined regimen. Notably, the reduction in spleen weight was most prominent in the mice in the combination group (Fig. 3d, e). IHC staining analysis of human CD45 + T cells in the bone marrow and spleen also revealed a greater reduction in the leukemia burden in the combination group than in the single agent group (Fig. 3f, g). No obvious body weight change was observed in the combination group, but slight weight loss was observed in the HPA-12 group (Fig. 3h). To further quantify the tumor burden in the bone marrow (BM), spleen (SP), and peripheral blood (PB), three mice in each group were euthanized for flow cytometry analysis of the human cell percentage after 2 weeks of drug administration. Flow cytometry analysis of human CD45+/CD33+ cells revealed a significantly lower AML burden in the Combo group than in all the other groups, including the Creno monotherapy group (Figs. 3i and S4). Taken together, these results suggest that HPA-12 in combination with Creno inhibited AML development and prolonged the survival of diseased mice in vivo, which may represent an effective combination strategy for AML therapy.
a Schematic diagram of the experimental design. The mice were randomly allocated into four groups and treated with Creno (15 mg/kg), HPA-12 (4 mg/kg), or Combo (n = 8 mice per group). b In vivo bioluminescence imaging of B-NDG mice on Day 7 and Day 27 (n = 5 per group). Five mice from each group were randomly chosen for bioluminescence imaging and survival analysis on Day 27. c Overall survival of the mice in each treatment group (n = 5 per group). The P values were calculated with a log-rank (Mantel‒Cox) test. Images of the spleen size (d) and weight (e) were acquired once the mice were euthanized on Day 27 (n = 3 per group). f, g Representative images of leukemia infiltration in the BM and spleen as analyzed by immunohistochemistry (IHC) staining of human CD45 (hCD45). Scale bar, 50 µm. Slides from one mouse per group were randomly chosen for IHC staining. BM, bone marrow. h Body weights of treated mice. Body weights were measured every two days and are presented as the means for each group (n = 8 mice). i Statistical analysis of the percentage of human CD33+/human CD45+ (hCD33+/hCD45+) cells in the BM (n = 3 mice). i Statistical analysis of the percentage of human CD33+/human CD45+ (hCD33+/hCD45+) cells in the BM. Representative flow plots are shown in Fig. S4a. Three mice in each group were sacrificed on Day 27 for leukemic burden analysis. The data are presented as the means ± SDs, and differences were compared by 2-tailed Student’s t tests. Multiple groups were analyzed with one-way ANOVA. ns: not significant, *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Source data are provided as a source data file.
The synergistic effect of the combination of HPA-12 with Creno involves pro-cell death Cer accumulation
As inhibiting FLT3 is reported to increase the generation of Cer and inhibiting CERT disrupts the transfer of Cer from the endoplasmic reticulum ER to Golgi bodies10,22, we expected that the combination of an FLT3 inhibitor with the CERT inhibitor HPA-12 would lead to the accumulation of ceramide prior to cell death. To determine whether Cer accumulated in the treated AML cells, we performed targeted lipidomic analysis on the Molm13 and MV4-11 cell lines treated with DMSO, HPA-12, Creno, or Combo for 48 h (Fig. S5a). We conducted single-dimensional screening for differentially abundant metabolites and found that 44 metabolites were upregulated and 103 were downregulated in the Combo group compared with those in the DMSO group (Fig. S5b). Interestingly, our data revealed that the Combo group contained higher levels of ceramides of various chain lengths (Figs. 4a, S5d, and Table S1–2) than did the control group. The addition of Creno to MV4-11 and Molm13 cells with CERT knockdown induced a similar increase in ceramides as the Combo treatment did (Figs. 4b, S5e, Table S3,4). In contrast, the level of SM, a resulting metabolite synthesized after the trafficking of Cer with CERT to the Golgi, was significantly lower in Combo-treated AML cells or CERT-KD cells treated with Creno (Fig. S5c and Table S5–8). Furthermore, to determine the subcellular distribution of this increased Cer lipid content in the Combo group cells, we performed transmission electron microscopy (TEM) on treated AML cells. We observed increased and aggregated flat vesicles in the Combo group cells (blue arrow), suggesting that the number and volume of the endoplasmic reticulum increased (Fig. 4c). In addition, increased and aggregated vacuole-like transparent structures (purple arrows) were observed in the Combo groups, suggesting lipid accumulation within the cells (Fig. 4d). We further validated the accumulation of Cer in the endoplasmic reticulum and Golgi bodies without and with the SPT inhibitor myriocin (Fig. 4e, Fig. S6). Consistently, the intensity of ceramide increased significantly in the Combo group compared with the control group (Fig. 4f). As expected, the colocalization of ceramide with the endoplasmic reticulum (PDI) was observed (Fig. 4g). In addition, the ceramide signal on the Golgi was weakened in the Combo group, indicating that less ceramide was transferred to the Golgi apparatus (Fig. 4h). Interestingly, the increase in total ceramide intensity and colocalization with the ER triggered by Combo treatment was attenuated by myriocin, which blocks de novo ceramide synthesis (Fig. S6b–e). These data collectively demonstrate that the combination of HPA-12 with Creno induced the accumulation of Cer in the endoplasmic reticulum and disrupted the trafficking of Cer to Golgi bodies within cells.
a, b Stacked bar chart of the relative abundance statistics of ceramide determined by LC‒MS. n = 3 independent experiments. a MV4-11 cells were treated with HPA-12 (80 µM) plus Creno (6 µM) (red bars) or vehicle control (0.1% DMSO; blue bars) for 48 h. b MV4-11/CERT-KD cells were treated with Creno (6 µM) (red bars) for 48 h. Transmission electron microscopy images of the endoplasmic reticulum (c) and lipid accumulation (d) in treated MV4-11 cells. The cells were treated as described in (a, b) and harvested for TEM sample preparation following 48 h of treatment. Blue arrows indicate flat vesicular structures representing the endoplasmic reticulum. The purple arrows indicate vacuole-like transparent lipid droplets. Scale bar, 2 μm. e Representative immunofluorescence images of ceramide distribution in the ER (PDI, green) and Golgi. Scale bar, 50 μm. f Statistical analysis of the green MFI representing the ceramide in (e). MFI, mean fluorescence intensity. g Statistical analysis of the colocalization of ceramide (green) with the Golgi (red). h Statistical analysis of the colocalization of ceramide (green) with the ER (yellow). One field per slide and three slides per group were chosen for analysis with ImageJ and subsequent statistical tests. n = 3 independent experiments. The data are presented as the means ± SDs, and differences were compared by 2-tailed Student’s t tests. Multiple groups were analyzed with one-way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001. Source data are provided as a source data file.
The combination of HPA-12 and Creno induces ER stress in AML cells
To gain further insight into the mechanism by which HPA-12 and Creno synergistically suppress AML cell growth, we performed RNA-seq on MV4-11 cells after treatment with Creno plus HPA-12 for 48 h. Differential genes in the Combo group revealed an enrichment of genes associated with ER stress (Figs. 5a, S7a). In response to ER stress, cells activate UPR (unfolded protein response) pathways, which involve three main UPR pathways: the IRE1α (inositol-requiring kinase 1) ‑XBP1 (X-box binding protein 1) pathway, the protein kinase R-like ER kinase (PERK)‑eIF2α (eukaryotic initiation factor-2α) pathway and the activating transcription factor-6 (ATF6) pathway. To determine the pathway responsible for the synergistic effect of HPA-12 and Creno, we performed a western blot analysis of the key components of these three pathways. We observed that HPA-12 and Creno treatment of MV411 cells induced activation of the ATF6 arm of the UPR, together with upregulation of its upstream regulator GRP78 and downstream effector CHOP (Fig. 5b–f). Notably, no activation of the IRE1 or PERK pathway was observed in response to HPA-12 and Creno treatment. Moreover, the expression level of CERT increased when GRP78 was inhibited (Fig. S7b). Intriguingly, the addition of 4-PBA, a potent endoplasmic reticulum stress inhibitor, significantly increased the viability of the cells in the Combo group (Fig. 5g, h). Consistent with these findings, 4-PBA partially reduced the percentage of apoptotic cells induced by Combo treatment (Fig. 5i–l). Taken together, these data indicate that HPA-12 in combination with Creno induced ER stress and activated the UPR to inhibit the growth and induce the apoptosis of AML cells.
a Schematic representation of the unfolded protein response pathways. Expression of GRP78, ATF6, CHOP, phospho-IRE1α (p-IRE1α), XBP1, phospho-PERK (p-PERK), phospho-eIF2α (p-eIF2α) and ATF4 was determined by western blotting. Molm13 (b) or (c) MV4-11 cells were treated with HPA-12 (80 µM), Creno (6 µM) or their combination for 48 h. Protein expression levels of GRP78 (d), ATF6 (e) and CHOP (f) in (b, c). n = 3 independent experiments. Effects of the ERS inhibitor 4-PBA on the viability of Molm13 (g) and MV4-11 (h) cells. n = 3 independent experiments. Representative flow plots of annexin V/PI staining of Molm13 (i) and MV4-11 (j) cells in (g, h). k, l Statistical analysis of the number of apoptotic cells in (i, j). n = 3 independent experiments. The data are presented as the means ± SDs, and differences were compared by 2-tailed Student’s t tests. Multiple groups were analyzed with one-way ANOVA. ns: not significant, *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Source data are provided as a source data file.
The GRP78/ATF6/CHOP axis is mainly responsible for the synergism between HPA-12 and Creno
Since our data indicated that GRP78, ATF6, and CHOP were involved in mediating the effects of the synergism between HPA-12 and Creno, we next examined the dependency on the GRP78/ATF6/CHOP axis by inducing the shRNA-mediated knockdown of GRP78, ATF6, and CHOP in AML cells. First, we constructed stable GRP78-knockdown cell lines. As shown in Figs. 6a and S8a, the knockdown of GRP78 increased the viability of Molm13 cells and MV4-11 cells in the Combo group. Notably, GRP78 knockdown decreased the levels of ATF6 and CHOP in the Combo group, dampening the ATF6 pathway (Figs. 6b and S8b). As expected, GRP78 knockdown mitigated the anti-AML effect of the Combo treatment (Figs. 6c, d and S8c-d). Next, we constructed stable ATF6-knockdown cell lines and examined their viability after drug treatment. The viability of the Molm13 and MV4-11 cells increased in the Combo group when ATF6 was knocked down (Figs. 6e and S8e). Not surprisingly, ATF6 knockdown reduced the level of CHOP, the downstream effector (Figs. 6f and S8f). Consistent with these findings, AML cell lines in which ATF6 was stably knocked down presented an increase in the annexin V- fraction, indicating that cell viability was restored in the Combo group (Figs. 6g, h and S8g, h). Finally, the knockdown of CHOP, the downstream effector of this axis, increased the viability and reduced the apoptosis of Combo-treated AML cells (Figs. 6i–l and S8i–l). Collectively, these data suggest that the synergistic anti-AML effect occurred through the GRP78/ATF6/CHOP axis.
a Viability of Molm13/shGRP78 cells and control cells treated with vehicle carrying scrambled sequences. n = 4 independent experiments. b Protein expression levels of GRP78, ATF6 and CHOP in (a). c Annexin V/PI staining in shGRP78 or scramble Molm13 cells. d Statistical analysis of the viable cells in (c). n = 3 independent experiments. e Viability of Molm13/shATF6 cells and control cells stably transduced with vehicle carrying scramble sequences. n = 4 independent experiments. f Protein expression levels of GRP78, ATF6 and CHOP in (e). g Annexin V/PI staining in shATF6 or scramble Molm13 cells. h Statistical analysis of the viable cells in (g). n = 3 independent experiments. i Cell viability of Molm13/shCHOP cells and control cells transduced with vehicle carrying scramble sequences. n = 4 independent experiments. j Protein expression levels of GRP78, ATF6 and CHOP in (i). k Annexin V/PI staining in shGRP78 or scramble Molm13 cells. l Statistical analysis of the number of viable cells in (k). n = 3 independent experiments. The data are presented as the means ± SDs, and differences were compared by 2-tailed Student’s t tests. Multiple groups were analyzed with one-way ANOVA. ns: not significant, *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Source data are provided as a source data file.
Ceramide accumulates in mitochondria and leads to mitochondrial dysfunction in response to combined treatment with HPA-12 and Creno
Because mitochondria are the most sensitive organelles within cells, it is reasonable to expect that mitochondria are affected by lipid accumulation and damage to the endoplasmic reticulum structure within the cell. To investigate whether the function of mitochondria was impaired, we assessed changes in the mitochondrial membrane potential (MMP). As expected, drug treatment induced ceramide accumulation in the mitochondria (Fig. 7a, b). Next, we investigated whether mitochondrial function was impaired by drug treatment. JC-1 staining revealed a significant decrease in red fluorescence and an increase in green fluorescence in the Combo group, indicating that the mitochondria were depolarized (Fig. S9a). Consequently, the alteration of the MMP led to an increase in ROS generation in the MV4-11 cells after drug treatment (Fig. 7c–e). Consistent with these findings, a significant increase in ROS was also observed in MV4-11/shCERT cells after drug treatment, similar to that in MV4-11 WT cells after Combo treatment (Fig. 7f). Collectively, these data suggest that the combination of HPA-12 and Creno led to impaired MMP and mitochondrial function, which may subsequently activate mitophagy.
a Representative immunofluorescence staining images of ceramide distribution in mitochondria. MV4-11 cells were treated with HPA-12 (80 µM) or Creno (6 µM) alone or in combination for 48 h. The treated cells were labeled with both the mitochondrial marker Tom 20 (red) and a ceramide antibody (green) and visualized by confocal microscopy. b Quantification of intensity of colocalization in (a). One field per slide and three slides per group were used for image analysis with ZEN and subsequent statistical analysis. Scale bar, 2 μm. c Mitochondrial ROS levels were analyzed by MitoSOX staining (red), with DAPI staining for nuclei (blue). MV4-11 cells were treated with HPA-12 (80 µM) and Creno (6 µM) alone or in combination for 48 h. Scale bar, 10 μm. d Quantification of MitoSOX fluorescence intensity in (c).One field per slide and three slides per group were used for ZEN and subsequent statistical analysis. n = 3 independent experiments. e Mitochondrial ROS levels were analyzed by MitoSOX staining (red), with DAPI staining for nuclei (blue) in MV4-11 stable cell lines. GFP indicates cells harboring scramble sequences or shCERT. Scale bar, 10 μm. f Quantification of MitoSOX fluorescence intensity in (e). One field per slide and three slides per group were used for ZEN and subsequent statistical analysis. n = 3 independent experiments. The data are presented as the means ± SDs, and differences were compared by 2-tailed Student’s t tests. Multiple groups were analyzed with one-way ANOVA. **P < 0.01; ***P < 0.001. Source data are provided as a source data file.
Mitophagy contributes to the synergistic inhibitory effect of HPA-12 and Creno in AML cells
Interestingly, in the Combo group, we detected a significant increase in the number of mitochondria, although structural damage was evident (red arrow, Figs. 8a and S9b). We also observed typical autophagosomes engulfing damaged mitochondria (blue arrows), indicating the occurrence of mitophagy (Figs. 8b and S9c). Next, we investigated whether mitophagy played a role in the synergistic inhibitory effect of Combo treatment. We first verified the recruitment of LC3II to the mitochondria. Indeed, confocal microscopy revealed significantly increased colocalization of LC3II (red) and mitochondria (green) in the Combo group (Fig. 8c). WB confirmed the upregulation of the autophagy-related markers Atg7 and P62 (Fig. 8d). The autophagy inhibitor CQ partially reversed the decrease in cell viability in the Combo group (Fig. 8e). Most importantly, the mitophagy inhibitor Mdivi-1 partially but significantly rescued AML cells treated with Combo (Fig. 8f, g). Collectively, these data suggest that the combination of HPA-12 and Creno activates mitophagy to exert an inhibitory effect on AML.
a, b Transmission electron microscopy images of morphological changes in organelles in MV4-11 cells in the DMSO and Combo groups. The cells were treated with HPA-12 (80 µM) or Creno (6 µM) for 48 h. Red arrows indicate mitochondria. Blue arrows indicate autophagosomes engulfing damaged mitochondrial structures. Scale bar, 2 μm. c Confocal microscopy of treated MV4-11 cells dual labeled with an anti-LC3B antibody (red) and the mitochondrial marker Tom20 (green). One field per slide and three slides per group were used for image analysis with ZEN and subsequent statistical analysis. n = 5 independent experiments. Scale bar, 10 μm. d Expression of Atg7 and P62 in MV4-11 cells. e Effect of the autophagy inhibitor CQ on the viability of MV4-11 cells. The cells were treated with DMSO, HPA-12 (80 µM), Creno (6 µM) or their combination for 24 h prior to the viability assay. CQ (10 µM) was added for 24 h of treatment with the other drugs. n = 3 independent experiments. f, g Effects of the mitophagy inhibitor Mdivi-1 on the viability of MV4-11 cells. The cells were treated with DMSO, HPA-12 (80 µM), Creno (6 µM) or their combination for 48 h prior to the viability assay. Mdivi-1 (10 µM) was added for 48 h of treatment with other drugs. n = 3 independent experiments. The data are presented as the means ± SDs, and differences were compared by 2-tailed Student’s t tests. Multiple groups were analyzed with one-way ANOVA. **P < 0.01; ***P < 0.001. Source data are provided as a source data file.
Cotreatment with HPA-12 and Creno is effective in FLT3-ITD+ and FLT3-TKD+ primary AML samples
To further explore the efficacy of HPA-12 and its combination with Creno in treating AML, we collected bone marrow samples from AML patients and treated these primary AML cells with HPA-12 or/and Creno (Fig. S10a). HPA-12 treatment reduced the viability of those primary AML cells from patient 1 ~ 5, 7 (FLT3-ITD+) and 6 (FLT3-TKD+) in a dose-dependent manner (Figs. 9a, and S10b–d). Notably, the combination of HPA-12 with Creno reduce the viability of CD34+ AML cells, whereas the same combination had a minimal effect on CD34+ hematopoietic stem and progenitor cells isolated from healthy donors (Figs. 9b, c). Moreover, HPA-12 treatment dose-dependently induced apoptosis in these primary AML cells (Figs. 9d, e and S10e). Intriguingly, although Creno monotherapy failed to reduce the viability of primary cells from patient 5, 7 (FLT3-ITD+) and 6 (FLT3-TKD+), HPA-12 alone or in combination with Creno decreased cell viability and promote the apoptosis of treated primary cells from these particular patients (Figs. 9f, g, and S10f–i). Moreover, we found that the GRP78/ATF6/CHOP axis was activated by cotreatment with HPA-12 and Creno in these FLT3-ITD+ and FLT3-TKD+ samples (Fig. 9h–j), demonstrating a similar pattern as that in AML cell lines. Next, we assessed the efficacy of HPA-12 and its combination with Creno in FLT3/WT primary AML cells. HPA-12 alone reduced cell viability and increased apoptosis in FLT3/WT primary AML cells of patient 8 (Fig. S10j–k). However, its combination with Creno failed to further reduced the viability of primary AML cells from patient 8, 13–16 (Fig. S10l–n). Further western blot analysis revealed no activation of the GRP78/ATF6/CHOP pathway (Fig. S10o). In addition, we further examined the effects of HPA-12 monotherapy alone and in combination with Creno via a colony formation assay. Consistent with the viability results, the combination of HPA-12 with Creno had a minimal effect on CD34+ hematopoietic stem and progenitor cells, whereas a significant reduction in colony number was observed for CD34+ AML cells (Fig. S11). In summary, these results indicate that HPA-12 alone demonstrated an anti-AML effect on primary AML cells, while its combination with Creno suppressed growth and induced apoptosis in FLT3-ITD+ and FLT3-TKD+ primary AML cells.
a Viability of primary FLT3-ITD+ AML blasts treated with increasing concentrations of HPA-12 (0, 60, 80, and 100 μM) for 48 h. Cell viability was measured by the CCK-8 assay. Viability of FLT3-ITD+ CD34+ primary AML cells (b) and CD34+ hematopoietic stem and progenitor cells (c). CD34+ cells were enriched with anti-hCD34 microbeads. Annexin V/PI staining of primary FLT3-ITD+ AML cells from Patient #5 (d) and FLT3-TKD+ AML cells from Patient #6 (e) treated with increasing concentrations of HPA-12 for 48 h. Annexin V/PI staining of primary FLT3-ITD + AML cells from Patient #5 (f) and FLT3-TKD + AML cells from Patient #6 (g) treated with with HPA-12 (80 µM) and Creno (6 µM) alone or in combination for 48 h. h Expression of GRP78, ATF6, and CHOP determined by western blotting of primary FLT3-ITD+ AML cells from Patient #5 in (h). Expression of GRP78 and ATF6 determined by western blotting in primary FLT3-TKD+ AML cells from Patient #6 (i) and primary FLT3-ITD+ AML cells from Patient #7 (j). The data are presented as the means ± SDs. The characteristics of the AML patients and healthy donors in this figure are shown in Table S9–10. Source data are provided as a source data file.
Discussion
Through transcriptional and pharmacological approaches, we revealed that the inhibition of CERT, a protein responsible for the transfer of Cer from the ER to the Golgi, has an antileukemic effect both in vitro and in vivo. This finding is consistent with the reported role of inhibiting the growth of other types of cancer cells, including ovarian cancer, colorectal cancer, and/or HER2positive breast cancer cells as well as HeLa cells14,24. Therefore, targeting CERT may represent a promising strategy to induce cell death in various cancer cells, including AML cells. Notably, HPA-12 has antileukemic effects but remains relatively safe within the range of 60–100 μM. HPA-12 treatment was well tolerated by normal hematopoietic stem and progenitor cells from healthy donors within this range. In contrast, HPA-12 alone was effective at inhibiting primary AML cells with FLT3 mutations and even those without FLT3 mutations.
Interestingly, we demonstrated that HPA-12 alone or in combination with Creno was more effective in AML cells harboring an FLT3 mutation. This is not surprising, as suppression of Cer generation has been reported for FLT3/ITD+ AML cells, and these cells are sensitive to the accumulation of Cer22. Therefore, the inhibition of Cer trafficking and clearance with HPA-12 in AML cells with FLT3/ITD+ mutations might further increase Cer accumulation. Our data support this possibility, and the combination of HPA-12 with the FLT3 inhibitor Creno led to an increase in Cer levels and a decrease in SM. Consequently, Cer accumulation initiated downstream effector pathways to induce cell death. Notably, AML can be stratified into two clinically relevant sphingolipidomic subtypes that are independent of the AML mutation status25. It would be interesting to explore whether CERT and FLT3 participate in the determination of this subtype in other larger cohorts and whether targeting CERT and FLT3 with our combination regimen would provide clinical benefit, especially in the subtype of AML patients with high SM levels. Another study also reported the heterogeneity of AML in sphingolipid metabolism and S1PR3 signaling in leukemic stem cells (LSCs)26. In that study, the authors reported that the activation of SIPR3 signaling decreased LSC function. The effects of CERT inhibition as well as its combination with FLT3 inhibition should be further explored in the context of other branches of Cer clearance, including S1PR signaling, and in more chemoresistant populations of LSCs.
From a molecular mechanism perspective, the increase in endogenous Cer induced ER stress and activated the UPR pathway. Our data indicated that the combination of HPA-12 with the Creno-activated GRP78/ATF6/CHOP axis inhibited growth and induced apoptosis in AML cells. This finding is different from that of another study in which the clearance of Cer was disrupted with MP-A08, an SPHK1 inhibitor. In that study, MP-A08 activated the integrated stress response (ISR) PKR/ATF4/Noxa/Mcl-1 axis and synergized with venetoclax to inhibit AML23. This might be attributed to the different target and combination regimens we chose. The cytotoxic role of the UPR in the induction of apoptosis has been reported as a therapeutic response in leukemia, when excessive and prolonged ER stress triggers proapoptotic signaling27,28. For example, an inspiring study with the conditional expression of XBP1 reported the activation of the IRE1-XBP1 arm of the UPR in AML in response to chemotherapy and further revealed the activation of the downstream effector miR-22/SIRT1 axis to induce apoptosis29. The roles of the UPR and ISR in normal hematopoiesis have also been investigated extensively. Elevated expression of the PERK arm of the UPR or high expression of ATF4 and low expression of eIF2α has been observed in hematopoietic stem cells (HSCs) compared with those in progenitor cells, and these signatures contribute to the maintenance of the integrity of the HSC pool30,31. Interestingly, the sphingolipid metabolism enzyme DEGS1 governs HSC function by altering the sphingolipid composition, which subsequently activates the UPR and autophagy to maintain stemness32. Taken together, these findings indicate that the UPR/ISR can play cytoprotective and cytotoxic roles in normal hematopoiesis and leukemia. Therefore, the roles of sphingolipid metabolism and the regulation of UPR/ISR in normal hematopoiesis and leukemia should be elucidated and delicately tuned to achieve therapeutic effects to eradicate AML cells while maintaining minimal impairment of normal hematopoiesis or transplanted HSCs. Further studies exploring the effects of UPR/ISR activation through the modulation of sphingolipid composition are warranted.
Our findings support a prominent role for the GRP78/ATF6/CHOP axis, which was demonstrated in this study to mediate Cer-induced AML cell death. However, other mechanisms may also be involved in this process. We observed the accumulation of lipids within treated AML cells in different subcellular compartments; therefore, lipids might also induce other pro-cell death cascades, such as mitophagy. Mitophagy has been reported to be involved in the inhibitory effect of Creno on FLT3/ITD+ AML cells22. In addition, necroptosis also participates in Cer-induced death in lung cancer cells33. Another possibility is lipid stress-induced ferritin deficiency, particularly in FLT3-ITD AML34. The underlying mechanism may be very complex and cellular- and disease-specific and warrants further investigation.
In summary, we have provided evidence for strategies targeting the clearance of Cer to treat AML, especially for patients with FLT3 mutations. An inhibitor of the Cer trafficking protein CERT in collaboration with FLT3 inhibitors may represent a promising combinatory regimen to reduce the possibility of relapse and resistance.
Methods
Ethics statement
The collection of bone marrow samples from AML patients and healthy donors was performed according to a protocol approved by the Institutional Review Board of The First Affiliated Hospital of Jinan University (IRB approval number: KYk-2021-030). Informed consent from all AML patients and healthy donors for sample collection were obtained. All animal experiments were performed in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals, the National Institute of Health guidelines and the ARRIVE guidelines on ethics. The study was authorized by the Institutional Animal Care and Use Committee of Jinan University (JNU-IACUC) and was performed in accordance with the guidelines issued by the committee (approval number: 20221206-02).
Human bone marrow samples
Human bone marrow (BM) samples from AML patients and healthy donors were collected, and the main clinical characteristics of these participants are shown in Table S3. Mononuclear cells were isolated by density gradient centrifugation (Ficoll-Paque, Solarbio, China) and cultured in base media of MEM (Gibco) supplemented with 10 nM human cytokines SCF, IL3, FLT, IL6 and TPO (PEPROTech). The media was supplemented with 20% fetal bovine serum (BI) and 1% penicillin/streptomycin (Gibco). CD34+ primary cells were enriched with anti-hCD34 microbeads (Miltenyi) following the manufacturer’s instructions.
Cell culture and reagents
The AML cell lines MOLM13, MV4-11, THP-1, HL-60, Kasumi-1, OCI-AML3, and KG1-α were cultured in RPMI 1640 medium (BI). The cell lines were authenticated by STR profiling (Guangzhou IGE Biotechnology Co., Ltd., Guangzhou, Guangdong, China) and routinely tested for mycoplasma contamination. Human embryonic kidney (HEK293T) cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with high glucose. All the media contained 10% fetal bovine serum (BI) plus 1% penicillin/streptomycin (Gibco). The cells were maintained in a 37 °C humidified atmosphere containing 5% CO2. Crenolanib and (1 R,3S)-HPA-12 were purchased from Selleck Chemicals (CP-868596, s2730, Houston, TX, USA) and TCI (Shanghai) Development Co., Ltd. (H1553-5MG, 383418-30-22, Shanghai, China), respectively. Stable cell lines were obtained by transduction of lentiviruses carrying shGRP78/shATF6/shCHOP and puromycin (0.5 μg/ml) selection for 14 days. The cell lines or stable cell lines were treated with DMSO, HPA-12 (80 µM), Creno (6 µM) or Combo (80 µM HPA-12 plus 6 µM Creno) for 48 h prior to subsequent assays, if not specified in the legends. The sequences of shCERT, shGRP78, shATF6, and shCHOP are listed in Supplementary Table 4.
Assessment of apoptosis by flow cytometry
The annexin-V and PI double-staining method was used. A total of 1× 106 cells treated with HPA-12 and Creno (at the indicated concentrations), either alone or in combination, were collected after 48 h of incubation, washed twice with cold PBS, resuspended in 300 µL of binding buffer, stained with 5 μL of Annexin-V and 10 μL of PI (Yeasen Biotechnology, Shanghai, China), incubated in the dark at room temperature for 15 min, and subjected to flow cytometry (Becton Dickinson Bioscience, Oxford, UK) to analyze apoptosis.
EdU proliferation assay
Cell proliferation was assessed with a Cell Light 5-ethynyl-2’-deoxyuridine (EdU) imaging kit (Beyotime) according to the manufacturer’s instructions. Treated cells were observed under a fluorescence microscope.
Western blot
Prepared protein lysates were separated by SDS–PAGE and transferred to PVDF membranes (ISEQ00010/IPVH00010, Merck Millipore, China). The membranes were subsequently blocked with fast blocking buffer (GF1815, Beyotime, China) for 30 min at room temperature prior to antibody staining. Specific primary antibodies (a GRP78 polyclonal antibody (11587-1-AP-50UL), ATF6 polyclonal antibody (24169-1-AP-50UL), CHOP polyclonal antibody (15204-1-AP-50UL), phospho-EIF2S1 (Ser51) monoclonal antibody (68023-1-Ig), ATF4 polyclonal antibody (10835-1-AP), and beta-actin recombinant antibody (81115-1-RR) from Proteintech (USA), a phospho-IRE1 (Ser724) antibody (AF7150), XBP1 antibody (AF5110), and tubulin beta antibody (AF7011) from Affinity (USA), and a P-PERK antibody (T982, WL05295) from Wanleibio (Shengyang)) were added overnight incubation at 4 °C. A secondary antibody (HRP-conjugated AffiniPure goat anti-rabbit lgG (H + L) (SA00001-2-100UL) or HRP-conjugated AffiniPure goat anti-mouse lgG (H + L) (SA00001-1-100UL), both from Proteintech, USA) was then added, and incubated with the membrane for 1 h at room temperature. Signal detection was performed with enhanced chemiluminescence (P0018FS, Beyotime, China). Quantitative analysis of proteins was performed with ImageJ software.
Cytotoxicity assay
To examine the toxicity of different drugs, the viability of treated cells was determined by a Cell Counting Kit (40203ES76) following the manufacturer’s manual (Yeasen, China). The cell lines were seeded in 96-well culture plates at a density of 5 × 104 cells/ml. After treatment, 10 µL of CCK-8 solution was added to each well, and the cells were further cultured at 37 °C for 3 h. The absorbance at a wavelength of 450 nm was determined with a spectrophotometer (Bio Tek Instruments, USA).
Immunohistochemical (IHC) staining analysis
The tissue was fixed in 4% PFA and embedded in paraffin. The embedded samples were sectioned into 5-µm-thick slices on a sliding microtome (Leica, Nussloch, Germany) and mounted on Superfrost Plus slides for IHC staining. Antibodies against CD45 were used for IHC staining. The tissue was visualized by optical microscopy (Olympus BX41, Japan).
Transmission electron microscopic analysis
Freshly excised tissues were cut into 1 mm cubes, fixed overnight in 2.5% glutaraldehyde, postfixed with 1% osmium tetroxide, dehydrated through a graded ethanol series, and embedded in epoxy resin. Ultrathin sections (70 nm) were generated and double-stained with uranyl acetate and lead citrate. The prepared samples were photographed with a TECNA110 transmission electron microscope (Philips, Netherlands).
Immunofluorescence staining/confocal microscopy
After the desired treatment, the MV4-11 and Molm13 cells were washed with PBS, fixed in 4% (w/v) paraformaldehyde for 20 min, permeabilized in 0.2% (v/v) Triton X-100 for 30 min, and blocked for 1 h in 3% BSA. Fixed and permeabilized cells were then incubated with appropriate primary antibodies in PBS + 1% BSA overnight at 4 °C, washed and stained with fluorescence-conjugated secondary antibodies (1:1000) for 1 h at room temperature. The cells were washed three times, and a final concentration of 2 mg/mL DAPI (Sigma) was included in the final wash to stain the nuclei. Images were captured with a Zeiss LSM880 Airyscan confocal microscope with a 100X objective (N.A. 1.46). A Ceramide antibody (Cat. No. ALX-804-196-T050, Enzo) at a 1:50 dilution was used for detection in nonadherent cells following a protocol previously described35. The antibodies used for immunofluorescence staining were as follows: GOLGA2/GM130 polyclonal antibody, Cat. No. 11308-1-AP; TOM20 polyclonal antibody, Cat. No. 11802-1-AP; PDI polyclonal antibody, Cat. No. 11245-1-AP; Alexa Fluor 488 anti-LC3B antibody (1:100 dilution) (clone EPR18709, Abcam, #ab225382); CoraLite594-conjugated goat anti-rabbit IgG, Cat. No. SA00013-4; and CoraLite488-conjugated goat anti-mouse IgG (H + L), Cat. No. SA00013-1. For triple staining of ceramide, the ER and the Golgi, the treated cells were resuspended in PBS and adjusted to a concentration of 1×106 cells/ml to 2×106 cells/ml. The cell suspensions were applied and fixed on the slides by the cytospin technique. Subsequent staining of ceramide, the ER, and the Golgi in combination with DAPI staining was performed with a TSA 7-color kit (abs50015, Absin Bioscience, Shanghai, China) following the manufacturer’s instructions. The following primary antibodies were used: a ceramide antibody (1:100), PDI antibody (1:100), and GOLGA2/GM130 (1:100). The HRP-labeled secondary antibody was added at a 1:400 dilution. The quantification of ceramide colocalization with the ER and Golgi was performed with ImageJ.
Mitochondrial function assays
JC-1 staining
JC-1 staining was used to assess mitochondrial damage. The cells (3×105/well) were seeded into 6-well plates, subjected to drug treatment for the indicated time, harvested and resuspended in PBS. Staining was conducted in PBS containing 40 μg/mL RNaseA at 37 °C and 10 μg/mL PI in the dark at room temperature for 30 min. The cells were washed twice with PBS and suspended in 500 μL of PBS. For each sample, 2 × 105 cells were collected and analyzed with a FACS-Canto cytometer (Becton Dickinson, San Jose, CA, USA). A JC-1 staining kit (#C2006) was purchased from Beyotime (Haimen, China).
MitoSOX staining
After drug treatment for 48 h, the MV4-11 cells were collected and washed twice with PBS, and the cell suspension was uniformly dropped onto an adsorbent slide (CITOTEST, 100105, China), followed by incubation until the cells were semidry. The cells were then fixed with 4% (w/v) paraformaldehyde (Biosharp, BL539A, China) for 20 min and incubated with a MitoSOX probe at 37 °C (Yeasen, 40778ES50, China). The cells were washed three times, and a final concentration of 2 mg/mL DAPI was included in the final wash to stain the nuclei. Images were captured with a Zeiss LSM880 Airyscan confocal microscope with a 100X objective. The MitoSox signal was quantified via ImageJ.
RNA-Seq analysis
Approximately 1 × 106 MV4-11 cells, including groups of cells treated with DMSO or HPA-12 (80 µM) and Creno (6 µM) alone or in combination for 48 h, were collected and suspended in TRIzol reagent. These cells were then sent to Personalbio Technologies (Shanghai, China) for RNA isolation, library preparation and sequencing.
Targeted lipidomics
MV4-11 cells were treated with DMSO, 60 μM HPA-12, 4 μM Creno, or Combo. After 48 h of treatment, 1 × 107 cells were collected and centrifuged. The culture medium was removed to retain the precipitate, which was then washed twice with PBS. The samples were blotted dry, and the precipitates were retained, quenched with liquid nitrogen, and stored temporarily in a –80 °C freezer. Three replicates were prepared for each group. Subsequent quality control and targeted lipidomics were performed by Metabo-Profile Biotechnology (Shanghai) Co., Ltd. (Shanghai, China).
Leukemia xenograft model
Animal experiments were conducted in a specific pathogen-free facility at the Laboratory Animal Center of Jinan University. All animal experiments were approved by and performed according to the guidelines of the Institutional Animal Care and Use Committee of Jinan University (IACUC-JNU, approval number: 20221206-02). Six- to eight-week-old B/NDG female mice were obtained from Zhuhai Bes Test Bio-Tech Co., Ltd. (Guangdong, China). The mice were kept on a 12/12 h light/dark cycle and given water and food ad libitum. On Day 0, each mouse was injected intravenously with 1 × 106 MV4-11 cells. On Day 4, the mice were randomly distributed into four groups: untreated (n = 8), Creno (15 mg/kg; n = 8), HPA-12 (4 mg/kg; n = 8), or combination (HPA-12 4 mg/kg plus Creno 15 mg/kg; n = 8). All treatments were administered I.P. or S.C. from Day 7 for a “5 days on, 2 days off” scheme for 2 cycles. The mice were weighed every two days. Three mice in each group were randomly selected and humanely euthanized after 2 treatment cycles; the bone marrow (BM) and spleen were harvested for flow cytometric measurements and immunohistochemical staining analysis. The other mice were maintained until a humane endpoint. The humane endpoint for these AML mice was achieved when severe clinical signs of leukemia were observed (hind limb paralysis), or when they became moribund. No animal exceeded the humane endpoint. The investigators were not blinded to group allocation.
Statistics and reproducibility
Statistical analyses were performed with GraphPad Prism 8.0c (GraphPad Software, La Jolla, CA), and the data are presented as the means ± SD. Student’s t test was used for comparisons between two groups. For survival analysis, Kaplan–Meier survival curves were generated, and the log-rank test was performed. P < 0.05 was considered statistically significant. Statistical significance is indicated with asterisks (* P < 0.05, ** P < 0.01, *** P < 0.005, **** P < 0.001). No statistical method was used to predetermine the sample size. No data were excluded from the analyses. Blinding was not applicable to this study.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The bulk RNA-seq data of this study have been deposited in the GEO database with the accession number GSE280391. All other data are available in the main text, supplementary information or source data files. Source data are provided with this paper.
References
Pan, C., Li, B. & Simon, M. C. Moonlighting functions of metabolic enzymes and metabolites in cancer. Mol. cell 81, 3760–3774 (2021).
Broadfield, L. A., Pane, A. A., Talebi, A., Swinnen, J. V. & Fendt, S. M. Lipid metabolism in cancer: New perspectives and emerging mechanisms. Developmental cell 56, 1363–1393 (2021).
Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 18, 33–50 (2018).
Schömel, N., Geisslinger, G. & Wegner, M. S. Influence of glycosphingolipids on cancer cell energy metabolism. Prog. lipid Res. 79, 101050 (2020).
Canals, D. & Clarke, C. J. Compartmentalization of Sphingolipid metabolism: Implications for signaling and therapy. Pharmacol. Ther. 232, 108005 (2022).
Morad, S. A. & Cabot, M. C. Ceramide-orchestrated signalling in cancer cells. Nat. Rev. Cancer 13, 51–65 (2013).
Bai, A. P. & Guo, Y. Ceramide is a potential activator of immune responses against tumors. Gastroenterology 155, 579–580 (2018).
Tallima, H., Azzazy, H. M. E. & El Ridi, R. Cell surface sphingomyelin: Key role in cancer initiation, progression, and immune evasion. Lipids health Dis. 20, 150 (2021).
Haddadi, N., Lin, Y., Simpson, A. M., Nassif, N. T. & McGowan, E. M. “Dicing and Splicing” sphingosine kinase and relevance to cancer. Int. J. Mol. Sci. 18, 1891 (2017).
Hanada, K. et al. Molecular machinery for non-vesicular trafficking of ceramide. Nature 426, 803–809 (2003).
Maceyka, M., Harikumar, K. B., Milstien, S. & Spiegel, S. Sphingosine-1-phosphate signaling and its role in disease. Trends cell Biol. 22, 50–60 (2012).
Kumagai, K. & Hanada, K. Structure, functions and regulation of CERT, a lipid-transfer protein for the delivery of ceramide at the ER-Golgi membrane contact sites. FEBS Lett. 593, 2366–2377 (2019).
Swanton, C. et al. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer cell 11, 498–512 (2007).
Lee, A. J. et al. CERT depletion predicts chemotherapy benefit and mediates cytotoxic and polyploid-specific cancer cell death through autophagy induction. J. Pathol. 226, 482–494 (2012).
Palau, V. E. et al. γ-Tocotrienol induces apoptosis in pancreatic cancer cells by upregulation of ceramide synthesis and modulation of sphingolipid transport. BMC cancer 18, 564 (2018).
Hanada, K., Sakai, S. & Kumagai, K. Natural ligand-mimetic and nonmimetic inhibitors of the ceramide transport protein CERT. Int. J. Mol. Sci. 23, 2098 (2022).
Yasuda, S. et al. A novel inhibitor of ceramide trafficking from the endoplasmic reticulum to the site of sphingomyelin synthesis. J. Biol. Chem. 276, 43994–44002 (2001).
Crivelli, S. M. et al. Synthesis, radiosynthesis, and preliminary in vitro and in vivo evaluation of the fluorinated ceramide trafficking inhibitor (HPA-12) for brain applications. J. Alzheimer’s. Dis.: JAD 60, 783–794 (2017).
Charruyer, A. et al. Decreased ceramide transport protein (CERT) function alters sphingomyelin production following UVB irradiation. J. Biol. Chem. 283, 16682–16692 (2008).
Crivelli, S. M. et al. CERT(L) reduces C16 ceramide, amyloid-β levels, and inflammation in a model of Alzheimer’s disease. Alzheimer’s. Res. Ther. 13, 45 (2021).
Chung, L. H., Liu, D., Liu, X. T. & Qi, Y. Ceramide transfer protein (CERT): An overlooked molecular player in cancer. Int. J. Mol. Sci. 22, 13184 (2021).
Dany, M. et al. Targeting FLT3-ITD signaling mediates ceramide-dependent mitophagy and attenuates drug resistance in AML. Blood 128, 1944–1958 (2016).
Lewis, A. C. et al. Ceramide-induced integrated stress response overcomes Bcl-2 inhibitor resistance in acute myeloid leukemia. Blood 139, 3737–3751 (2022).
Hullin-Matsuda, F. et al. Limonoid compounds inhibit sphingomyelin biosynthesis by preventing CERT protein-dependent extraction of ceramides from the endoplasmic reticulum. J. Biol. Chem. 287, 24397–24411 (2012).
Paudel, B. B. et al. Acute myeloid leukemia stratifies as 2 clinically relevant sphingolipidomic subtypes. Blood Adv. 8, 1137–1142 (2024).
Xie, S. Z. et al. Sphingosine-1-phosphate receptor 3 potentiates inflammatory programs in normal and leukemia stem cells to promote differentiation. Blood cancer Discov. 2, 32–53 (2021).
Féral, K. et al. ER stress and unfolded protein response in Leukemia: Friend, foe, or both? Biomolecules 11, 199 (2021).
Khateb, A. & Ronai, Z. A. Unfolded protein response in Leukemia: From basic understanding to therapeutic opportunities. Trends cancer 6, 960–973 (2020).
Philippe, C. et al. Pivotal role of the endoplasmic reticulum stress-related XBP1s/miR-22/SIRT1 axis in acute myeloid leukemia apoptosis and response to chemotherapy. Leukemia 38, 1764–1776 (2024).
van Galen, P. et al. The unfolded protein response governs integrity of the haematopoietic stem-cell pool during stress. Nature 510, 268–272 (2014).
van Galen, P. et al. Integrated stress response activity marks stem cells in normal hematopoiesis and Leukemia. Cell Rep. 25, 1109–1117.e1105 (2018).
Xie, S. Z. et al. Sphingolipid modulation activates proteostasis programs to govern human hematopoietic stem cell self-renewal. Cell Stem Cell 25, 639–653.e637 (2019).
Saddoughi, S. A. et al. Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A-RIPK1-dependent necroptosis. EMBO Mol. Med. 5, 105–121 (2013).
Sabatier, M. et al. C/EBPα confers dependence to fatty acid anabolic pathways and vulnerability to lipid oxidative stress-induced ferroptosis in FLT3-mutant leukemia. Cancer Discov. 13, 1720–1747 (2023).
Vaena, S. et al. Aging-dependent mitochondrial dysfunction mediated by ceramide signaling inhibits antitumor T cell response. Cell Rep. 35, 109076 (2021).
Acknowledgements
We are grateful to Dr. Xuejie Jiang and Dr. Lingling Liu for providing human BM samples from AML patients and healthy donors samples. This work was supported by the National Natural Science Foundation of China (Grant No. 81970143 and No. 82270167), the Talent Young Program of Guangdong Province (2021B1515020017), Municipal-School Joint Program from Guangzhou Science and Technological Project (202201020012) and the Leading Talents Program from The First Affiliated Hospital of Jinan University to H.Z.
Author information
Authors and Affiliations
Contributions
Conceptualization: H.Z.; Methodology: X.F.S., Y.L., F.S.L., C.P.W., W.H.X., G.P.Y., X.W.C.; Investigation: H.Z., J.D.; Visualization: X.F.S., Y.L., J.D.; Funding acquisition: H.Z.; Project administration: H.Z.; Supervision: H.Z., J.D.; Writing original draft: X.F.S.; Writing review & editing: H.Z., J.D., R.P.G.
Corresponding author
Ethics declarations
Competing interests
The Authors declare the following competing interests: R.P.G. is a consultant to NexImmune Inc. Nanexa Pharma Ascentage Pharm Group and Antengene Biotech LLC, Medical Director of FFF Enterprises Inc.; Partner in AZCA Inc.; Board of Directors of Russian Foundation for Cancer Research Support and Scientific Advisory Board of StemRad Ltd. Other authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Besim Ogretmen, Kentaro Hanada and Stephanie Xie for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, X., Li, Y., Du, J. et al. Targeting ceramide transfer protein sensitizes AML to FLT3 inhibitors via a GRP78-ATF6-CHOP axis. Nat Commun 16, 1358 (2025). https://doi.org/10.1038/s41467-025-56520-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-56520-7