Abstract
Immunometabolism, encompassing metabolic processes within the immune system, plays a pivotal role in modulating the development, activity, and function of immune cells. Oxidative stress, resulting from an imbalance between pro-oxidants and antioxidants, is a critical factor in the pathogenesis of various diseases, including cancer and aging. This review synthesizes current knowledge on the interplay between immunometabolism and oxidative stress, highlighting their mechanisms in cancer progression and the aging process. We discuss how metabolic reprogramming in our body can influence immune cell function and promoting ageing and cancer development. Additionally, we examine the impact of aging on immune metabolism, leading to a decline in immune function and a predisposition to chronic diseases. The review also explores the potential of traditional Chinese medicine in targeting oxidative stress to delay aging and combat cancer, underscoring the need for further research to elucidate the molecular mechanisms underlying these effects. Our findings suggest that interventions targeting immunometabolism and oxidative stress could offer novel therapeutic avenues for cancer and aging-related diseases.
Similar content being viewed by others
Introduction
Immunometabolism refers to the metabolic processes and molecular regulation involved in the immune system, playing a crucial role in regulating the development, activity, and function of immune cells. It involves various aspects, including energy production, substance transport, signal transduction, and molecular regulation, all of which are essential for maintaining the normal functions of immune cells. The key processes include: glucose metabolism (immune cells, especially activated T cells and macrophages, are highly dependent on glucose. The regulation of lipid metabolism is critical for immune cell function and response), and amino acid metabolism (certain amino acids, such as glutamate, arginine, cysteine, etc., play important roles in anti-tumor, anti-aging, antioxidant, and anti-infection responses)1,2,3,4.
Biochemical reactions in metabolism are closely linked with redox reactions. In biological systems, redox reactions occur through the transfer of electrons from reduced donor molecules (including NADPH and thiols in reduced glutathione (GSH) and the amino acid cysteine found in many proteins) to acceptor molecules (such as NADP+ and disulfide bonds in cysteine, the oxidized dimeric form of cysteine)5. During oxidative phosphorylation (OXPHOS) metabolism, the electron transport chain (ETC) delivers electrons to molecular oxygen, a process that drives energy production in aerobic organisms’ mitochondria. This process inevitably generates oxygen radicals (also known as reactive oxygen species (ROS), such as hydrogen peroxide and superoxide). Since radicals can induce damage to DNA, proteins, and lipids, aerobic organisms have developed complex antioxidant systems, such as thioredoxin (TRX), GSH, and the NF-E2-related factor 2 (NRF2) system, to balance oxidant levels in each cell and maintain redox homeostasis6.
Normal immunometabolism is often accompanied by redox reactions, but when the balance between oxidation and antioxidant defense is disrupted, oxidative stress occurs7. OS is a state of imbalance between oxidation and antioxidant defense in the body, tending towards oxidation, leading to neutrophil inflammatory infiltration, increased protease secretion, and the production of large amounts of oxidative intermediates8. Oxidative stress is a negative effect caused by the generation of free radicals in the body and is a significant factor contributing to aging and disease9,10. In immunometabolism, ROS can damage lipids, nucleic acids, and proteins, altering their functions. When the balance between ROS production and antioxidant defenses is disrupted, oxidative stress ensues, leading to various diseases11,12.
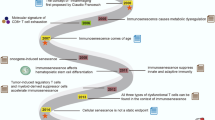
This review focuses on the interaction and regulatory mechanisms between immunometabolism and oxidative stress in aging and tumor promotion research, summarizing existing reports on the intervention of active components of traditional Chinese medicine (Fig. 1).
This schematic elucidates the mechanistic convergence of oxidative stress and immunometabolic dysregulation in aging and cancer pathogenesis, alongside TCM interventions bridging both diseases. The upper panel illustrates anti-aging TCM formulations (e.g., Astragali Radix, Lycii Fructus), while the lower panel highlights anti-tumor TCM agents (e.g., Astragalus polysaccharides, Baicalin), with overlapping components (e.g., Astragali Radix-derived compounds) emphasizing their dual therapeutic potential. Physiological redox balance (left): Controlled oxidative eustress (ROS/antioxidant equilibrium) sustains redox-sensitive signaling and immunometabolic homeostasis in innate/adaptive immune cells (NK cells, T cells, macrophages). This enables efficient immunosurveillance against damaged or transformed cells through balanced glycolysis and mitochondrial respiration. ROS sources (middle): Mitochondrial dysfunction, NADPH oxidases, and xenobiotics generate ROS subtypes (e.g., O2−, H2O2) that disrupt redox signaling. Notably, aging-associated mitochondrial decay and cancer-driven Warburg effect synergistically amplify ROS production. Pathological crosstalk (right): Sustained oxidative stress triggers a vicious cycle: Immunometabolic paralysis: Excessive ROS impair immune cell metabolic adaptation (e.g., suppressed glucose utilization, defective lipid oxidation), compromising cytotoxic activity and antigen presentation. Redox signaling collapse: Dysregulated redox networks deplete antioxidant defenses (e.g., SOD, CAT) while amplifying pro-inflammatory cytokine release, fostering a chronic inflammatory microenvironment. Disease convergence: This dual failure enables the survival of redox-damaged cells, driving senescent cell accumulation and malignant clone expansion through oxidative DNA damage and impaired repair mechanisms, thereby accelerating both tissue aging and tumor progression. Anti-aging interventions (top): Classical formulations (e.g., Astragali Radix, Lycii Fructus) ameliorate age-related pathologies by modulating oxidative stress-immunometabolic crosstalk, particularly through mitochondrial ROS regulation and metabolic reprogramming of senescent immune cells. Anti-aging interventions (bottom): Multi-herb formulations (e.g., Bu Shen Huo Xue Decoction) and bioactive compounds (e.g., Baicalin) counteract tumor progression via dual regulation of redox homeostasis and immunometabolic rewiring in tumor-associated immune cells. TCM therapeutic advantages: TCM leverages shared components (e.g., Astragali Radix, Huangqi Baihe Granules) to concurrently mitigate oxidative damage in aging tissues and tumor microenvironments through redox homeostasis regulation, rejuvenate immunometabolic function. This synergistic strategy positions TCM as a unique paradigm for managing aging-cancer comorbidities, particularly in elderly patients with age-related malignancies.
Fundamental definition and core mechanisms of oxidative stress
Oxidative stress refers to a pathological state in which the rate of ROS and reactive nitrogen species (RNS) production exceeds the scavenging capacity of endogenous antioxidant systems, leading to oxidative damage of biomolecules (e.g., DNA, proteins, lipids)7,13. Its core characteristic is the disruption of the dynamic balance between oxidation and antioxidant systems, resulting in the accumulation of free radicals that cause cellular dysfunction or even death. When the body is under oxidative stress, the generation rate of ROS and RNS surpasses the clearance capacity of endogenous antioxidant systems (e.g., the glutathione system), resulting in excessive ROS production, which serves as a key driver of tumor initiation, progression, metastasis, and drug resistance14.
Reactive oxygen species and reactive nitrogen species are not only effectors of oxidative damage but also critical cellular signaling molecules. At physiological concentrations, ROS/RNS regulate processes such as cell proliferation, differentiation, and immune responses through reversible modifications of target proteins (e.g., thiol oxidation or nitrosylation of cysteine residues)15,16,17. For example, low concentrations of H₂O₂ promote cell survival by inhibiting the phosphatase PTEN and activating the PI3K/Akt pathway, while superoxide anions (O₂⁻) produced by NOX2 in macrophages induce pro-inflammatory cytokine expression via NF-κB signaling to enhance antimicrobial immunity18,19,20. However, when ROS/RNS production surpasses the antioxidant system’s clearance capacity, irreversible oxidative damage (e.g., DNA strand breaks, lipid peroxidation) dominates the pathological process, leading to oxidative stress. The effects of ROS/RNS must also be dynamically assessed based on their concentration and microenvironment. In the tumor microenvironment, chronic oxidative stress promotes PD-L1 upregulation and T cell exhaustion, while localized low levels of ROS may enhance angiogenesis via HIF-1α signaling. During aging, mitochondrial ROS (mtROS) accumulation triggers DNA damage and senescence-associated secretory phenotype (SASP) secretion, whereas physiological ROS fluctuations may delay aging through Nrf2 activation21.
When the pathological progression of oxidative stress surpasses the cellular repair threshold, the resulting lipid peroxidation cascade may drive an iron-dependent cell death modality—ferroptosis, a molecular-level causal nexus that manifests divergent biological destinies in aging and tumorigenesis. Ferroptosis is an iron-dependent, non-apoptotic form of cell death characterized by lipid peroxidation-driven loss of plasma membrane integrity, primarily mediated through three interconnected mechanisms: iron metabolism dysregulation, glutathione peroxidase 4 (GPX4) inactivation with concomitant glutathione depletion, and lipoxygenase (LOX)-catalyzed lipid peroxidation. Iron metabolism dysregulation involves labile Fe²⁺ ions catalyzing lipid peroxidation via the Fenton reaction, generating hydroxyl radicals (·OH) that attack polyunsaturated fatty acid (PUFA) double bonds to initiate a self-propagating lipid peroxidation chain reaction22. Concurrently, GPX4—the sole enzyme responsible for repairing membrane lipid peroxidation—loses activity when its cofactor GSH is depleted, such as through inhibition of the cystine-glutamate antiporter System Xc⁻ (SLC7A11/SLC3A2), leading to irreversible lipid peroxide (LPO) accumulation23. Additionally, LOXs (e.g., ALOX15) directly oxidize PUFAs into lipid hydroperoxides (PUFA-OOH), accelerating ferroptosis execution, a process that can be pharmacologically inhibited by LOX blockers like zileuton or mitigated through structural PUFA modifications such as bis-allylic deuteration24.
Mechanisms of oxidative stress in aging
Functional changes induced by aging
Human aging is a complex physiological process characterized by the gradual deterioration, damage, and even loss of tissue and organ functions over time. This process involves various molecular functions and cellular changes, including mitochondrial dysfunction, DNA damage, telomere shortening, lipid peroxidation, and protein oxidation modifications. These changes not only accelerate the aging process but also serve as significant risk factors for age-related diseases such as cancer, type II diabetes, autoimmune diseases, infections, and cardiovascular diseases25.
Nuclear DNA damage is a critical mechanism of aging, with signaling accumulating in the activation of p53, leading to cell cycle arrest. Prolonged DNA damage response (DDR) promotes aging26. Persistent activation of DDR at telomeres is sufficient to trigger replicative senescence27. Additionally, the upregulation of p21 and p16 halts the cell cycle, influencing the aging process28. Furthermore, the accumulation of senescence-associated β-galactosidase (SA-β-gal) due to increased lysosomal content indicates metabolic changes associated with aging29. Different levels of post-transcriptional regulatory pathways influence aging, including the actions of mRNA-binding proteins (RBP) and non-coding RNAs30, and dysregulation of splicing factor expression31. Oncogene activation, partially determined by reactive oxygen species production, alters proliferation and DNA replication profiles26. As cells age, there is a notable increase in lysosomal mass, which is accompanied by marked changes in the activity of β-galactosidase within lysosomes. The subsequent buildup of lipofuscin, characteristic of lysosomal aggregates, is associated with metabolic disruptions and impaired autophagic function, establishing it as a key biomarker for cellular aging29. all contribute to accelerated aging. Immunosenescence is another crucial factor in aging, where the secretion of pro-inflammatory cytokines, chemokines, proteases, and growth factors affects neighboring cells, weakening the immune system’s resistance to tumors and pathogens, increasing the risk of autoimmune diseases, and leading to chronic inflammation32.
As people age, the immune system gradually declines, often resulting in a chronic inflammatory state known as “inflammaging.” This is characterized by a reduction in the number and function of immune cells, as well as a decrease in immune response and regulation. The senescence-associated impairment of clearance mechanisms results in the pathological accumulation of senescent cells, extracellular debris, and infectious pathogens. This process, coupled with the exhaustion of myeloid and lymphoid cells, synergistically induces a significant upregulation in the production of pro-inflammatory cytokines, including interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α). This phenomenon is linked to age-related thymic atrophy, resulting in diminished thymopoiesis and a reduced T cell repertoire, which hampers effective immune responses to new antigens33. Thymic atrophy, imbalance in the ratios of naive to memory cells, metabolic dysregulation, and epigenetic alterations are significant features of immunosenescence. Disrupted T cell pools and chronic antigen stimulation lead to premature aging of immune cells, which develop a pro-inflammatory senescence-associated secretory phenotype, exacerbating inflammaging34. Older adults are unable to mount sufficient immune responses to microbial infections and tumors, leading to the accumulation of senescent T cells, B cells, and myeloid cells. Age-associated B cells can efficiently produce autoantibodies. T cell senescence promotes the accumulation of terminally differentiated effector T cells with potent cytotoxic and pro-inflammatory capabilities, while senescent myeloid cells contribute to a state of low-grade, sterile chronic inflammation (inflammaging)35. Additionally, the deterioration of macrophage function is a key factor in immunosenescence, as the ability of macrophages to effectively clear senescent cells from tissues declines with age. Senescent macrophages exhibit a significant increase in Pro-inflammatory and immunomodulatory cytokines and chemokines (including TNF-α, IL-6, and IL-1β), diminished autophagic function, impaired antiviral capabilities, and downregulation of glycolysis and mitochondrial oxidative phosphorylation, ultimately leading to a state of energy depletion36. Such immune dysfunction and excessive inflammatory mediators reduce the body’s ability to recognize and eliminate pathogens, such as the decline in CD28 expression and the increase in senescence markers like CD5737,38. These changes elevate the risk of infections and diseases, leading to tissue damage and functional impairment39. During aging, the immune system’s ability to recognize self-tissues declines, increasing the likelihood of autoimmune reactions and the development of autoimmune diseases37. Aging cells are critical drivers of the aging process, characterized by cell cycle arrest and the development of chronic inflammation. They primarily induce damage to adjacent tissues by secreting a variety of pro-inflammatory cytokines and chemokines. These inflammatory mechanisms are implicated in the pathogenesis of various age-related disorders, including autoimmune diseases. With advancing age, the immune system undergoes a functional decline—termed immunosenescence—characterized by a reduced ability to effectively respond to pathogens and eliminate malignant cells. This decline includes altered ratios of naive to memory T cells, disruptions in the CD4/CD8 balance, impaired calcium signaling, and thymic involution40.
Inflammaging fundamentally differs from acute inflammation in triggers, molecular mechanisms, and functional outcomes. Acute inflammation is transiently activated by pathogens or tissue damage via TLRs/NF-κB, leading to a burst of pro-inflammatory cytokines (e.g., IL-1β, TNF-α) to eliminate threats, followed by rapid resolution via anti-inflammatory signals (e.g., IL-10, TGF-β)18. In contrast, inflammation arises from persistent accumulation of senescent cells, mitochondrial dysfunction (e.g., excessive mtROS), and immunometabolic dysregulation, characterized by chronic, low-level secretion of pro-inflammatory factors (e.g., IL-6, TGF-β)41. Inflammation, a maladaptive chronic inflammatory state distinct from the self-limiting acute inflammatory response, is primarily driven by three interconnected mechanisms. Senescent cells contribute through the senescence-associated secretory phenotype (SASP), which involves the sustained secretion of pro-inflammatory mediators such as IL-6 and matrix metalloproteinases (MMPs)42. These factors perpetuate inflammation by activating NF-κB and JAK-STAT signaling pathways, creating a self-amplifying feedback loop. Concurrently, mitochondrial dysfunction and oxidative stress exacerbate inflammaging via mitochondrial reactive oxygen species (mtROS), which not only trigger NLRP3 inflammasome activation and IL-1β maturation but also suppress SIRT1 deacetylase activity, further reinforcing NF-κB-driven inflammatory cascades43. Additionally, age-related immunometabolic dysregulation to diminish glycolytic flux and enhanced fatty acid oxidation in aging T cells promotes the expansion of pro-inflammatory Th17 subsets while impairing regulatory T cell (Treg) function44. Collectively, these mechanisms drive tissue damage and accelerate age-related pathologies, including cancer and atherosclerosis, underscoring the systemic harm caused by chronic inflammation in aging.
The impact of oxidative stress on aging
The free radical theory of aging suggests that free radicals generated by external factors and internal metabolism can damage cellular structures, disrupt cellular functions, and ultimately trigger apoptosis and organismal aging. OS, as a physiological process induced by free radicals, is a key factor in causing oxidative damage to cells and tissues and a major driver of aging45. Oxidative stress occurs when the excessive production of reactive oxygen species and reactive nitrogen species overwhelms the antioxidant system’s ability to eliminate them31. Oxidative stress results from the excessive production of free radicals and oxidants within cells, surpassing the cell’s antioxidant defenses and leading to oxidative damage that hinders cellular proliferation46.
The oxidative damage and DNA damage induced by oxidative stress can also trigger apoptotic stress, leading to a reduction in cell numbers. As shown in Fig. 2, the oxidative stress state of cells can influence the tissue microenvironment through various pathways, exacerbating telomere dysfunction and accelerating cellular aging. Key pathways include iNOS-related oxidative damage and Nrf2-associated mitochondrial dysfunction, which also mediate pathways related to cell death. Additionally, failure of antioxidant cascades, influenced by defects in the transcription factor Nrf247, and the activation of redox-sensitive pathways regulated by the transcription factor NF-κB contribute to this process48 This activation triggers molecular cascades associated with p53/p21 (due to persistent double-strand breaks and telomere shortening) and p16/Rb (due to epigenetic modifications)49. In summary, cellular aging results from a multifaceted interplay of these factors.
Oxidative stress influences cellular aging through various mechanisms and signaling pathways, resulting in a decline in cellular function and physiological impairment. This phenomenon is primarily characterized by elevated levels of ROS and RNS, which surpass the capacity of the antioxidant defense system50. The ROS generated during oxidative stress can directly damage cellular DNA and lead to mitochondrial dysfunction, exhibiting features of mitochondrial apoptotic stress, which ultimately results in decreased cellular functionality51. Oxidative stress also activates key signaling pathways, such as p53 and ATM/ATR, promoting cell cycle arrest and apoptosis. The modulation of AMPK and mTOR signaling pathways further impacts cellular metabolism and survival. Then, oxidative stress can trigger chronic inflammation by activating inflammatory factors such as IL-1β, thereby accelerating the aging process. These signaling pathways represent critical mechanisms through which oxidative stress affects aging.
Immune cells also produce inflammatory compounds to perform their defense functions. However, if their production is not well controlled by anti-inflammatory compounds, it can lead to inflammatory responses. Additionally, chronic inflammation can accelerate the aging of immune cells, resulting in impaired immune function that is unable to clear senescent cells and inflammatory factors, thus establishing a vicious cycle of inflammation and aging25,40. In addition, studies have shown that inflammatory stimuli can lead to irreversible damage to hematopoietic stem cell (HSC) function, which does not recover to pre-inflammation levels even one year after the resolution of inflammation in mice. Moreover, sporadic and temporally disjointed inflammation can cumulatively exacerbate harm to HSC function, leading to a series of aging-related changes at both the molecular and cellular levels in HSCs25. Since oxidation and inflammation are interconnected processes with many feedback loops50, excessive production of ROS and RNS by leukocytes can also trigger inflammatory responses in these immune cells. Studies have found that high oxidative stress levels (e.g., increased intracellular superoxide anion, oxidized glutathione, XO activity, etc.) and oxidative damage (e.g., elevated malondialdehyde, 8-oxodeoxyguanosine levels) in leukocytes from aged mice and peripheral blood immune cells from elderly humans are consistent with impaired immune responses. In contrast, healthy centenarians and long-lived mice maintain preserved immune function, with low expression of pro-inflammatory genes and well-controlled oxidative stress in their immune cells, which may explain their delayed aging51. Rich lipofuscin granules have been observed in lymphocytes from aged mice, and it is known that lipofuscin may act as a danger signal, stimulating the release of pro-inflammatory chemokines and cytokines and activating macrophages, thereby leading to a chronic oxidative-inflammatory process52. Recent research has indicated that the removal of lipofuscin significantly reduces neuroinflammation and neuronal apoptosis, suggesting its potential role in mitigating age-related neurodegenerative processes53. Therefore, if the immune system fails to regulate the “oxidation-inflammation-aging” process effectively, it will further accelerate the aging process.
Telomerase, composed of TERT and TERC subunits, is a reverse transcriptase that extends telomeres53. DNA damage caused by oxidative stress can activate ATM/ATR kinases, which in turn activate Chk1/Chk2. Telomere shortening leads to cellular senescence, which is associated with the activation of the p16INK4a/Rb signaling pathway that inhibits cell cycle progression. Both telomere shortening and oxidative stress can activate inflammasomes, resulting in the release of inflammatory factors, which is related to the chronic inflammatory state observed in the aging process53,54.
Furthermore, mitochondrial dysfunction may lead to increased ROS/RNS levels, causing telomere damage and epigenetic modifications. Finally, alterations in the NAD+/sirtuin pathway may induce aging via the p53/p21 pathway but could also negatively affect the specific functions of forkhead box O (FOXO) and peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), leading to increased ROS and mitochondrial dysfunction55. These diseases include retinal diseases, neurodegenerative diseases, osteoarthritis, cardiovascular diseases, cancer, and various reproductive disorders, significantly impacting the quality of life and lifespan of the elderly56.
Critical role of oxidative stress in tumorigenesis
Oxidative stress promotes tumorigenesis
The relationship between oxidative stress and tumors includes: ① oxidative stress can cause DNA oxidative damage, leading to gene mutations and cancer initiation; ② it can interfere with apoptotic mechanisms, allowing abnormal cells to evade programmed cell death, thus promoting tumor development; ③ high levels of oxidative stress may lead to immune evasion, protecting tumor cells from immune system attacks. (① Causes cancer ➔ ② Promotes cancer ➔ ③ Immune evasion)
When the body is under oxidative stress, it leads to excessive production of ROS, which is one of the key drivers of tumor initiation, progression, metastasis, and drug resistance57,58.
① Excess ROS in the TME attacks biomolecules such as lipids, proteins59, and DNA. This produces mutagenic and carcinogenic byproducts, including 8-hydroxy-2’-deoxyguanosine (8-OHdG), 4-hydroxy-nonenal (4HNE) modifications, and malondialdehyde (MDA), resulting in lipid peroxidation, protein modification, and DNA oxidative damage60.
② Tumor growth and survival depend on angiogenesis, and tumor cells release endothelial growth factors that play a crucial role in tumor angiogenesis61,62. Research has shown that in prostate cancer tissues, vascular endothelial growth factor-A (VEGF-A) regulates the Rac1 pathway through VEGFR2 on the cell membrane, inducing NOX2 oxidase activation. Other cytokines, such as fibroblast growth factor (FGF) and hepatocyte growth factor (HGF), also act after inducing ROS, and ROS itself may affect growth factor receptor signaling, further promoting tumor development63. Additionally, ROS promotes tumor metastasis by interacting with VEGF under the influence of hypoxia-inducible factor-1 (HIF-1), inducing angiogenesis and facilitating tumor spread64.
③ Intracellular ROS primarily consists of mtROS65. MtROS can cause oxidative damage to mtDNA and induce IFN signaling, upregulating PD-L1 expression, thereby inhibiting T cell activation, DCs, and M1 macrophage functions. Under ROS stimulation, cancer cell mitochondrial Lon (a mitochondrial protease) triggers NF-κB-dependent inflammatory cytokine secretion (IL-6, IFN-γ, TGFβ, VEGF, IL-4, and IL-10) via the mtROS-NF-κB pathway, inducing immune suppression in macrophages and DCs and promoting T cell differentiation into Tregs66,67,68,69. ROS-induced Lon enhances downstream signaling through the NF-κB axis, accelerating tumor progression70.The expression of mitochondrial Lon protease is directly regulated by the NF-κB signaling pathway66,71. In the tumor microenvironment, chronic inflammatory factors (e.g., TNF-α, IL-1β) or ROS activate NF-κB, which binds to the promoter region of the Lon gene to enhance its transcription, forming a “NF-κB → Lon upregulation” positive feedback loop. Overexpression of Lon further exacerbates mtROS production, leading to the leakage of oxidatively damaged mtDNA into the cytoplasm. The released mtDNA activates the cGAS-STING-TBK1 pathway, inducing the secretion of type I interferons (IFN-α/β), which subsequently upregulate PD-L1 and IDO-1 expression via JAK-STAT signaling, directly suppressing the antitumor function of CD8+ T cells. Additionally, Lon-dependent mtROS accumulation promotes the secretion of extracellular vesicles (EVs) carrying oxidized mtDNA and PD-L1. Upon uptake by tumor-associated macrophages (TAMs), these EVs trigger IFN-γ and IL-6 release through the TLR9-MyD88 pathway, inhibiting T cell activity and driving polarization of macrophages toward the M(IL-4) phenotype71. ROS-induced Lon enhances downstream signaling through the NF-κB axis, accelerating tumor progression.
Moreover, the hypoxia factor HIF-1α induces upregulation of mitochondrial Lon and promotes IL-6 secretion through indirect effects on STAT372. ROS and hypoxia-induced upregulation of Lon lead to the secretion of extracellular vesicles (EVs) carrying mtDNA and PD-L173. MtROS further induces macrophages to produce IFN-γ and IL-6, weakening T cell cytotoxicity in the TME74. Simultaneously, ROS significantly affects the expression of programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1)70,75.
The dual role of ROS in tumors: balancing arrest and pro-tumorigenic effects
The cell cycle arrest induced by ROS is closely associated with tumorigenesis and progression, representing a critical mechanism through which ROS regulates tumor development. This process highlights the dual role of ROS in tumors76: while they may promote tumor evolution under specific conditions (e.g., inducing DNA damage, facilitating angiogenesis, and activating immunosuppressive pathways), they may also exert anti-tumor effects by triggering cell cycle arrest. For instance, reactive oxygen species (ROS) regulate the arrest of various cell cycle phases (G0-G1-S-G2-M) through multi-layered molecular interactions77 (Fig. 3). ROS levels fluctuate during the cell cycle, peaking during mitosis78. During the G0 phase, ROS activate the NRF2 and p53 signaling pathways79, induce CDKN1A (p21) expression80, inhibit CDK4/6 and Cyclin D1 activity, and maintain G0 phase arrest via the involvement of p27 and p3880,81,82,83 In the G1 phase, ROS activate the PI3K/AKT84,85 and SIRT1/FOXO3 signaling pathways86,87,88, thereby inducing the expression of p27, p53, and p16INK4A86, suppressing CDK4/6 and Cyclin D activity89, and preventing cell entry into the S phase. During the S phase, ROS activate the ATR/Chk1 signaling pathway90, inhibit CDC25A91 and PCNA activity92, and block progression to the G2 phase through the involvement of ATM93 and MTH192. In the G2 phase, ROS activate the WEE1/Myt1 and Chk1/2 signaling pathways, suppress CDC25C and CDK1/Cyclin B activity, and prevent transition to the M phase via the participation of p38, p53, p21, SAC, and APC/C²¹⁻²³. These mechanisms collectively maintain cell cycle homeostasis, thereby preventing cellular damage and aberrant proliferation.
This figure illustrates the regulatory roles of ROS in modulating cell cycle phases (G0, G1, S, G2, and M) and their complex interplay with p53 and CDKs. Distinct cell cycle phases are demarcated by color-coded boxes, with detailed annotations highlighting ROS-mediated activation of specific signaling pathways and molecules (e.g., p53, CDKs, CKIs) to influence cell cycle progression or arrest. Examples include: G0 phase: ROS induces G0 arrest via activation of NRF2 and ATR/Chk1 signaling pathways. G1 phase: ROS triggers G1 arrest through PI3K/AKT and p53-dependent mechanisms. S phase: ROS promotes S phase arrest via ATM and p53-mediated pathways. G2 phase: ROS causes G2 arrest by activating WEE1/Myt1 and p53-related signaling. The schematic underscores ROS as a dual-functional modulator that orchestrates phase-specific cell cycle checkpoints through interactions with DNA damage sensors (e.g., ATM/ATR) and CDK inhibitors.
Oxidative stress impacts post-transcriptional and protein modifications
Post-transcriptional modifications refer to a series of chemical processes that occur after RNA synthesis, including RNA splicing, RNA editing, and RNA methylation. These modifications can influence RNA molecule stability and translation efficiency, thereby affecting protein expression and function. Protein modifications involve a series of chemical processes occurring after protein synthesis, including phosphorylation, glycosylation, methylation, acetylation, sulfation, and palmitoylation. These modifications affect protein activity, stability, conformation, and interactions, thereby impacting protein function94.
In tumor cells, extensive oxidative stress processes generate peroxides that attack guanine nucleotides in RNA molecules, converting them to 8-oxoguanine nucleotides95. This affects mRNA stability and translation efficiency. Peroxides also attack amino acid residues in proteins, causing oxidative damage. For example, peroxides can target tyrosine residues in proteins, converting them to hydroxylated tyrosine. This oxidative damage impacts protein activity, stability, conformation, and interactions, thereby affecting protein function.
Oxidative stress: a central player in aging and tumorigenesis
In the pathological processes of aging and cancer, oxidative stress and changes in immune metabolism are two critical factors. Both aging and cancer development are associated with the accumulation of ROS. Aging results in decreased antioxidant capacity, contributing to ROS accumulation. Excessive ROS not only causes DNA damage and genetic instability but also affects immune metabolism pathways, weakening the immune system’s normal function, thus promoting cancer development and progression as well as cellular aging and tissue function decline96.
Oxidative stress drives shared pathological processes in aging and tumorigenesis through multiple mechanisms97. First, ROS directly induce DNA damage. In aging, ROS activate the p53/p16 pathway via telomere shortening and DNA damage response (DDR), leading to cell cycle arrest98,99,100,101. In cancer, ROS promote genomic instability, facilitating proto-oncogene activation (e.g., RAS) and tumor suppressor gene inactivation (e.g., PTEN), thereby accelerating malignant transformation98,102. Second, oxidative stress links aging and cancer by modulating inflammatory phenotypes: ROS activate NF-κB and mTOR pathways, driving pro-inflammatory factors (e.g., IL-6, IL-8) in aging103,104; in tumors, ROS stabilize HIF-1α to promote angiogenesis (VEGF) and immune checkpoint molecule expression (e.g., PD-L1), shaping an immunosuppressive microenvironment104,105. Additionally, metabolic reprogramming and oxidative stress form a positive feedback loop: enhanced glycolysis in senescent cells increases mitochondrial ROS accumulation106,107, while cancer cells sustain high ROS levels via the Warburg effect, promoting proliferation and inhibiting apoptosis108. Moreover, antioxidant defense system failure exacerbates pathological progression—declining NAD+ in aging reduces SIRT1 activity, impairing mitochondrial repair109, whereas tumors upregulate glutathione systems to balance ROS, but excessive oxidative stress still causes genomic instability104. Finally, strategies targeting oxidative stress (e.g., senolytics to clear senescent cells, NOX inhibitors to block ROS generation) show significant potential in delaying aging110 and enhancing chemosensitivity in cancer therapy111.
Immunometabolism: bridging the gap between aging and tumorigenesis
The Impact of Immunometabolism on Aging
More importantly, metabolic function declines with age, including energy metabolism, lipid metabolism, and glucose metabolism. For example, during aging, the levels of O-GlcNAcylated proteins increase, and the O-GlcNAcylation of IKKβ protein affects NF-κB activity112, leading to elevated lactate production and glucose consumption113. The tricarboxylic acid (TCA) cycle, glycolysis, fatty acid metabolism, and nucleotide synthesis are key metabolic pathways that maintain intracellular homeostasis and regulate DNA repair mechanisms. Activation of the DDR reduces glutamine sensitivity while promoting nucleotide synthesis and expanding glucose metabolism. Metabolic changes further impact DNA replication and repair, accelerating cellular aging. This decline in metabolic function affects the function and metabolic activity of immune cells, thereby impairing the normal operation of the immune system. Recent research has indicated that IgG accumulates in adipose tissue with aging, leading to fibrosis and metabolic dysfunction within the adipose microenvenvironment. This metabolic impairment is closely associated with chronic inflammation and dysfunction of adipocytes, subsequently affecting the overall function of the immune system114.
Senescent cells can also produce various pro-inflammatory chemokines and cytokines, leading to the accumulation of senescent cells that impair normal tissue function, including tissue dysfunction, limited regeneration, and tumor development113. The activity and function of immune cells are directly influenced by aging. Age-related damage to the immune system, known as immunosenescence, involves remodeling changes in both structure and function, negatively impacting the health of older individuals115. Vida et al. observed that aging is associated with lower antioxidant defense capacity (catalase activity and GSH levels), higher levels of oxidative compounds (XO activity/expression, superoxide, ROS, and GSSG levels), and increased lipofuscin accumulation, along with impaired immune cell functions such as macrophages116.
Therefore, immune cells may experience functional decline under when faced with insufficient energy supply or metabolic disorders, making the body more vulnerable to infections. Notable features of immunosenescence include thymic involution, imbalance in the ratio of naïve to memory cells, metabolic dysregulation, and epigenetic changes. These characteristics manifest as increased susceptibility to infections, diminished vaccine responsiveness, the onset of age-related diseases, and the progression of tumors34.
Immunometabolism affects the tumor microenvironment
Tumor cells perform aerobic glycolysis under normoxic conditions—a phenomenon known as the Warburg effect. Glycolysis converts glucose to pyruvate, leading to the production of lactate and CO2, which results in lactate accumulation and acidification of the TME117,118. This acidic environment suppresses mTOR activity, inhibits IFN-γ production, and affects cancer-related cells. The acidic environment created by the accumulation of lactate and CO2 degrades IFN-γ, weakening the differentiation of naive T cells into anti-tumor Th1 cells, while promoting their differentiation into pro-tumor Th2 cells. Studies have shown that under acidic conditions, IFN-γ undergoes irreversible conformational changes, reducing its binding capacity to the IFN-γ receptor (IFNGR)119,120. Simultaneously, the acidic environment activates G protein-coupled receptors (e.g., GPR65) in T cells, suppressing the mTORC1 signaling pathway, which inhibits the expression of T-bet (a key transcription factor for Th1 differentiation) and enhances the activity of GATA3 (a critical transcription factor for Th2 differentiation)121,122. Furthermore, lactate itself acts as a signaling molecule by inhibiting histone deacetylase (HDAC) activity, thereby promoting the opening of epigenetic modifications in Th2-related genes (e.g., IL-4, IL-5)123. Additionally, CO₂ is converted to bicarbonate (HCO₃⁻), which further suppresses Th1 differentiation and enhances Th2 polarization124. This multi-layered regulatory mechanism weakens the differentiation of naive T cells into anti-tumor Th1 cells while promoting their differentiation into pro-tumor Th2 cells. TAMs tend to shift towards a pro-tumor M2 phenotype125. Aerobic glycolysis is a major metabolic pathway for activating T cells in the TME and is required for the effector functions of activated T cells126. Different T cell subsets have distinct metabolic pathways. Activated CD8+ T cells primarily use glycolysis for energy metabolism, while CD4+ T cells increase both glycolysis and fatty acid (FA) metabolism. Therefore, aerobic glycolysis can suppress T cell activity and efficacy.
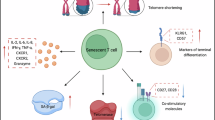
Sialic acids are carbohydrates commonly found as acidic residues on glycoproteins and glycolipids on many cell surfaces. In the TME, sialic acids can help tumor cells evade the immune system by binding to immune cell surface ligands (e.g., Siglec family), thereby inhibiting immune cell activity and function (Fig. 4). Additionally, sialic acids can promote tumor cell proliferation and growth by activating the PI3K/Akt and MAPK pathways through binding to tumor cell receptors127, or indirectly affect tumor growth and angiogenesis by influencing intercellular signaling in the TME.
Different subtypes of TAMs exhibit distinct metabolic processes: M1 macrophages tend toward fatty acid synthesis (FAS), while M2 macrophages tend toward fatty acid oxidation (FAO). In the TME, high levels of fatty acids lead TAMs to polarize towards the M2 phenotype128,129. The polarization of TAMs is a continuous process and progressively shifts towards an M2-like state with tumor malignancy, creating a positive feedback loop that supports tumor growth and metastasis. The accumulation of lipid droplets in tumor-derived dendritic cells (DCs) with tolerogenic functions leads to impaired antigen presentation and reduced anti-tumor activity130,131. Increased cholesterol levels in the TME can lead to TIL (tumor-infiltrating lymphocyte) exhaustion and weaken T cell anti-tumor responses131,132 (Fig. 5).
Tumor cells generate ROS and other metabolites that facilitate the differentiation and proliferation of immune cells toward pro-tumorigenic phenotypes within the tumor microenvironment, while simultaneously suppressing the anti-tumor functions of specific immune cell populations, ultimately leading to immune evasion. Through metabolic reprogramming, neoplastic cells deplete critical nutrients including glutamine and arginine to produce glutamate, which induces the differentiation of TAMs and DCs into M2-polarized macrophages and DCregs. Hypoxia-induced alterations in the TCA cycle result in excessive lactate and CO2 production by tumor cells. Concurrently, ROS-mediated signaling promotes T cell differentiation into immunosuppressive regulatory T cells and T helper 2 (Th2) cells. Furthermore, tumor-derived fatty acids in abundance significantly impair the release of anti-cancer-related cytokines from natural killer (NK) cells216.
Tumor cells in the TME require more exogenous non-essential amino acids for cellular activities133. Glutamine is the most abundant amino acid in circulation after glucose and is one of the fastest-depleted nutrients in many cultured cancer cells134,135. It is crucial for cell biosynthesis, maintaining redox balance, and regulating cell signaling pathways. Glutamine enters cells via transporters SLC1A5 and SLC7A5, is converted to glutamate by glutaminase (GLS). Gln-derived glutathione helps maintain ROS balance136. Tumor cells competitively acquire Gln, which inhibits T cell IFN-γ secretion. Gln deficiency in tumor cells induces PD-L1 expression through the EGFR/ERK/c-Jun pathway, leading to immune evasion. GLS deficiency promotes the proliferation, differentiation, and tumor-killing efficacy of Th1 cells and CD8+ cytotoxic T lymphocytes (CTLs), while GLS absence inhibits Th17 cell differentiation137. Under the catalytic action of IDO1/2 and TDO, tryptophan is metabolized to kynurenine (Kyn). Intermediate cells in the TME (e.g., endothelial cells, TAMs) express high levels of IDO and TDO, leading to tryptophan depletion, which inhibits T cell proliferation and function, causing cell cycle arrest at the G1 phase. Additionally, the accumulation of Kyn promotes Treg differentiation, enhancing immune suppression.
Common mechanisms of aging and cancer in antioxidant systems
Aging and cancer share common features of immunometabolic dysregulation97. First, both drive disease progression through chronic inflammation: senescent cells secrete the pro-inflammatory chemokines and cytokines (e.g., IL-6, IL-8) to trigger “inflammaging,“103 while cancer cells and stromal cells in the tumor microenvironment release pro-inflammatory factors via similar mechanisms, forming immunosuppressive inflammation138. Second, immunosuppressive cells like regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) are significantly expanded in both contexts. In aging tissues, Tregs suppress immune responses by inducing senescence in effector T cells139,140, whereas MDSCs in tumors block T cell activation through molecules like IL-1RA138,141. Third, metabolic reprogramming exhibits shared characteristics. Both senescent cells and cancer cells rely on glycolysis, with senescent cells showing increased glucose consumption and cancer cells exhibiting the Warburg effect. Mitochondrial dysfunction in immune cells further drives metabolic exhaustion, manifested as declined OXPHOS in aged T cells and metabolic suppression in tumor-infiltrating T cells. Finally, amino acid metabolic imbalance exacerbates immunosuppression. For example, elevated activity of ARG1 (arginase 1) and IDO1 (indoleamine 2,3-dioxygenase 1) in aging and cancer restricts T cell function, establishing “metabolic checkpoints” that collectively weaken immune surveillance.
In the tumor microenvironment, significant metabolic changes occur in immune cells, with tumor cells using metabolic reprogramming to suppress the antitumor functions of immune cells, thereby achieving immune escape (Fig. 6). Similarly, during aging, changes in immune metabolism lead to weakened immune function, characterized by a chronic inflammatory state and a decreased response to pathogens. Persistent elevation of inflammation levels in organs such as the bone marrow, liver, and lungs, if not resolved promptly, can result in organ damage and the development of age-related diseases. Consequently, targeting inflammation for resolution is regarded as a promising strategy for aging intervention25. Both cancer and aging are associated with inflammatory responses. In cancer development, inflammatory cells and factors (e.g., IL-6, TNF-α) play roles in the tumor microenvironment, promoting tumor cell proliferation, invasion, and metastasis, leading to chronic inflammation. In aging, chronic low-grade inflammation (“inflammaging”) impairs tissue function and may contribute to age-related diseases such as atherosclerosis, diabetes, and cancer142.
ROS affects NRF2 activation through PI3K-Akt pathway. ROS can affect the NF-κB pathway by affecting IκB kinase. ROS also promotes transcription and translation HIF-1α positive feedback via promoting the phosphorylation of NF-κB and affects the expression of NOX to promote the generation and aggregation of reactive oxygen species ROS. ROS affects the expression of NLRP3 inflammasome through NF-κB, induces the production of IL-18 and IL-1β, and affects the immune activity of T cells and dendritic cells. ROS promotes the production of IFN-γ and IL-6 and induces the expression of PD-L1 through the JAK-STAT pathway to achieve immune escape217,218,219,220,221,222,223,224,225,226,227,228,229,230,231.
The metabolic pathways of immune cells are closely related to their functions, including glycolysis, the tricarboxylic acid cycle, OXPHOS, and fatty acid metabolism. The redox reactions generated during metabolism inevitably produce ROS, leading to oxidative stress. There is a reciprocal regulatory relationship between oxidative stress and immune metabolism. NADPH is a critical reductant produced during immune metabolism, maintaining the function of intracellular antioxidant systems (e.g., thioredoxin and glutathione systems) and bidirectionally regulating ROS production and clearance in certain cells such as macrophages143,144,145,146.
The crosstalk between oxidative stress and immunometabolism represents a shared mechanistic nexus in both aging and tumorigenesis
The role of antioxidant systems in oxidative stress
A.TRX1 maintains nucleotide biosynthesis
The rate-limiting step in nucleotide biosynthesis during the final phase of the PPP is catalyzed by ribonucleotide reductase (RNR), which reduces ribonucleotides to the corresponding 2’-deoxyribonucleotides (dNTPs), which are essential components of DNA147,148,149. Due to the very low expression of GRX1 in T cells, which does not upregulate upon activation, the GSH/GRX system cannot compensate for the loss of the TRX1 pathway, making TRX1 the sole electron donor for dNTP production in proliferating T cells. Therefore, the TRX1 system is crucial for rapid T cell proliferation. Aerobic glycolysis fuels the PPP to produce NADPH, which in turn provides reducing equivalents to the TRX1 system, ultimately promoting the maintenance of RNR activity in the PPP to regenerate dNTPs, thus completing a full cycle.
B.TXNIP plays a central role in negative regulation of glucose metabolism
TXNIP is another component of the TRX1 system, negatively regulating TRX1 function by inhibiting reduced TRX through intermolecular disulfide bond interactions150,151. While it remains to be confirmed whether TXNIP also plays a key role in the negative regulation of glucose metabolism in immune cells, increasing evidence supports this idea152. These complexes can sense glycolytic metabolite levels, and increased glycolysis has been shown to deplete complex formation, leading to reduced TXNIP gene transcription153. Furthermore, in activated T cells, increased aerobic glycolysis and glucose uptake result in rapid downregulation of TXNIP, further promoting glucose uptake and metabolism154. Additionally, TXNIP also plays a gatekeeping role in acute lymphoblastic leukemia B cells by inhibiting GLUT1 expression and glucose uptake. The transcription factor PAX5 regulates TXNIP expression and directly suppresses glucose transporters154. The downregulation of TXNIP and PAX5 in activated B cells and plasma cells promotes glucose uptake to drive glycolysis and enhance the PPP, which is crucial for B cell development and response155.
C.GSH buffers ROS and regulates cellular metabolism
The GSH system plays a crucial role in buffering ROS in every cell. Increased metabolic activity in activated T cells leads to ROS production by the mitochondrial ETC, which, if not properly cleared, can result in DNA damage and cell death156,157,158. To maintain redox balance and increase GSH uptake, T cell activation induces the upregulation of the glutamate-cysteine ligase catalytic subunit (GCLC), which primarily catalyzes the rate-limiting step of GSH biosynthesis. Conditional knockout of the GCLC gene in T cells leads to defects in metabolic reprogramming. ROS accumulation occurs in GCLC-deficient T cells, where excessive ROS impairs mTOR activation, affecting the expression of c-MYC and nuclear factor of activated T cells (NFAT), and adversely impacts T cell metabolic reprogramming. mTOR integrates signals related to energy and nutrient levels, stress pathways, and TCR signaling to play a central role in reprogramming T cell metabolism, increasing glycolysis and glutamine catabolism158. For example, mTOR promotes the induction of c-MYC and HIF-1α, which supports aerobic glycolysis and glutamine catabolism. NFAT directly controls the expression of key metabolic regulators, including c-MYC159,160,161. Calcineurin, a phosphatase that activates NFAT, can also be inhibited by ROS161. In GCLC-deficient T cells, excessive ROS accumulation due to impaired GSH biosynthesis inhibits calcineurin function, thus suppressing NFAT activation and impairing T cell metabolic reprogramming. Overall, T cell activation leads to upregulation of the GSH pathway to prevent excessive ROS accumulation, ensuring the activation of mTOR and NFAT, and facilitating proper T cell metabolic reprogramming and cell fate decisions.
How does immune metabolism maintain homeostasis against oxidative stress?
A.NADPH provides reductive power to maintain the function of antioxidant pathways including TRX and GSH systems
NADPH is a crucial product of the immune metabolic pentose phosphate pathway (PPP) and has been shown to maintain redox homeostasis in immune cells while reducing ROS production. NADPH acts as an electron donor for antioxidant pathways including the TRX and GSH systems162. An important component of the TRX system is TRX-interacting protein (TXNIP), which acts as a negative regulator. TXNIP binds to TRX through intermolecular disulfide bonds, inhibiting its reducing activity and thus negatively regulating the TRX system. After T cell stimulation, TXNIP is rapidly downregulated, relieving the inhibition on the TRX1 system.
GSH is regenerated from its oxidized form (GSSG) to its reduced form (GSH) using NADPH electrons provided by glutathione reductase (GSR)163. The GSH system not only removes ROS but also provides reductive capacity for other antioxidant enzymes. For instance, it can reduce glutaredoxins (GRXs) and glutathione peroxidases (GPXs)164. The GPX4 protein family clears lipid peroxides, preventing ferroptosis, while GPX1 catalyzes the reduction of hydrogen peroxide and organic hydroperoxides to water and alcohol29. In macrophages, these systems collectively coordinate to maintain cellular redox homeostasis in an inflammatory context.
B.Glutamine metabolism involves GSH biosynthesis
GSH is a widely present tripeptide composed of glutamate, glycine, and cysteine, with substrate availability determining the rate of GSH biosynthesis in cells. After T cell stimulation, glutamine is rapidly taken up through the upregulation of the transporter SLC1A5 (ASCT2)165. mTORC1 and c-MYC are key participants in inducing glutamine uptake, promoting T cell proliferation and effector functions166. Glutamate, derived from glutamine hydrolysis into α-ketoglutarate167, can serve as a carbon source for the TCA cycle and as a precursor for de novo synthesis of GSH. T cells must regulate glutamine flux via glutamine hydrolysis to maintain effective glutamine synthesis. Recent studies have shown that deletion of mitochondrial pyruvate carriers in cancer cells disrupts this balance, reducing GSH synthesis and negatively impacting cell proliferation168. Glutamine uptake and conversion to glutamate are significantly increased during B cell activation, and blocking this process negatively affects B cell growth, proliferation, differentiation, and antibody responses169.
C.TCA cycle metabolites regulate ROS and reactive nitrogen species, inducing NRF2 activation
NRF2 is another major regulator of cellular antioxidant responses. Macrophages need to adjust their metabolic processes to achieve optimal functional responses to different pathogens and tissue environments169. Similar to glycolysis, intermediates from the TCA cycle in M1 macrophages also participate in biosynthesis processes. After LPS stimulation, TCA cycle intermediates undergo two breaks, leading to the accumulation of succinate and citrate. The accumulation of succinate stabilizes HIF-1α, promoting the transcription of IL-1β. Additionally, succinate dehydrogenase (SDH) catalyzes the oxidation of succinate, resulting in reverse electron transfer at complex I and the production of ROS170.
Activated macrophages in a high metabolic state use glutamine to provide nutrients for the TCA cycle, resulting in the accumulation of oxaloacetate in the cytosol, which stabilizes HIF-1α and enhances IL-1β secretion171. NO in inflammatory macrophages also forms a positive feedback loop, reducing IDH1 and IDH2 activity and further promoting citrate accumulation. Furthermore, citrate can be converted to itaconate, with TCA cycle intermediate cis-aconitate decarboxylated to form itaconate. Itaconate can exert anti-inflammatory effects by modifying key glycolytic enzymes and NLRP3 inflammasome proteins, inducing ATF3 to activate NRF2. NRF2 activation alleviates cellular oxidative stress by inducing the production of GSH, TRX1, and TRXR1. For instance, in T cells, stimulation leads to ROS generation and activation of the NRF2 pathway. NRF2 target genes also involve PPP products such as G6PD, 6PGD, transketolase, and transaldolase, indicating that NRF2 activation is crucial for participating in and/or maintaining the PPP, especially for producing NADPH to fuel FAS, TRX, and GSH systems172.
The role and function of traditional Chinese medicine and its extracts in antioxidant aging and antitumor effects
Antioxidants are a broad category of substances (bioactive compounds and enzyme complexes) present in small amounts (micronutrients) in organisms, including both natural (phospholipids, proteins, DNA) and synthetic (plastics, oils) substances, which protect against free radical damage. All antioxidants that can inhibit or reduce the formation of free radicals are considered preventive agents, as they act by preventing the formation of free radicals173. The human antioxidant defense system comprises endogenous (enzymatic and non-enzymatic) antioxidants, such as SOD, CAT, and glutathione peroxidase (GSH-Px), as well as exogenous antioxidants mainly from the diet. These exogenous antioxidants include hydrophilic free radical scavengers (e.g., vitamin C and glutathione (GSH)) and hydrophobic ones (e.g., tocopherols, flavonoids, carotenoids, and coenzyme Q, which directly scavenge O₂−•, •OH, and ¹O₂). Endogenous and exogenous antioxidants work together, often synergistically, to maintain or restore redox balance174. SOD and CAT are central to the cellular antioxidant defense. The SOD family (Cu/Zn-SOD1 in the cytoplasm, Mn-SOD2 in mitochondria, and extracellular SOD3) converts superoxide anions (O₂−•) into H₂O₂ and O₂ via dismutation. Subsequently, CAT, with iron protoporphyrin as a cofactor, decomposes H₂O₂ into water in a two-step reaction175,176. Antioxidants, as defensive factors, combat free radicals, eliminate reactive oxygen species (ROS), and modulate cellular redox states, facilitating redox signaling. By inhibiting initiation and propagation steps, they terminate reactions, delay oxidation, and counteract oxidative mechanisms in disease progression177,178.
Many natural and synthetic compounds have been studied for their anti-aging and antitumor potential in cell and animal models, as well as in humans, demonstrating antioxidant effects through various mechanisms and pathways. These compounds reduce intracellular oxidative stress and free radical damage, thereby helping to slow down the aging process and the development of tumors179. Traditional Chinese medicine herbs and their extracts have been found in numerous studies to offer multiple health benefits. By improving oxidative stress conditions, they can effectively delay the aging process and protect cells from oxidative stress, thereby reducing cancer risk. Recent studies have also focused on the role of various TCM herbs in modulating oxidative stress to slow aging. To elucidate the potential applications of TCM in anti-aging and antitumor contexts, this review summarizes the anti-aging and antitumor effects and mechanisms of TCM and its effective monomeric components (Table 1). Traditional Chinese Medicine demonstrates multi-target and multi-pathway mechanisms in regulating oxidative stress-mediated aging and tumorigenesis. Numerous studies indicate that TCM and its active components effectively delay aging and suppress tumor progression by enhancing antioxidant enzyme activity, modulating immunometabolism, and inhibiting inflammatory signaling pathways. For example, Wang et al.180 found that Astragali Radix (Huangqi) significantly alleviates oxidative stress in a D-galactose-induced aging animal model by activating the PI3K/Akt signaling pathway, reducing malondialdehyde (MDA) levels, and elevating superoxide dismutase (SOD) and catalase (CAT) activity, thereby mitigating age-related damage. Similarly, Xiong et al.181 demonstrated in a nematode model that Lycii Fructus (Gouqizi) upregulates SOD and CAT expression while reducing lipofuscin accumulation, with its antioxidant effects strongly correlating with lifespan extension. In cancer therapy, TCM components exert antitumor effects by modulating the redox balance and immunometabolism in the tumor microenvironment (TME). Tan et al.182 revealed that baicalin induces RelB/p52-dependent autophagy, promoting the polarization of TAMs from pro-tumor M(IL-4) to antitumor M(IFN-γ) phenotypes, thereby inhibiting hepatocellular carcinoma progression. Additionally, Lan et al.183 reported that saikosaponin A triggers endoplasmic reticulum stress to activate ATF3 expression, depletes GSH, and induces ferroptosis in liver cancer cells, markedly suppressing tumor proliferation. Mitigation significantly decreases mitochondrial reactive oxygen species (mtROS) by enhancing Sirt3-mediated mitochondrial DNA (mtDNA) transcription, leading to marked increases in ATP synthesis and Complex I activity. It also activates AMPK and upregulates PGC-1α and Sirt3 protein expression both in vivo and in vitro184. Ginsenosides Rg3 and Rh2 from ginseng suppress breast and lung cancer growth, inhibit angiogenesis, and enhance chemotherapeutic drug sensitivity (e.g., cisplatin) by modulating Bcl-2 family proteins185,186,187. Ginseng polysaccharides alter gut microbiota and the kynurenine/tryptophan ratio to enhance PD-1 therapeutic efficacy188. These mechanisms highlight that TCM not only directly scavenges ROS but also reprograms the immunosuppressive TME via key metabolic pathways (Fig. 1).
Shared mechanisms between aging and cancer include oxidative stress-induced DNA damage and chronic inflammation. Chen189 showed in murine models that Bu Shen Huo Xue Tang activates the Nrf2/Keap1 pathway, upregulates HO-1 and NQO1 expression, reduces MDA levels in premature ovarian insufficiency models, and improves oocyte function. Similarly, Zeng et al.190 demonstrated that Huangqi Baihe Granules inhibit the TLR4/NF-κB/NLRP3 inflammatory axis, attenuating ROS production and alleviating oxidative stress and inflammation in acute lung injury. These studies illustrate TCM’s dual efficacy in combating aging and cancer through synergistic regulation of redox homeostasis and inflammatory signaling networks. Notably, some TCM components influence aging and tumor progression via epigenetic modifications. Wu et al.191 identified that dendrobine enhances autophagy and reduces oxidative damage via the Sch9/Rim15/Msn2 pathway, extending yeast lifespan. In oncology, Lin et al.192 confirmed that acetylshikonin activates the RIPK1/RIPK3/MLKL cascade to induce necroptosis in lung cancer cells, a mechanism linked to ROS-dependent DNA damage. These findings further validate TCM’s multi-dimensional intervention in oxidative stress-related pathways for disease modulation. This information can assist clinicians and scientists in developing new targeted and effective therapeutic drugs, thereby establishing better treatment strategies to combat aging and cancer.
The treatment strategy of traditional Chinese medicine focuses on overall conditioning, which can not only improve oxidative stress, but also regulate the internal immune balance of the body and improve the resistance of the body to tumors193. In summary, TCM has important clinical application prospects in the treatment of oxidative stress-related aging and cancer, providing an effective and natural therapeutic option for human health.
Conclusion
This article provides a comprehensive review of the pivotal roles of immunometabolism and oxidative stress in cancer and aging, as well as their interplay. Immunometabolism involves metabolic processes within immune cells, including glucose, lipid, and amino acid metabolism, which are essential for maintaining the normal function of immune cells. Oxidative stress occurs when the balance between pro-oxidants and antioxidants in the body is disrupted, leading to an overproduction of ROS, causing damage to cellular components. In the tumor microenvironment, cancer cells undergo metabolic reprogramming to meet the demands of rapid proliferation, a process that affects the function of immune cells, particularly through influencing the polarization of TAMs, thereby promoting tumor immune evasion and progression. Additionally, oxidative stress plays a significant role in the initiation and development of cancer by causing DNA damage, disrupting apoptosis mechanisms, and promoting immune evasion. In the context of aging, changes in immunometabolism lead to a decline in immune function, a phenomenon known as immunosenescence. The accumulation of senescent cells and the production of the SASP further exacerbate the inflammatory state and oxidative stress, accelerating the aging process. Oxidative stress also promotes cellular aging by affecting the cellular redox state and post-translational modifications, such as DNA damage and protein oxidation. Traditional Chinese medicine shows potential in modulating oxidative stress and immunometabolism, with various active components of Chinese herbs proven to alleviate the aging process and inhibit tumor growth through their antioxidant and anti-inflammatory effects. These components modulate key signaling pathways and transcription factors, such as NF-κB, NRF2, and SIRT1, to mitigate oxidative stress and immunometabolic imbalances. In summary, the mechanisms of action of immunometabolism and oxidative stress in cancer and aging are complex and interconnected. By regulating these processes, new strategies may be developed for the treatment of cancer and aging-related diseases. Future research should further explore the molecular mechanisms of these processes and develop effective therapeutic interventions.
Data availability
No datasets were generated or analysed during the current study.
References
Boothby, M. & Rickert, R. C. Metabolic regulation of the immune humoral response. Immunity 46, 743–755, (2017).
Zheng, Y. et al. Amino acid metabolism reprogramming: shedding new light on T cell anti-tumor immunity. J. Exp. Clin. Cancer Res 42, 291, (2023).
Wettersten, H. I., Aboud, O. A., Lara, P. N. Jr. & Weiss, R. H. Metabolic reprogramming in clear cell renal cell carcinoma. Nat. Rev. Nephrol. 13, 410–419 (2017).
Martínez-Reyes, I. & Chandel, N. S. Cancer metabolism: looking forward. Nat. Rev. Cancer 21, 669–680, (2021).
Muri, J. & Kopf, M. Redox regulation of immunometabolism. Nat. Rev. Immunol. 21, 363–381 (2021).
Kalinina, E. V., Chernov, N. N. & Novichkova, M. D. Role of glutathione, glutathione transferase, and glutaredoxin in regulation of redox-dependent processes. Biochem. Biochim. 79, 1562–1583 (2014).
Sen, C. K., Packer, L. & Baeuerle, P. A. Antioxidant and redox regulation of genes. (Academic Press, 1999).
Sies, H. Oxidative eustress: On constant alert for redox homeostasis. Redox Biol. 41, 101867 (2021).
Bratic, A. & Larsson, N. G. The role of mitochondria in aging. J. Clin. Investig. 123, 951–957 (2013).
Biala, A. K., Dhingra, R. & Kirshenbaum, L. A. Mitochondrial dynamics: Orchestrating the journey to advanced age. J. Mol. Cell Cardiol. 83, 37–43 (2015).
Fimognari, C. Role of oxidative RNA damage in chronic-degenerative diseases. Oxid. Med Cell Longev. 2015, 358713 (2015).
Guillaumet-Adkins, A. et al. Epigenetics and oxidative stress in aging. Oxid. Med Cell Longev. 2017, 9175806 (2017).
Sies, H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 4, 180–183 (2015).
Gorrini, C., Harris, I. S. & Mak, T. W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 12, 931–947 (2013).
Araki, E. & Nishikawa, T. Oxidative stress: A cause and therapeutic target of diabetic complications. J. Diab Investig. 1, 90–96 (2010).
Hajam, Y. A. et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 11, https://doi.org/10.3390/cells11030552 (2022).
Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 82, 47–95(2002).
Habib, R. Multifaceted roles of Toll-like receptors in acute kidney injury. Heliyon 7, e06441 (2021).
Schieber, M. & Chandel, N. S. ROS function in redox signaling and oxidative stress. Curr. Biol: CB 24, R453–R462 (2014).
Satoh, M. et al. Requirement of Rac1 in the development of cardiac hypertrophy. Proc. Natl Acad. Sci. USA 103, 7432–7437 (2006).
Hybertson, B. M., Gao, B., Bose, S. K. & McCord, J. M. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol. Asp. Med 32, 234–246 (2011).
Huang, Y. X., Lin, K. H., Chiang, J. C., Chen, W. M. & Lee, H. Lysophosphatidic Acid Receptor 3 Activation Is Involved in the Regulation of Ferroptosis. Int. J. Mol. Sci. 25, https://doi.org/10.3390/ijms25042315 (2024).
Mazhar, M. et al. Implication of ferroptosis in aging. Cell Death Discov. 7, 149, https://doi.org/10.1038/s41420-021-00553-6 (2021).
Ghasemitarei, M. et al. Effects of nitro-oxidative stress on biomolecules: Part 1-non-reactive molecular dynamics simulations. Biomolecules 13, https://doi.org/10.3390/biom13091371 (2023).
Li, X. et al. Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct. Target Ther. 8, 239 (2023).
Di Micco, R., Krizhanovsky, V., Baker, D. & d’Adda di Fagagna, F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 22, 75–95, https://doi.org/10.1038/s41580-020-00314-w (2021).
Gorgoulis, V. et al. Cellular senescence: defining a path forward. Cell 179, 813–827 (2019).
Kumari, R. & Jat, P. Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front. Cell Dev. Biol. 9, 645593 (2021).
Roger, L., Tomas, F. & Gire, V. Mechanisms and Regulation of Cellular Senescence. Int. J. Mol. Sci. 22, https://doi.org/10.3390/ijms222313173 (2021).
Lettieri-Barbato, D., Aquilano, K., Punziano, C., Minopoli, G. & Faraonio, R. MicroRNAs, long non-coding RNAs, and circular RNAs in the Redox control of cell senescence. Antioxidants 11, https://doi.org/10.3390/antiox11030480 (2022).
Harries, L. W. Dysregulated RNA processing and metabolism: a new hallmark of ageing and provocation for cellular senescence. FEBS J. 290, 1221–1234 (2023).
Wei, W. & Ji, S. Cellular senescence: Molecular mechanisms and pathogenicity. J. Cell Physiol. 233, 9121–9135 (2018).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: An expanding universe. Cell 186, 243–278 (2023).
Liu, Z. et al. Immunosenescence: molecular mechanisms and diseases. Signal Transduct. Target Ther. 8, 200 (2023).
Zheng, Y., Liu, Q., Goronzy, J. J. & Weyand, C. M. Immune aging - A mechanism in autoimmune disease. Semin Immunol. 69, 101814 (2023).
Stranks, A. J. et al. Autophagy controls acquisition of aging features in macrophages. J. Innate Immun. 7, 375–391 (2015).
Lian, J., Yue, Y., Yu, W. & Zhang, Y. Immunosenescence: a key player in cancer development. J. Hematol. Oncol. 13, 151 (2020).
Tang, X. et al. Characterization of age-related immune features after autologous NK cell infusion: Protocol for an open-label and randomized controlled trial. Front Immunol. 13, 940577 (2022).
Walker, K. A., Basisty, N., Wilson, D. M., 3rd & Ferrucci, L. Connecting aging biology and inflammation in the omics era. J. Clin. Investig. 132, https://doi.org/10.1172/jci158448 (2022).
Yousefzadeh, M. J. et al. An aged immune system drives senescence and ageing of solid organs. Nature 594, 100–105 (2021).
Koenig, A. & Buskiewicz-Koenig, I. A. Redox activation of mitochondrial DAMPs and the metabolic consequences for development of autoimmunity. Antioxid. Redox Signal 36, 441–461 (2022).
Sławińska, N. & Krupa, R. Molecular aspects of senescence and organismal ageing-DNA damage response, Telomeres, inflammation and Chromatin. Int. J. Mol. Sci. 22, https://doi.org/10.3390/ijms22020590 (2021).
Ortega, M. A. et al. Reframing the link between metabolism and NLRP3 inflammasome: therapeutic opportunities. Front Immunol. 14, 1232629 (2023).
Martin, D. E., Torrance, B. L., Haynes, L. & Bartley, J. M. Targeting aging: lessons learned from immunometabolism and cellular senescence. Front. Immunol. 12, 714742 (2021).
Picca, A., Faitg, J., Auwerx, J., Ferrucci, L. & D’Amico, D. Mitophagy in human health, ageing and disease. Nat. Metab. 5, 2047–2061 (2023).
Wang, L. et al. Oxidative stress in oocyte aging and female reproduction. J. Cell Physiol. 236, 7966–7983 (2021).
Yu, C. & Xiao, J. H. The Keap1-Nrf2 system: a mediator between oxidative stress and aging. Oxid. Med Cell Longev. 2021, 6635460 (2021).
Lopes-Paciencia, S. et al. The senescence-associated secretory phenotype and its regulation. Cytokine 117, 15–22 (2019).
Collado, M. & Serrano, M. Senescence in tumours: evidence from mice and humans. Nat. Rev. Cancer 10, 51–57 (2010).
Vida, C., González, E. M. & De la Fuente, M. Increase of oxidation and inflammation in nervous and immune systems with aging and anxiety. Curr. Pharm. Des. 20, 4656–4678 (2014).
Arranz, L., Caamaño, J. H., Lord, J. M. & De la Fuente, M. Preserved immune functions and controlled leukocyte oxidative stress in naturally long-lived mice: possible role of nuclear factor kappa B. J. Gerontol. Ser. A Biol. Sci. Med Sci. 65, 941–950 (2010).
Anderson, O. A., Finkelstein, A. & Shima, D. T. A2E induces IL-1ß production in retinal pigment epithelial cells via the NLRP3 inflammasome. PloS one 8, e67263 (2013).
Qi, W. et al. Inhibition of miR-4763-3p expression activates the PI3K/mTOR/Bcl2 autophagy signaling pathway to ameliorate cognitive decline. Int J. Biol. Sci. 20, 5999–6017 (2024).
Luo, J., Mills, K., le Cessie, S., Noordam, R. & van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 57, 100982 (2020).
Zhang, J. et al. Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Res. Rev. 25, 55–69 (2016).
Campisi, J. et al. From discoveries in ageing research to therapeutics for healthy ageing. Nature 571, 183–192 (2019).
Morry, J., Ngamcherdtrakul, W. & Yantasee, W. Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Biol. 11, 240–253 (2017).
Muguruma, M. et al. Possible involvement of oxidative stress in piperonyl butoxide induced hepatocarcinogenesis in rats. Toxicology 236, 61–75 (2007).
Wu, D. & Yotnda, P. Production and detection of reactive oxygen species (ROS) in cancers. J. Vis. Exp.: JoVE, https://doi.org/10.3791/3357 (2011).
Wu, J. et al. TNF antagonist sensitizes synovial fibroblasts to ferroptotic cell death in collagen-induced arthritis mouse models. Nat. Commun. 13, 676 (2022).
Napoli, C. et al. Directed in vivo angiogenesis assay and the study of systemic neoangiogenesis in cancer. Int J. cancer 128, 1505–1508 (2011).
Ushio-Fukai, M. et al. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ. Res. 91, 1160–1167 (2002).
Harrison, I. P. et al. NOX2 oxidase expressed in endosomes promotes cell proliferation and prostate tumour development. Oncotarget 9, 35378–35393 (2018).
Cheng, J. et al. Melatonin restricts the viability and angiogenesis of vascular endothelial cells by suppressing HIF-1α/ROS/VEGF. Int J. Mol. Med 43, 945–955 (2019).
Murphy, M. P. How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13 (2009).
Pinti, M. et al. Functional characterization of the promoter of the human Lon protease gene. Mitochondrion 11, 200–206 (2011).
Alissafi, T. et al. Mitochondrial oxidative damage underlies regulatory T cell defects in autoimmunity. Cell Metab. 32, 591–604.e597 (2020).
Guo, Z. et al. DCAF1 regulates Treg senescence via the ROS axis during immunological aging. J. Clin. Investig. 130, 5893–5908 (2020).
Tanaka, A. & Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res 27, 109–118 (2017).
Su, P. et al. Enhanced lipid accumulation and metabolism are required for the differentiation and activation of tumor-associated macrophages. Cancer Res 80, 1438–1450 (2020).
Cheng, A. N. et al. Mitochondrial Lon-induced mtDNA leakage contributes to PD-L1-mediated immunoescape via STING-IFN signaling and extracellular vesicles. J. Immunother. Cancer 8, https://doi.org/10.1136/jitc-2020-001372 (2020).
Lu, H. et al. Chemotherapy-Induced Ca(2+) release stimulates breast cancer stem cell enrichment. Cell Rep. 18, 1946–1957 (2017).
Garg, A. D. et al. ROS-induced autophagy in cancer cells assists in evasion from determinants of immunogenic cell death. Autophagy 9, 1292–1307 (2013).
Cheng, C. W. et al. Overexpression of Lon contributes to survival and aggressive phenotype of cancer cells through mitochondrial complex I-mediated generation of reactive oxygen species. Cell Death Dis. 4, e681 (2013).
Cao, Y. Adipocyte and lipid metabolism in cancer drug resistance. J. Clin. Investig. 129, 3006–3017 (2019).
Srivastava, R., Fernández-Ginés, R., Encinar, J. A., Cuadrado, A. & Wells, G. The current status and future prospects for therapeutic targeting of KEAP1-NRF2 and β-TrCP-NRF2 interactions in cancer chemoresistance. Free Radic. Biol. Med 192, 246–260 (2022).
Mackova, V., Raudenska, M., Polanska, H. H., Jakubek, M. & Masarik, M. Navigating the redox landscape: reactive oxygen species in regulation of cell cycle. Redox Rep: Commun. free Radic. Res 29, 2371173 (2024).
Patterson, J. C. et al. ROS and oxidative stress are elevated in mitosis during asynchronous cell cycle progression and are exacerbated by mitotic arrest. Cell Syst. 8, 163–167.e162 (2019).
Hong, Y., Boiti, A., Vallone, D. & Foulkes, N. S. Reactive oxygen species signaling and oxidative stress: transcriptional regulation and evolution. Antioxidants 13, https://doi.org/10.3390/antiox13030312 (2024).
Huang, Z. et al. Artesunate inhibits the cell growth in colorectal cancer by promoting ROS-dependent cell senescence and autophagy. Cells 11, https://doi.org/10.3390/cells11162472 (2022).
Li, X. L. et al. Dioscin inhibits human endometrial carcinoma proliferation via G0/G1 cell cycle arrest and mitochondrial-dependent signaling pathway. Food Chem. Toxicol. 148, 111941 (2021).
Liu, J. et al. Jaceosidin induces apoptosis and inhibits migration in AGS gastric cancer cells by regulating ROS-mediated signaling pathways. Redox Rep.: Commun. Free Radic. Res. 29, 2313366 (2024).
Becker, W. A. wake-up call to quiescent cancer cells - potential use of DYRK1B inhibitors in cancer therapy. FEBS J. 285, 1203–1211 (2018).
Xu, S., Li, Z., Xin, X. & An, F. Curdepsidone A Induces intrinsic apoptosis and inhibits protective autophagy via the ROS/PI3K/AKT signaling pathway in HeLa cells. Marine Drugs 22, https://doi.org/10.3390/md22050227 (2024).
Wang, L. et al. A Marine Alkaloid, Ascomylactam A, Suppresses lung tumorigenesis via inducing cell cycle G1/S arrest through ROS/Akt/Rb pathway. Marine Drugs 18, https://doi.org/10.3390/md18100494 (2020).
Yuan, X. et al. PM(2.5) induces embryonic growth retardation: Potential involvement of ROS-MAPKs-apoptosis and G0/G1 arrest pathways. Environ. Toxicol. 31, 2028–2044 (2016).
Chung, S. et al. Regulation of SIRT1 in cellular functions: role of polyphenols. Arch. Biochem. Biophys. 501, 79–90 (2010).
Rahman, I., Kinnula, V. L., Gorbunova, V. & Yao, H. SIRT1 as a therapeutic target in inflammaging of the pulmonary disease. Prev. Med. 54, S20–S28 (2012).
Vijayaraghavan, S. et al. CDK4/6 and autophagy inhibitors synergistically induce senescence in Rb positive cytoplasmic cyclin E negative cancers. Nat. Commun. 8, 15916 (2017).
Liu, H. et al. Sodium fluoride causes hepatocellular S-phase arrest by activating ATM-p53-p21 and ATR-Chk1-Cdc25A pathways in mice. Oncotarget 9, 4318–4337 (2018).
Zhang, Y. Y. et al. Mechanism of Juglone-induced cell cycle arrest and apoptosis in Ishikawa human endometrial cancer cells. J. Agric Food Chem. 67, 7378–7389 (2019).
Qin, J. L. et al. Oxoaporphine metal complexes (Co(II), Ni(II), Zn(II)) with high antitumor activity by inducing mitochondria-mediated Apoptosis and S-phase Arrest in HepG2. Sci. Rep. 7, 46056 (2017).
Srinivas, U. S., Tan, B. W. Q., Vellayappan, B. A. & Jeyasekharan, A. D. ROS and the DNA damage response in cancer. Redox Biol. 25, 101084 (2019).
Zhao, B. S., Roundtree, I. A. & He, C. Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell Biol. 18, 31–42 (2017).
Zhou, B. et al. RNA modification writer expression profiles predict clinical outcomes and guide neoadjuvant immunotherapy in non-small cell lung cancer. EBioMedicine 84, 104268 (2022).
Jelic, M. D., Mandic, A. D., Maricic, S. M. & Srdjenovic, B. U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 17, 22–28 (2021).
Calcinotto, A. et al. Cellular senescence: aging, cancer, and injury. Physiol. Rev. 99, 1047–1078 (2019).
Counter, C. M. et al. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 11, 1921–1929 (1992).
Courtois-Cox, S., Jones, S. L. & Cichowski, K. Many roads lead to oncogene-induced senescence. Oncogene 27, 2801–2809 (2008).
d’Adda di Fagagna, F. Living on a break: cellular senescence as a DNA-damage response. Nat. Rev. Cancer 8, 512–522 (2008).
Richter, T. & von Zglinicki, T. A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Exp. Gerontol. 42, 1039–1042 (2007).
Sanchez-Prieto, R., Rojas, J. M., Taya, Y. & Gutkind, J. S. A role for the p38 mitogen-acitvated protein kinase pathway in the transcriptional activation of p53 on genotoxic stress by chemotherapeutic agents. Cancer Res. 60, 2464–2472 (2000).
Coppé, J. P., Desprez, P. Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
Kuilman, T. et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133, 1019–1031 (2008).
Laberge, R. M. et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 17, 1049–1061 (2015).
Chien, Y. et al. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev. 25, 2125–2136 (2011).
Dörr, J. R. et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature 501, 421–425 (2013).
Kaplon, J. et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature 498, 109–112 (2013).
Luo, J. et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 107, 137–148 (2001).
Hecker, L. et al. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci. Transl. Med. 6, 231ra247 (2014).
Gewinner, C. et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell 16, 115–125 (2009).
Balistreri, C. R., Candore, G., Accardi, G., Colonna-Romano, G. & Lio, D. NF-κB pathway activators as potential ageing biomarkers: targets for new therapeutic strategies. Immun. Ageing. 10, 24 (2013).
Shmulevich, R. & Krizhanovsky, V. Cell senescence, DNA Damage, and metabolism. Antioxid. Redox Signal 34, 324–334 (2021).
Yu, L. et al. IgG is an aging factor that drives adipose tissue fibrosis and metabolic decline. Cell Metab. 36, 793–807.e795, https://doi.org/10.1016/j.cmet.2024.01.015 (2024).
Bauer, M. E. & Fuente Mde, L. The role of oxidative and inflammatory stress and persistent viral infections in immunosenescence. Mech. ageing Dev. 158, 27–37 (2016).
Vida, C. et al. Role of macrophages in age-related oxidative stress and lipofuscin accumulation in mice. Redox Biol. 12, 423–437 (2017).
Panyard, D. J., Yu, B. & Snyder, M. P. The metabolomics of human aging: Advances, challenges, and opportunities. Sci. Adv. 8, eadd6155 (2022).
Ye, L., Jiang, Y. & Zhang, M. Crosstalk between glucose metabolism, lactate production and immune response modulation. Cytokine Growth Factor Rev. 68, 81–92, https://doi.org/10.1016/j.cytogfr.2022.11.001 (2022).
Bosticardo, M. et al. Biased activation of human T lymphocytes due to low extracellular pH is antagonized by B7/CD28 costimulation. Eur. J. Immunol. 31, 2829–2838 (2001).
Boedtkjer, E. & Pedersen, S. F. The acidic tumor microenvironment as a driver of cancer. Annu Rev. Physiol. 82, 103–126 (2020).
Lin, R. et al. GPR65 promotes intestinal mucosal Th1 and Th17 cell differentiation and gut inflammation through downregulating NUAK2. Clin. Transl. Med. 12, e771 (2022).
Fan, J. et al. GPR65 contributes to constructing immunosuppressive microenvironment in glioma. Neurosurg. Rev. 47, 417 (2024).
Zhang, W. et al. Targeted intervention of tumor microenvironment with HDAC inhibitors and their combination therapy strategies. Eur. J. Med Res 30, 69 (2025).
Rotin, D., Steele-Norwood, D., Grinstein, S. & Tannock, I. Requirement of the Na+/H+ exchanger for tumor growth. Cancer Res. 49, 205–211 (1989).
Vitale, I., Manic, G., Coussens, L. M., Kroemer, G. & Galluzzi, L. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 30, 36–50 (2019).
Macintyre, A. N. et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 20, 61–72 (2014).
Zeng, H. & Chi, H. Metabolic control of regulatory T cell development and function. Trends Immunol. 36, 3–12 (2015).
Geeraerts, X., Bolli, E., Fendt, S. M. & Van Ginderachter, J. A. Macrophage metabolism as therapeutic target for cancer, atherosclerosis, and obesity. Front. Immunol. 8, 289, https://doi.org/10.3389/fimmu.2017.00289 (2017).
Ge, W. & Wu, W. Influencing factors and significance of tumor-associated macrophage polarization in tumor microenvironment. Zhongguo Fei Ai Za Zhi 26, 228–237 (2023).
Ramakrishnan, R. et al. Oxidized lipids block antigen cross-presentation by dendritic cells in cancer. J. Immunol. 192, 2920–2931 (2014).
Herber, D. L. et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat. Med. 16, 880–886 (2010).
Ma, X. et al. Cholesterol induces CD8(+) T cell exhaustion in the tumor microenvironment. Cell Metab. 30, 143–156.e145 (2019).
Zhu, L., Zhu, X. & Wu, Y. Effects of glucose metabolism, lipid metabolism, and glutamine metabolism on tumor microenvironment and clinical implications. Biomolecules 12, https://doi.org/10.3390/biom12040580 (2022).
Mao, X. et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol. Cancer 20, 131 (2021).
Li, T., Copeland, C. & Le, A. Glutamine metabolism in cancer. Adv. Exp. Med Biol. 1311, 17–38 (2021).
Menegon, S., Columbano, A. & Giordano, S. The dual roles of NRF2 in cancer. Trends Mol. Med 22, 578–593 (2016).
Johnson, M. O. et al. Distinct regulation of Th17 and Th1 cell differentiation by Glutaminase-dependent metabolism. Cell 175, 1780–1795.e1719 (2018).
Di Mitri, D. & Alimonti, A. Non-cell-autonomous regulation of cellular senescence in cancer. Trends Cell Biol. 26, 215–226 (2016).
Liu, X. et al. Regulatory T cells trigger effector T cell DNA damage and senescence caused by metabolic competition. Nat. Commun. 9, 249 (2018).
Angelin, A. et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 25, 1282–1293.e1287 (2017).
Di Mitri, D. et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature 515, 134–137 (2014).
Choi, K., Kim, M., Ryu, J. & Choi, C. Ginsenosides compound K and Rh(2) inhibit tumor necrosis factor-alpha-induced activation of the NF-kappaB and JNK pathways in human astroglial cells. Neurosci. Lett. 421, 37–41 (2007).
Varesi, A. et al. The role of antioxidants in the interplay between oxidative stress and senescence. Antioxidants 11, https://doi.org/10.3390/antiox11071224 (2022).
Reis, J. et al. A closer look into NADPH oxidase inhibitors: Validation and insight into their mechanism of action. Redox Biol. 32, 101466 (2020).
Crosas-Molist, E., Bertran, E. & Fabregat, I. Cross-talk between TGF-β and NADPH oxidases during liver fibrosis and hepatocarcinogenesis. Curr. Pharm. Des. 21, 5964–5976 (2015).
Crosas-Molist, E. & Fabregat, I. Role of NADPH oxidases in the redox biology of liver fibrosis. Redox Biol. 6, 106–111 (2015).
Holmgren, A. & Sengupta, R. The use of thiols by ribonucleotide reductase. Free Radic. Biol. Med. 49, 1617–1628 (2010).
Muri, J. et al. The thioredoxin-1 system is essential for fueling DNA synthesis during T-cell metabolic reprogramming and proliferation. Nat. Commun. 9, 1851 (2018).
Wu, N. et al. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol. Cell 49, 1167–1175 (2013).
Kaadige, M. R., Looper, R. E., Kamalanaadhan, S. & Ayer, D. E. Glutamine-dependent anapleurosis dictates glucose uptake and cell growth by regulating MondoA transcriptional activity. Proc. Natl Acad. Sci. USA 106, 14878–14883 (2009).
Brigelius-Flohé, R. & Maiorino, M. Glutathione peroxidases. Biochim. Biophys. 1830, 3289–3303 (2013).
Stoltzman, C. A. et al. Glucose sensing by MondoA:Mlx complexes: a role for hexokinases and direct regulation of thioredoxin-interacting protein expression. Proc. Natl Acad. Sci. USA 105, 6912–6917 (2008).
Patwari, P. et al. Thioredoxin-independent regulation of metabolism by the alpha-arrestin proteins. J. Biol. Chem. 284, 24996–25003 (2009).
Chan, L. N. et al. Metabolic gatekeeper function of B-lymphoid transcription factors. Nature 542, 479–483 (2017).
Cobaleda, C., Schebesta, A., Delogu, A. & Busslinger, M. Pax5: the guardian of B cell identity and function. Nat. Immunol. 8, 463–470 (2007).
Sena, L. A. et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38, 225–236 (2013).
Yi, J. S., Holbrook, B. C., Michalek, R. D., Laniewski, N. G. & Grayson, J. M. Electron transport complex I is required for CD8+ T cell function. J. Immunol. 177, 852–862 (2006).
Mak, T. W. et al. Glutathione Primes T cell metabolism for inflammation. Immunity 46, 675–689 (2017).
Laplante, M. & Sabatini, D. M. mTOR signaling in growth control and disease. Cell 149, 274–293 (2012).
Namgaladze, D., Hofer, H. W. & Ullrich, V. Redox control of calcineurin by targeting the binuclear Fe(2+)-Zn(2+) center at the enzyme active site. J. Biol. Chem. 277, 5962–5969 (2002).
Vaeth, M. et al. Store-operated Ca(2+) entry controls clonal expansion of T cells through metabolic reprogramming. Immunity 47, 664–679.e666 (2017).
Rhee, S. G. & Kil, I. S. Multiple functions and regulation of mammalian Peroxiredoxins. Annu Rev. Biochem. 86, 749–775 (2017).
Nakaya, M. et al. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity 40, 692–705 (2014).
Pollizzi, K. N. et al. Asymmetric inheritance of mTORC1 kinase activity during division dictates CD8(+) T cell differentiation. Nat. Immunol. 17, 704–711 (2016).
Verbist, K. C. et al. Metabolic maintenance of cell asymmetry following division in activated T lymphocytes. Nature 532, 389–393 (2016).
Wang, R. et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882 (2011).
Blagih, J. et al. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity 42, 41–54 (2015).
Tompkins, S. C. et al. Disrupting mitochondrial Pyruvate uptake directs Glutamine into the TCA cycle away from glutathione synthesis and impairs hepatocellular tumorigenesis. Cell Rep. 28, 2608–2619.e2606 (2019).
Waters, L. R., Ahsan, F. M., Wolf, D. M., Shirihai, O. & Teitell, M. A. Initial B cell activation induces metabolic reprogramming and mitochondrial remodeling. iScience 5, 99–109 (2018).
Jha, A. K. et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42, 419–430 (2015).
West, A. P. et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472, 476–480 (2011).
Mills, E. L. et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556, 113–117 (2018).
Coling, D., Chen, S., Chi, L. H., Jamesdaniel, S. & Henderson, D. Age-related changes in antioxidant enzymes related to hydrogen peroxide metabolism in rat inner ear. Neurosci. Lett. 464, 22–25 (2009).
Ames, B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science 221, 1256–1264 (1983).
Kwon, K., Jung, J., Sahu, A. & Tae, G. Nanoreactor for cascade reaction between SOD and CAT and its tissue regeneration effect. J. Control. Rel. Soc. 344, 160–172 (2022).
Kinnula, V. L. & Crapo, J. D. Superoxide dismutases in the lung and human lung diseases. Am. J. Respir. Crit. Care Med 167, 1600–1619 (2003).
Peoples, J. N., Saraf, A., Ghazal, N., Pham, T. T. & Kwong, J. Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 51, 1–13 (2019).
Gülçin, I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 78, 803–811 (2006).
Lin, M. T. & Beal, M. F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795 (2006).
Wang, Y. C., Hui, J. R., Xiao, G. & Ma, Q. L. Mechanism of key ingredient of Astragalus membranaceus on Lung Adenocarcinoma via PI3K/AKT signaling clarified by utilizing network pharmacology approach and experimental validation. Chin. J. Integr. Med. 29, 244–252 (2023).
Xiong, L., Deng, N., Zheng, B., Li, T. & Liu, R. H. HSF-1 and SIR-2.1 linked insulin-like signaling is involved in goji berry (Lycium spp.) extracts promoting lifespan extension of Caenorhabditis elegans. Food Funct. 12, 7851–7866 (2021).
Tan, H. Y. et al. Autophagy-induced RelB/p52 activation mediates tumour-associated macrophage repolarisation and suppression of hepatocellular carcinoma by natural compound baicalin. Cell Death Dis. 6, e1942 (2015).
Lan, T. et al. Saikosaponin A triggers cell ferroptosis in hepatocellular carcinoma by inducing endoplasmic reticulum stress-stimulated ATF3 expression. Biochem. Biophys. Res. Commun. 674, 10–18 (2023).
Wang, D. et al. Mitigation of honokiol on fluoride-induced mitochondrial oxidative stress, mitochondrial dysfunction, and cognitive deficits through activating AMPK/PGC-1α/Sirt3. J. Hazard Mater. 437, 129381 (2022).
Sun, C. et al. Additive antiangiogenesis effect of ginsenoside Rg3 with low-dose metronomic temozolomide on rat glioma cells both in vivo and in vitro. J. Exp. Clin. Cancer Res: CR 35, 32 (2016).
Li, K. F. et al. Ginsenoside Rh2 inhibits human A172 glioma cell proliferation and induces cell cycle arrest status via modulating Akt signaling pathway. Mol. Med. Rep. 17, 3062–3068 (2018).
Gu, B., Wang, J., Song, Y., Wang, Q. & Wu, Q. The inhibitory effects of ginsenoside Rd on the human glioma U251 cells and its underlying mechanisms. J. Cell Biochem. 120, 4444–4450 (2019).
Huang, J. et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut 71, 734–745 (2022).
Chen, S. et al. The effect of Bu Shen Huo Xue Tang on autoimmune premature ovarian insufficiency via Modulation of the Nrf2/Keap1 signaling pathway in mice. J. Ethnopharmacol. 273, 113996 (2021).
Zeng, Y. et al. Huangqi Baihe Granules alleviate hypobaric hypoxia-induced acute lung injury in rats by suppressing oxidative stress and the TLR4/NF-κB/NLRP3 inflammatory pathway. J. Ethnopharmacol. 324, 117765 (2024).
Wu, E. et al. Involvement of the Sch9/Rim15/Msn2 signaling pathway in the anti-aging activity of dendrobine from Dendrobium nobile Lindl. via modification of oxidative stress and autophagy. Chin. Med 18, 111 (2023).
Lin, S. S. et al. Acetylshikonin induces necroptosis via the RIPK1/RIPK3-dependent pathway in lung cancer. Aging 15, 14900–14914 (2023).
Liu, Z. Y., Luo, J. Y., Lu, Y. & Du, S. Y. [Application of traditional Chinese medicine theory in modern traditional Chinese medicine nano-preparation: taking tumor treatment as an example]. China J. Chin. Mater. Med. 48, 1455–1462 (2023).
Peng, X. et al. Red ginseng has stronger anti-aging effects compared to ginseng possibly due to its regulation of oxidative stress and the gut microbiota. Phytomedicine: Int J. Phytother. Phytopharmacol. 93, 153772 (2021).
Azami, S. H. et al. The antioxidant curcumin postpones ovarian aging in young and middle-aged mice. Reprod., Fertil. Dev. 32, 292–303 (2020).
Yang, S. et al. Tea polyphenols alleviate tri-ortho-cresyl phosphate-induced autophagy of mouse ovarian granulosa cells. Environ. Toxicol. 35, 478–486 (2020).
McKay, D. L. & Blumberg, J. B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother. Res: PTR 20, 619–633 (2006).
Liu, P., Zhao, H. & Luo, Y. Anti-aging implications of Astragalus Membranaceus (Huangqi): A well-known Chinese Tonic. Aging Dis. 8, 868–886 (2017).
Liang, T. et al. Anti-senescence effects of Rhodiola crenulate extracts on LO(2) cells and bioactive compounds. J. Ethnopharmacol. 306, 116179 (2023).
Niu, Y. et al. Angelica sinensis polysaccharides alleviate the oxidative burden on hematopoietic cells by restoring 5-fluorouracil-induced oxidative damage in perivascular mesenchymal progenitor cells. Pharm. Biol. 61, 768–778 (2023).
Li, R. et al. Crataegus pinnatifida: A botanical, ethnopharmacological, phytochemical, and pharmacological overview. J. Ethnopharmacol. 301, 115819 (2023).
Min, F. et al. Hepatoprotective effects of hydroxysafflor yellow A in D-galactose-treated aging mice. Eur. J. Pharm. 881, 173214 (2020).
Lee, H. & Park, E. Perilla frutescens extracts enhance DNA repair response in UVB-damaged HaCaT cells. Nutrients 13, https://doi.org/10.3390/nu13041263 (2021).
Zeng, Z. et al. Anoectochilus roxburghii flavonoids extract ameliorated the memory decline and reduced neuron apoptosis via modulating SIRT1 signaling pathway in senescent mice. J. Ethnopharmacol. 296, 115361 (2022).
Pan, M. et al. Polygonati Rhizoma: A review on the extraction, purification, structural characterization, biosynthesis of the main secondary metabolites and anti-aging effects. J. Ethnopharmacol. 327, 118002 (2024).
He, J. et al. Gastrodin extends the lifespan and protects against neurodegeneration in the Drosophila PINK1 model of Parkinson’s disease. Food Funct. 12, 7816–7824 (2021).
Xu, S. et al. The extract of buds of Chrysanthemum morifolium ramat alleviated UVB-induced skin photoaging by regulating MAPK and Nrf2/ARE pathways. J. Ethnopharmacol. 332, 118352 (2024).
Yu, Z. et al. Baicalin promoted site-2 protease and not site-1 protease in endoplasmic reticulum stress-induced apoptosis of human hepatocellular carcinoma cells. FEBS Open Bio 6, 1093–1101 (2016).
Zhang, Y. Z., Wang, C. F. & Zhang, L. F. Cucurbitacin D impedes gastric cancer cell survival via activation of the iNOS/NO and inhibition of the Akt signalling pathway. Oncol. Rep. 39, 2595–2603 (2018).
Pei, J., Velu, P., Zareian, M., Feng, Z. & Vijayalakshmi, A. Effects of Syringic acid on apoptosis, inflammation, and AKT/mTOR signaling pathway in gastric cancer cells. Front Nutr. 8, 788929 (2021).
Cheng, C. et al. A novel Chinese medicine formula inhibits non-small cell lung cancer by triggering oxidative stress dependent on Pentose phosphate pathway. Zhongguo Fei Ai Za Zhi 26, 639–649 (2023).
Que, Z. et al. Jingfukang induces anti-cancer activity through oxidative stress-mediated DNA damage in circulating human lung cancer cells. BMC Complement. Altern. Med. 19, 204 (2019).
Nguyen, C. T., Luong, T. T., Kim, G. L., Pyo, S. & Rhee, D. K. Korean Red Ginseng inhibits apoptosis in neuroblastoma cells via estrogen receptor β-mediated phosphatidylinositol-3 kinase/Akt signaling. J. Gnseng Res. 39, 69–75 (2015).
Chuang, M. H., Jan, M. S., Chang, J. T. & Lu, F. J. The Chinese medicine JC-001 enhances the chemosensitivity of Lewis lung tumors to cisplatin by modulating the immune response. BMC Complement. Altern. Med 17, 210 (2017).
Chen, Y. et al. Water extract of ginseng and astragalus regulates macrophage polarization and synergistically enhances DDP’s anticancer effect. J. Ethnopharmacol. 232, 11–20 (2019).
Terrén, I., Orrantia, A., Vitallé, J., Zenarruzabeitia, O. & Borrego, F. NK cell metabolism and tumor microenvironment. Front. Immunol. 10, 2278 (2019).
Wu, K. C., Cui, J. Y. & Klaassen, C. D. Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol. Sci: J. Soc. Toxicol. 123, 590–600 (2011).
Wang, X. J. et al. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis 29, 1235–1243 (2008).
Majmundar, A. J., Wong, W. J. & Simon, M. C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. cell 40, 294–309 (2010).
Semenza, G. L. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharm. Sci. 33, 207–214 (2012).
Chen, Z. J., Parent, L. & Maniatis, T. Site-specific phosphorylation of IkappaBalpha by a novel ubiquitination-dependent protein kinase activity. Cell 84, 853–862 (1996).
Talty, R. & Olino, K. Metabolism of Innate Immune Cells in Cancer. Cancers 13, https://doi.org/10.3390/cancers13040904 (2021).
van Uden, P., Kenneth, N. S. & Rocha, S. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem. J. 412, 477–484 (2008).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013).
Grivennikov, S. I. & Karin, M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 21, 11–19 (2010).
Li, C. W. et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun. 7, 12632 (2016).
Ataie-Kachoie, P., Pourgholami, M. H. & Morris, D. L. Inhibition of the IL-6 signaling pathway: a strategy to combat chronic inflammatory diseases and cancer. Cytokine Growth Factor Rev. 24, 163–173 (2013).
Kishimoto, T. Interleukin-6: from basic science to medicine–40 years in immunology. Annu. Rev. Immunol. 23, 1–21 (2005).
Barsoum, I. B., Smallwood, C. A., Siemens, D. R. & Graham, C. H. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 74, 665–674 (2014).
Zhang, Y. et al. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 23, 898–914 (2013).
Yu, H., Pardoll, D. & Jove, R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer 9, 798–809 (2009).
Acknowledgements
We thank all of the study participants. All data were generated in-house, and no paper mill was used. All authors agree to be accountable for all aspects of work, ensuring integrity and accuracy. This work was financially supported by a grant from the National Natural Science Foundation of China (82204677, 82474267), the National Science and Technology Major Project of China (2024ZD0521405), Major Project of Guangzhou National Laboratory(No. GZNL2023A02009), Traditional Chinese Medicine Bureau of Guangdong Province Project (NO. 20241112), State Key Laboratory of Traditional Chinese Medicine Syndrome (QZ2023ZZ11), Chinese Medicine Guangdong Laboratory (HQL2024PZ005).
Author information
Authors and Affiliations
Contributions
Zhou and Bao and Zhang wrote the main manuscript text Jiang and Liang and Ma gathered the information. Li and Pan and Liu are in charge of the overall idea of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, Nj., Bao, WQ., Zhang, Cf. et al. Immunometabolism and oxidative stress: roles and therapeutic strategies in cancer and aging. npj Aging 11, 59 (2025). https://doi.org/10.1038/s41514-025-00250-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41514-025-00250-z