Abstract
Chronic respiratory diseases (CRD) are major contributors to mortality. The “obesity paradox” suggests that higher body mass index (BMI) may confer survival benefits in CRD patients. This study investigates the association between BMI and mortality risk in CRD patients, focusing on the mediating role of the triglyceride-glucose (TyG) index. A cross-sectional analysis of 7689 participants with CRD was conducted. Participants were categorized by BMI into <25.0, 25.0–29.9, 30.0–34.9, 35.0–39.9, and ≥40 kg/m2. Outcomes included all-cause mortality, cardiovascular disease (CVD) mortality, and non-CVD mortality. Cox regression models assessed associations, and mediation analysis evaluated the role of the TyG index. Among 7689 CRD patients, higher BMI was associated with lower all-cause mortality (HR for BMI 25.0–29.9: 0.81, 95% CI 0.70–0.94; HR for BMI 30.0–34.9: 0.72, 95% CI 0.61–0.85; HR for BMI 35.0–39.9: 0.72, 95% CI 0.59–0.88; HR for BMI ≥ 40: 0.82, 95% CI 0.66–1.02) and non-CVD mortality (HR for BMI 25.0–29.9: 0.77, 95% CI 0.65–0.91; HR for BMI 30.0–34.9: 0.65, 95% CI 0.54–0.79; HR for BMI 35.0–39.9: 0.66, 95% CI 0.52–0.83; HR for BMI ≥ 40: 0.69, 95% CI 0.53–0.89), but not CVD mortality. The TyG index mediated a significant proportion of the association between BMI and mortality (mediation effects: −22.39 to −18.49%). Kaplan-Meier survival curves and restricted cubic spline regression further illustrated the significant associations between BMI and all-cause mortality and non-CVD mortality, while no significant association was observed for CVD mortality. Higher BMI is associated with lower mortality risk in CRD patients, particularly for non-CVD causes, mediated by the TyG index. This highlights the potential role of insulin resistance in the “obesity paradox” and suggests that metabolic health interventions may improve outcomes in CRD.
Similar content being viewed by others
Introduction
Chronic respiratory diseases (CRDs), including conditions such as chronic obstructive pulmonary disease (COPD) and asthma, have emerged as significant global health issues. According to the World Health Organization (WHO), over 500 million individuals are affected by CRDs globally, with COPD affecting approximately 64 million and asthma affecting around 235 million people1. These conditions account for approximately 4 million deaths annually, with the burden of CRDs particularly pronounced in low- and middle-income countries, where higher prevalence rates and limited healthcare resources exacerbate the issue2.
Emerging evidence suggests that elevated body mass index (BMI) may confer survival benefits in specific CRD populations. The obesity paradox in COPD has garnered attention due to findings indicating that obese patients experience fewer exacerbations and lower mortality rates compared to their non-obese counterparts. For instance, research has shown that individuals with a higher BMI may possess greater energy reserves and a more favorable immune profile, which could confer a survival advantage during acute exacerbations of COPD3. This counterintuitive observation has led to investigations into the underlying mechanisms that might explain the protective effect of obesity in CRD patients. One potential explanation involves the role of insulin resistance, often assessed using the triglyceride-glucose (TyG) index, which is a reliable measure of metabolic health and has been linked to various chronic diseases4,5.
Insulin resistance is characterized by the body’s diminished ability to respond to insulin, leading to elevated levels of triglycerides and glucose in the bloodstream. The TyG index, derived from fasting triglyceride and glucose levels, has been shown to predict adverse health outcomes in diverse populations, including those with chronic diseases4,5. For instance, a meta-analysis by Zhou et al. demonstrated that elevated TyG index is associated with an increased prevalence of diabetic retinopathy in patients with diabetes mellitus (DM)6. Similarly, in different populations, pooled analyses reveal that higher TyG index correlates with a higher incidence of heart failure (HF)7. Emerging evidence also links TyG index severity to adverse outcomes in obstructive sleep apnea (OSA) patients8. In PAD cohorts, the TyG index has been identified as a predictor of disease progression9. These findings collectively emphasize the TyG index as a cross-disease biomarker of metabolic dysregulation, supporting its relevance in exploring the obesity paradox in CRD. Elevated levels of insulin resistance could contribute to a chronic pro-inflammatory state, which is known to exacerbate respiratory conditions10. Furthermore, the interplay between obesity, insulin resistance, and inflammation suggests that metabolic health should be a critical consideration in the management of patients with CRD11,12.
Despite the intriguing findings surrounding the obesity paradox and the role of the TyG index, the relationship between BMI, the TyG index, and mortality in individuals with CRD remains underexplored. Understanding this relationship is crucial for developing targeted interventions aimed at improving outcomes in this high-risk population. Given these gaps, our study aims to address the following key questions: (1) What is the relationship between BMI and mortality risk in patients with CRD? (2) Does the TyG index mediate this relationship, and if so, to what extent? By investigating these questions, our research seeks to provide novel insights into the mechanisms underlying the obesity paradox in CRD and to highlight the importance of considering metabolic health in the management of these patients.
Methods
Data source
NHANES employs a multistage, stratified probability sampling design to select a nationally representative sample of the U.S. non-institutionalized civilian population. Survey cycles (2001–2018) included household interviews, physical examinations, and laboratory tests. Sampling weights were applied to account for non-response and ensure population representativeness.
Study population
The study population consisted of adults aged 18 years and older who had a diagnosis of asthma. Initially, a total of 91,351 participants were included in the NHANES survey cycles from 2001 to 2018. However, after excluding participants without complete data on CRD status, BMI, TyG index, and other covariates, a final sample of 7689 CRDs patients was included in the analysis (Fig. 1).
Assessment of mortality
Mortality data were obtained by linking NHANES participants to the National Death Index (NDI) through December 31, 2019, using probabilistic matching of Social Security Numbers, name, birthdate, and sex. The NDI is regarded as the “gold standard” for mortality ascertainment in U.S. cohort studies, with over 98% sensitivity and specificity for death identification. Cause of death was coded according to the International Classification of Diseases, Tenth Revision (ICD-10).
Statistical analysis
The TyG index was determined by taking the natural logarithm of the product of glucose and triglyceride levels, both measured in mg/dL. TyG index was calculated using the formula Ln [fasting triglycerides (mg/dL) × fasting glucose (mg/dL)]/213. Additionally, participants were divided into quartiles by BMI categories: underweight/normal weight (BMI < 24.0 kg/m2), overweight (24.0–27.9 kg/m2), and obese (BMI ≥ 28.0 kg/m2).
The Cox proportional hazards regression model was utilized to compute and present the adjusted hazard ratios (HRs) along with their corresponding 95% confidence intervals (95% CIs). The current observational study utilized three models following the guidelines of Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)14. An initial model was adjusted for none (model 1). Model 2 was adjusted for age, sex, and race. Model 3 was adjusted for all variables in model 2 plus other risk factors for marital status, education level, and smoking status.
Mediation analysis was performed to assess the mediating role of the TyG index in the association between BMI and mortality. The analysis was conducted using a regression-based approach, where the total effect of the exposure on the outcome was decomposed into direct and indirect effects mediated by the TyG index. The proportion of the total effect mediated by the TyG index was calculated as the ratio of the indirect effect to the total effect.
Kaplan–Meier survival curves for all-cause mortality stratified by the values of BMI was generated. We used a penalized spline method for smooth curve fitting to examine any possible non-linear association between BMI level and mortality. A P value of less than 0.05 is considered statistically significant. All statistical analyses were performed with R version 4.2.2 and Free Statistics software version V2.1Beta.
Results
Baseline characteristics of the study population
The study included a total of 7689 patients with CRD. The participants were categorized into five BMI groups: underweight/normal weight (<25.0 kg/m2), overweight (25.0–29.9 kg/m2), Class I obesity (30.0–34.9 kg/m2), Class II obesity (35.0–39.9 kg/m2), and Class III obesity (≥40 kg/m2). Table 1 presents the demographic and clinical characteristics of the study population according to BMI level. The mean BMI was 30.4 ± 7.9 kg/m2, with significant differences observed across the BMI groups (P < 0.001). The mean TyG index was 4.9 ± 0.2, which also varied significantly among the BMI groups (P < 0.001). The average age of the participants was 49.7 ± 18.3 years, with a higher proportion of females (57.8%) than males (42.2%). The distribution of race, education levels, and marital status also showed significant differences across the BMI groups (P < 0.001).
Associations between BMI and mortality
Table 2 presents the results of the multivariate Cox regression analysis for the association between BMI and mortality outcomes. For all-cause mortality, compared to the reference group (BMI < 25.0 kg/m2), participants in the overweight, Class I, II, and III obesity groups had significantly lower hazard ratios (HRs) in all models. Specifically, in Model 3 (adjusted for age, sex, race, marital status, education level, and smoking status), the HRs were 0.81 (95% CI 0.70–0.94, P = 0.005) for the overweight group, 0.72 (95% CI 0.61–0.85, P < 0.001) for Class I obesity, 0.72 (95% CI 0.59–0.88, P = 0.002) for Class II obesity, and 0.82 (95% CI 0.66–1.02, P = 0.07) for Class III obesity. A significant trend was observed for decreasing all-cause mortality with increasing BMI (P for trend <0.001).
For cardiovascular disease (CVD) mortality, no significant associations were observed between BMI and mortality across all models. In Model 3, the HRs for the overweight, Class I, II, and III obesity groups were 0.93 (95% CI 0.68–1.27, P = 0.647), 0.95 (95% CI 0.67–1.34, P = 0.766), 0.88 (95% CI 0.57–1.36, P = 0.566), and 1.32 (95% CI 0.85–2.04, P = 0.211), respectively. The P for trend was 0.474, indicating no significant trend.
For non-cardiovascular disease (non-CVD) mortality, participants in the overweight, Class I, II, and III obesity groups had significantly lower HRs compared to the reference group in all models. In Model 3, the HRs were 0.77 (95% CI 0.65–0.91, P = 0.002) for the overweight group, 0.65 (95% CI 0.54–0.79, P < 0.001) for Class I obesity, 0.66 (95% CI 0.52–0.83, P < 0.001) for Class II obesity, and 0.69 (95% CI 0.53–0.89, P = 0.005) for Class III obesity. A significant trend was observed for decreasing non-CVD mortality with increasing BMI (P for trend <0.001).
Survival analysis
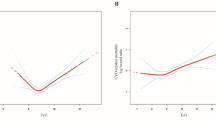
Kaplan-Meier survival curves stratified by BMI categories showed significant differences in survival rates for all-cause mortality and non-CVD mortality, with higher survival rates observed in the overweight and obese groups compared to the underweight/normal weight group (Fig. 2). The restricted cubic spline regression further illustrated the non-linear relationship between BMI and mortality outcomes, confirming the significant associations observed in the Cox regression models (Fig. 3).
Associations between BMI and TyG index
Table 3 shows the results of the linear regression analysis for the association between BMI and the TyG index. Compared to the reference group (BMI < 25.0 kg/m2), participants in the overweight, Class I, II, and III obesity groups had significantly higher TyG index values in all models. In Model 3, the β values were 0.05 (95% CI 0.04–0.06, P < 0.001) for the overweight group, 0.08 (95% CI 0.07–0.09, P < 0.001) for Class I obesity, 0.10 (95% CI 0.08–0.11, P < 0.001) for Class II obesity, and 0.10 (95% CI 0.09–0.11, P < 0.001) for Class III obesity. A significant trend was observed for increasing TyG index with increasing BMI (P for trend <0.001).
Mediation analysis
Table 4 presents the results of the mediation analysis, which assessed the role of the TyG index in the association between BMI and all-cause mortality. The total effect of BMI on all-cause mortality was partially mediated by the TyG index. In Model 3, the mediation effects were −22.39% for the overweight group and −18.49% for the obesity groups (Class I, II, and III combined). This indicates that the TyG index mediated a significant proportion of the association between BMI and mortality, suggesting that insulin resistance may play a role in the observed “obesity paradox” in CRD patients.
Discussion
Our study provides novel insights into the complex relationship between BMI and mortality in patients with CRD. The findings confirm the existence of the “obesity paradox,” where higher BMI is associated with lower all-cause and non-CVD mortality. This paradox has been previously observed in various chronic diseases, including COPD15,16. The underlying mechanisms remain incompletely understood, but several hypotheses have been proposed to explain this phenomenon. One potential explanation is that individuals with higher BMI may have greater energy reserves, which could be beneficial during acute exacerbations of CRD. This is particularly relevant in the context of COPD, where exacerbations can significantly impact mortality rates. Research indicates that patients with higher BMI may possess a metabolic advantage, allowing them to better withstand the physiological stress associated with acute respiratory events17,18. Furthermore, the presence of additional adipose tissue may provide a protective effect by serving as an energy reservoir during periods of increased metabolic demand, such as during exacerbations or infections19. Additionally, obese individuals may possess a more favorable immune profile, which could contribute to their improved survival. Studies have suggested that obesity can lead to alterations in immune function, potentially enhancing the body’s ability to respond to infections and inflammatory processes20. For instance, the presence of certain adipokines, which are signaling molecules secreted by adipose tissue, may modulate immune responses and reduce the severity of inflammation associated with CRD. This immune modulation could play a critical role in the observed survival advantage among obese patients with chronic respiratory conditions. Moreover, the obesity paradox has been documented in other chronic diseases, reinforcing the notion that higher BMI may not always correlate with poorer health outcomes. This suggests that the relationship between BMI and health outcomes is complex and may be influenced by various factors, including comorbidities and the overall health status of the individual.
Our study highlights the mediating role of the TyG index in the association between BMI and mortality in patients with CRD. The TyG index, a reliable measure of insulin resistance, was found to mediate a significant proportion of the observed associations. This finding suggests that insulin resistance may be an important factor contributing to the “obesity paradox” in CRD patients, where higher BMI is paradoxically associated with lower mortality rates. Insulin resistance is characterized by elevated levels of triglycerides and glucose, which can lead to a chronic pro-inflammatory state. This pro-inflammatory state may exacerbate respiratory conditions and contribute to mortality risk, as inflammation plays a critical role in the pathophysiology of CRD21. Our results indicate that the protective effect of higher BMI may be partially explained by its association with lower insulin resistance, as measured by the TyG index. The TyG index has been shown to be a reliable biomarker for assessing insulin resistance and has been linked to various adverse health outcomes, including CVDs and metabolic disorders22,23. Elevated levels of triglycerides and glucose, which comprise the TyG index, are indicative of metabolic dysregulation and can lead to systemic inflammation, further complicating the clinical picture for patients with CRD24. Moreover, the relationship between the TyG index and mortality risk underscores the importance of metabolic health in understanding the obesity paradox. Previous studies have suggested that individuals with higher BMI may have greater energy reserves, which could be beneficial during acute exacerbations of CRD. This is particularly relevant in the context of COPD, where exacerbations can significantly impact mortality rates25. Furthermore, the presence of insulin resistance, as indicated by the TyG index, may influence the severity of respiratory conditions and the overall health status of patients, thereby affecting their mortality risk26.
Recent evidence emphasizes the heterogeneity of adipose tissue, revealing that different fat depots exert distinct metabolic effects. Epicardial adipose tissue (EAT), for example, plays a dual role; in moderate amounts, it acts as a cardioprotective metabolic buffer by storing free fatty acids and secreting anti-inflammatory adipokines like adiponectin, which can mitigate vascular inflammation and stabilize coronary arteries27. However, excessive EAT volume is associated with detrimental outcomes, such as coronary plaque progression, arrhythmias, and heart failure, primarily due to the secretion of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6)28. In addition to EAT, visceral abdominal fat is strongly linked to insulin resistance and heightened cardiovascular risk, while subcutaneous fat may exhibit a neutral or even protective metabolic profile29. Our findings, grounded on BMI as a general measure of adiposity, do not differentiate between these various fat depots. However, the observed inverse association between BMI and non-CVD mortality may suggest a predominance of metabolically favorable adipose tissue—such as subcutaneous or moderate EAT—in CRD patients. This aligns with the concept of “protective fat,” wherein certain fat depots enhance energy reserves and immune modulation while reducing systemic inflammation30. For instance, subcutaneous adipose tissue may function as a benign energy reservoir during acute respiratory exacerbations, in contrast to visceral or ectopic fat, which tends to exacerbate insulin resistance and inflammatory processes. The elevated TyG index, noted in higher BMI groups yet paradoxically associated with improved survival, reflects potential metabolic adaptations in CRD patients that could mitigate some adverse effects related to adipose dysfunction.
Recent studies indicate that inflammation in adipose tissue significantly drives insulin resistance and metabolic abnormalities associated with obesity, creating a complex interrelationship that impacts CRD31. In this context, inflamed adipose tissue releases pro-inflammatory cytokines, such as TNF-α and IL-6, which impair insulin signaling in skeletal muscle and liver, leading to reduced glucose uptake and increased hepatic glucose production32. This inflammatory environment also contributes to dysfunctional adipocytes that exhibit elevated lipolytic activity, thus increasing circulating triglyceride levels and affecting TyG index33 The interaction between these metabolic changes creates a vicious cycle where increased inflammation perpetuates insulin resistance, linking obesity to heightened cardiovascular risks and metabolic dysfunction, ultimately influencing CRD progression. Our findings align with this framework, as higher BMI was associated with elevated TyG index values, yet paradoxically linked to improved survival. This suggests that in CRD patients, the benefits of energy reserves and adipokine-mediated immune modulation may outweigh the detrimental effects of adipose tissue dysfunction. However, the absence of a significant association between BMI and CVD mortality implies that the cardiometabolic risks of obesity (e.g., atherosclerosis, hypertension) may counterbalance these protective mechanisms in cardiovascular contexts.
Our findings hold significant clinical implications for the management of CRD patients. First, they emphasize the importance of considering metabolic health in the management of these patients. Given the significant role that insulin resistance, as measured by the TyG index, plays in the relationship between BMI and mortality, interventions aimed at improving insulin sensitivity may be beneficial. Lifestyle modifications, such as dietary changes and increased physical activity, have been shown to enhance insulin sensitivity and may lead to improved outcomes in patients with CRD. Additionally, pharmacological treatments that target insulin resistance, such as metformin or thiazolidinediones, could be explored as potential adjunct therapies in this population. Second, the study suggests that the traditional focus on weight reduction in obese patients with CRD may need to be reconsidered. While weight loss has been a common recommendation for managing obesity-related health issues, our findings indicate that a more nuanced approach is warranted. This approach should take into account not only BMI but also metabolic health indicators, such as the TyG index. For instance, some obese individuals may have a favorable metabolic profile and experience better health outcomes despite their weight. Therefore, clinicians should consider individual metabolic health when developing treatment plans, rather than solely focusing on weight reduction as a primary goal. Moreover, the implications of these findings extend to public health initiatives aimed at preventing and managing CRD. Strategies that promote metabolic health, such as community-based programs encouraging physical activity and healthy eating, could play a crucial role in improving the overall health of individuals at risk for or currently living with CRD. In summary, the findings of this study underscore the need for a paradigm shift in the management of patients with CRD. By prioritizing metabolic health and considering the complexities of the obesity paradox, healthcare providers can better tailor interventions to improve patient outcomes and enhance quality of life.
Limitations and strengths
The strengths of this study include the use of a large, nationally representative dataset from NHANES, which allows for generalizability of the findings. Additionally, the study utilized robust statistical methods, including mediation analysis and survival analysis, to assess the associations between BMI, the TyG index, and mortality. However, there are also several limitations. The cross-sectional design of the study limits the ability to establish causality. Additionally, the study relied on self-reported data for some variables, which may introduce bias. Future longitudinal studies are needed to further explore the underlying mechanisms of the “obesity paradox” and the role of insulin resistance in CRD patients.
Conclusions
In conclusion, our study confirms the “obesity paradox” in CRD patients, where higher BMI is associated with lower mortality risk, particularly for non-CVD causes. The TyG index, a measure of insulin resistance, mediated a significant proportion of this association. These findings highlight the potential role of insulin resistance in the “obesity paradox” and suggest that metabolic health interventions may improve outcomes in patients with CRD. Future research should focus on elucidating the underlying mechanisms and exploring targeted interventions to optimize patient care.
Data availability
All data used in this study are available upon reasonable request from the corresponding author.
References
Habib, G. M. et al. Systematic review (Protocol) of clinical effectiveness and models of care of low-resource pulmonary rehabilitation. NPJ Prim. Care Respir. Med. 29, 10 (2019).
Meghji, J., Jayasooriya, S., Khoo, E. M., Mulupi, S. & Mortimer, K. Chronic respiratory disease in low-income and middle-income countries: from challenges to solutions. J. Pan Afr. Thorac. Soc. 3, 92–97 (2022).
Li, J. et al. High fat-to-muscle ratio was associated with increased clinical severity in patients with abdominal trauma. J. Clin. Med. 12, 1503 (2023).
Gao, W. et al. Discordance between the triglyceride glucose index and HOMA-IR in incident albuminuria: a cohort study from China. Lipids Health Dis. 20, 176 (2021).
Liu, L. et al. Association of triglyceride–glucose index and traditional risk factors with cardiovascular disease among non-diabetic population: a 10-year prospective cohort study. Cardiovasc. Diabetol. 21, 256 (2022).
Zhou, J., Zhu, L. & Li, Y. Association between the triglyceride glucose index and diabetic retinopathy in type 2 diabetes: a meta-analysis. Front. Endocrinol. 14, 1302127 (2023).
Khalaji, A. et al. Triglyceride-glucose index and heart failure: a systematic review and meta-analysis. Cardiovasc. Diabetol. 22, 244 (2023).
Behnoush, A. H. et al. Triglyceride-glucose index and obstructive sleep apnea: a systematic review and meta-analysis. Lipids Health Dis. 23, 4 (2024).
Samavarchitehrani, A. et al. Investigating the association between the triglyceride-glucose index and peripheral artery disease: a systematic review and meta-analysis. Nutr. Diabetes 14, 80 (2024).
Guo, Z., Wang, X., Wang, Y., Xing, G. & Liu, S. “Obesity Paradox” in acute respiratory distress syndrome: asystematic review and meta-analysis. PLoS One. 11, e0163677 (2016).
Yao, S., Zeng, L., Wang, F. & Ke-jie, C. Obesity paradox in lung diseases: what explains it?. Obes. Facts. 16, 411–426 (2023).
Cheung, Y.-M. M., Joham, A. E., Marks, S. & Teede, H. The obesity paradox: an endocrine perspective. Intern. Med. J. 47, 727–733 (2017).
Simental-Mendía, L. E. & Guerrero-Romero, F. The correct formula for the triglycerides and glucose index. Eur. J. Pediatr. 179, 1171 (2020).
Rao, A. et al. Quality of reporting and study design of CKD cohort studies assessing mortality in the elderly before and after STROBE: a systematic review. PLOS One. 11, e0155078 (2016).
Putcha, N. et al. Mortality and exacerbation risk by body mass index in patients with COPD in TIOSPIR and UPLIFT. Ann. Am. Thorac. Soc. 19, 204–213 (2022).
Benslimane, A. et al. Association between obesity and chronic obstructive pulmonary disease in moroccan adults: evidence from the BOLD study. SAGE Open. Med. 9, 20503121211031428 (2021).
Niedziela, J. et al. The obesity paradox in acute coronary syndrome: a meta-analysis. Eur. J. Epidemiol. 29, 801–812 (2014).
Wei, Y. et al. The impact of overweight and obesity on acute exacerbations of COPD - subgroup analysis of the Taiwan obstructive lung disease cohort. Int. J. Chron. Obstruct Pulmon Dis. 12, 2723–2729 (2017).
Humińska-Lisowska, K. et al. Implications of adipose tissue content for changes in serum levels of exercise-induced adipokines: a quasi-experimental study. Int. J. Environ. Res. Public. Health 19, 8782 (2022).
Oesch, L., Tatlısumak, T., Arnold, M. & Sarıkaya, H. Obesity paradox in stroke – myth or reality? a systematic review. PLoS One 12, e0171334 (2017).
Zhang, S. et al. The triglyceride and glucose index (TyG) is an effective biomarker to identify nonalcoholic fatty liver disease. Lipids Health Dis. 16, 15 (2017).
Öztürk, S. Ö Comparison of triglyceride-glucose index between patients with gallstone and healthy individuals. Iran. J. Public. Health 53, 888–894 (2024).
Dorcely, B. Continuous glucose monitoring captures glycemic variability in obesity after sleeve gastrectomy: a prospective cohort study. Obes. Sci. Pract. 10, e729 (2024).
Qian, T. et al. Mets-Ir as a predictor of cardiovascular events in the middle-aged and elderly population and mediator role of blood lipids. Front. Endocrinol. 14, 1224967 (2023).
Khan, S. H. et al. Metabolic clustering of risk factors: evaluation of triglyceride-glucose index (TyG Index) for evaluation of insulin resistance. Diabetol. Metab. Syndr. 10, 74 (2018).
Kim, M. K. et al. Relationship between the triglyceride glucose index and coronary artery calcification in korean adults. Cardiovasc. Diabetol. 16, 108 (2017).
Monti, C. B. et al. Subcutaneous, paracardiac, and epicardial fat CT density before/after contrast injection: any correlation with CAD? J. Clin. Med. 10, 735 (2021).
Rosenquist, K. J. et al. Visceral and subcutaneous fat quality and cardiometabolic risk. Jacc Cardiovasc. Imaging 6, 762–771 (2013).
Sequeira, D. I. et al. The correlation of epicardial adipose tissue on postmortem CT with coronary artery stenosis as determined by autopsy. Forensic Sci. Med. Pathol. 11, 186–192 (2015).
Uysal, F. et al. Epicardial adipose tissue is increased in patients with inflammatory bowel disease. J. Ultrasound Med. 35, 1859–1864 (2016).
Yan, K. Recent advances in the effect of adipose tissue inflammation on insulin resistance. Cell. Signal. 120, 111229 (2024).
Wada, H. et al. Low BMI and weight loss aggravate COPD mortality in men, findings from a large prospective cohort: the JACC study. Sci. Rep. 11, 1531 (2021).
Yang, L., Zhu, Y., Huang, J., Jin, J. & Zhang, X. A low lean-to-fat ratio reduces the risk of acute exacerbation of chronic obstructive pulmonary disease in patients with a normal or low body mass index. Med. Sci. Monit. 25, 5229–5236 (2019).
Acknowledgements
The authors thank the participants of the NHANES survey for their contributions. This study was supported by grants from the Shenzhen Science and Technology Program (JCYJ20240813152030039).
Author information
Authors and Affiliations
Contributions
Feng and Pan Jiang conceived an designed the study, performed the initial data analysis, and drafted the original manuscript. During the revision process, Yongwen Feng and Jibo Li contributed to the validation of data and optimization of statistical methods. Xinlong Liu assisted in refining the interpretation of results and updating the survival analysis. Haoda Liang and Zhongsheng Tan provided critical revision to the manuscript, particularly in strengthening the discussion of the obesity paradox and the role the TyG index. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study utilized publicly available data from NHANES, which does not require individual ethics approval.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, F., Feng, Y., Li, J. et al. Exploring the obesity paradox in chronic respiratory disease: the mediating effect of triglyceride-glucose index on mortality. npj Prim. Care Respir. Med. 35, 25 (2025). https://doi.org/10.1038/s41533-025-00431-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41533-025-00431-z