Abstract
Cell replacement therapy is a promising therapeutic option for dry age-related macular degeneration (AMD). In this study, we outline our design for scalable manufacture with appropriate quality gates and present in vivo data for establishing preclinical safety and efficacy of an induced pluripotent stem cell (iPSC)-derived retinal pigment epithelium (RPE) product, thus laying the foundation for Phase 1/2a trial approval in India (ClinicalTrials.gov ID: NCT06394232; date of registration: 23rd September 2024). Escalating doses of RPE cell suspension in immunocompromised animals demonstrated absence of tumor formation up to 9 months post-injection. Good Laboratory Practices (GLP) toxicology and tolerability studies in rabbits and non-human primates (NHP) respectively showed no major adverse events. RPE transplanted into immune suppressed RCS rats showed integration, neuroprotection and rescue of visual function. In addition, we provide a detailed description of the modifications in GMP manufacturing protocol to create a final product with a unique composition and Chemistry, Manufacturing and Controls (CMC) studies performed during product development.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) caused as a result of dysfunction and loss of RPE cells is one of the leading causes of blindness in people over the age of 601. Early to intermediate phases of AMD known as “dry AMD” or non-exudative AMD is diagnosed by the formation of drusen accumulation of proteins and extracellular debris beneath RPE cells2,3. The exudative, or “wet” form of AMD is characterized by subretinal choroidal neovascularization, resulting in fragile blood vessels that penetrate Bruch’s membrane, causing bleeding and disruption of the RPE layer1,4. Though intravitreal injections of angiogenesis inhibitors like anti-VEGF is used to treat wet AMD, it is not suitable for dry AMD5.
Geographic atrophy (GA) is a non-exudative form of advanced dry AMD leading to irreversible loss of vision. GA begins with atrophic lesions in the outer retina that gradually spreads to envelop the macula and fovea. Chronic inflammation caused by excessive complement protein activation leads to gradual death of RPE cells followed by photoreceptor degeneration and the underlying choriocapillaris3. Recently, United States Food and Drug Administration (US FDA) has approved a complement therapy using Pegcetacoplan treat GA secondary to AMD which inhibits complement C3 and prevents inflammatory response6,7. In addition, National Eye Institute, NIH and Lineage Therapeutics are currently conducting Phase 1 and Phase 2a trials for dry AMD using an RPE patch and RPE cell suspension respectively8,9.
RPE is a monolayer of cells located between the outer segments of photoreceptors and the choroid. The RPE’s primary functions include nutrient secretion and transport, phototransduction, maintaining retina structural integrity and photoreceptor outer segment phagocytosis thus maintaining retinal homeostasis. Particularly, loss of phagocytic functions in the macula leads to central vision impairment which worsens over time10,11.
The microenvironment inside the retina is made up of soluble and cell-bound immunosuppressive substances which inhibits immune reactions. RPE cells induce T-cells to develop into T-regulatory cells via contact-dependent processes which leads to secretion of soluble substances to directly suppress immune cells. In this way, RPE cells contribute to the blood-ocular barrier thereby preserving a particular immunological privilege that supports exogenous cell transplantation8. However, there could be circumstances where ocular immune privilege might change leading to rejection of allogeneic cells by the host immune system. For example, Mcgill et al. 2018, reported inflammation only at cell transplanted sites in NHPs12. According to Sohn et al. 2015, the iPSC-RPE cells persisted in the subretinal space of pigs for about 3 weeks, during which time T-cell and macrophage activation was noted in the choroid and subretinal space13. However, the ongoing Lineage Therapeutics Phase 2a trial managed by Genentech8 using allogeneic RPE cells has never reported immune reaction as major obstacle, while demonstrating good efficacy after 2 years of follow up14. Therefore, off late, allogeneic RPE cell transplantation sparked considerable interest over the conventional autologous approach also for additional advantages like scalability and affordability. Though several groups have been developing RPE-based cell therapy for retinal degeneration since last 15 years9,10,11,12,13,14, none of them reached Phase 3 trial. Our focus in this manuscript has been on the production of bonafide RPE cells, ability to manufacture to scale, address CMC issues and perform preclinical safety, toxicology and efficacy studies.
We have previously reported successful generation of RPE cells from hiPSCs that mimic the morphological, molecular, and functional characteristics of native RPEs15,16. In the present study, we have designed an improved protocol that generates enriched hiPSC-RPE cells with enhanced purity and maturity (termed as Eyecyte-RPE). Before any newly developed therapy is introduced into the clinic, it is important to evaluate its safety and efficacy in animal models. We confirmed that Eyecyte-RPE cells are safe in immunocompromised animals and efficacious in disease models. Further, in NHP, we showed cell survival without severe adverse events following parsplana vitrectomy, a routine surgical technique in clinical practice. This technique has been specially designed to avoid human induced variability during injection that could be potentially adopted in Phase I/II trials. Additionally, Eyecyte-RPE allowed for cryopreservation, facilitating long-term storage and providing flexibility in transplantation scheduling. This comprehensive assessment laid the groundwork for Investigational New Drug (IND) approval to test the safety and efficacy of our product in GA patients in India.
Results
Small but significant changes to the differentiation protocol led to development of a clinical grade product, Eyecyte-RPE
Previously, we had developed a research grade protocol for generating RPE cells that could restore vision in animal models of retinal degeneration16. In this study, we developed a modified GMP protocol to generate clinical grade RPE cells in adequate numbers in a cost-effective manner. In the GMP protocol we eliminated the rosette selection step at day 20–22 and instead incorporated two enrichment steps around days 40–45 (P1) and 80–90 (P2), for better purity and to scale-up of RPE production (Fig. 1A). These additional enrichment steps led to generation of uniform and intensely pigmented (deep brown to black) monolayer of RPE cells with cobblestone morphology, in every batch (Fig. 1B, F). This resulted in considerable improvement both in terms of purity and scale across multiple iPSC lines tested (data not shown) which are critical attributes for GMP production. Our final drug product is a frozen vial of RPE cell suspension called Eyecyte-RPE. A cryopreserved cell product allowed shipping the cells to our collaborators for preclinical studies. Prior to batch release, extensive quality control testing was carried out with respect to identity, purity, potency, safety, and stability (Table-S1).
A Comparison of the three protocols used over years of product development. B Representative images of RPE cultures showing different levels of pigmentation based on the protocol used. GMP protocol shows the highest level of pigmentation. C Comparison of markers involved in melanin production by FACS analysis demonstrating the purity of RPE cell population. Cells generated by GMP protocol have the highest percentage of TYRP1 and PMEL17 positive cells with 90 ± 5% cells expressing the markers. D Comparison of expression of RPE specific markers by qPCR analysis. 20–40 fold increase in expression of the markers is seen in GMP protocol. E Quantification of PEDF by ELISA in RPE cells generated using the three protocols. Secreted PEDF level is 100 times more in GMP protocol compared to research and GLP protocol cells. F–N Characterization of RPE generated using GMP protocol. F Representative phase contrast images depicting the hexagonal morphology of RPE cells generated using GMP protocol. G–J Representative fluorescence microscopy images of markers specific to melanin production (PMEL17, TYRP1), RPE cells (CRALBP, RPE65), and primary cilia (acetyated tubulin). K PEDF and VEGF levels produced from basal and apical sides of polarized RPE cells. PEDF level is elevated in apical side and VEGF level is elevated in basal side. L Higher fold expression of RPE specific markers in GMP protocol, as analyzed by qPCR. M Scanning electron microscopy image showing cilia on RPE cells. N Transmission electron microscopy image showing the presence of cilia and abundance of stage III/IV melanosomes on the apical side. Scale bars indicate 100 μm, unless specified. p-value < 0.05 is considered statistically significant (* indicates p < 0.05; ** indicates p < 0.01; *** indicates p < 0.001; **** indicates p < 0.0001).
Purity was tested based on the expression of crucial RPE specific markers such as tyrosinase related protein 1 TYRP1, pre melanosome protein PMEL17 and visual cycle associated protein RPE65 by flow cytometry. TYRP1 and PMEL17 levels in GLP (70 ± 5%) and GMP (90 ± 5%) protocols are significantly higher than research protocol (50 ± 5%) (Fig. 1C, S1A). This finding corresponds with the pigmentation intensity and cellular homogeneity as shown in Fig. 1B. However, cultures from all three protocols showed presence of RPE65 positive cells (Fig. 1C, S1A). Expression of melanocyte inducing transcription factor (MITF), tyrosinase (TYR), RPE65 and TYRP1 by qPCR showed 20–40 fold increase in RPE cells generated using GMP protocol compared to research protocol (Fig. 1D, S1B). Functional RPE cells secrete pigment epithelium derived factor (PEDF) to protect them from apoptotic cell death. We found that Eyecyte-RPE cells generated from GMP protocol secreted ~100 times more PEDF in supernatant collected on the day of cryopreservation (final drug product) than those generated using research protocol highlighting their purity and potency (Fig. 1E, S1C).
RPE cells generated by GMP protocol was further characterized by qPCR, ELISA, immunofluorescence and electron microscopy. Immunostaining revealed that PMEL17 and CRALBP, key late-stage RPE specific markers, were expressed abundantly in the pigmented colonies (Fig. 1G). Other important RPE markers such as TYRP1, RPE65 and progenitor markers like MITF, ZO-1 were also present consistently (Fig. 1H, I, S1E). Notably, presence of acetylated tubulin, a key marker for primary cilia was a clear indication of the ciliated and native RPE-like state of these cells (Fig. 1J).
We also measured both PEDF and VEGF secretion of RPE grown using transwell chambers. PEDF secretion was higher on the apical side than the basal side thus maintaining the apical to basal ratio of 2.2 (Fig. 1K). Similarly, VEGF that is secreted by RPE cells to maintain the integrity of choriocapillaris was detected more at the basal side indicating that cells are highly polarized17,18 (Fig. 1K). Next, a panel of genes associated with retinal development and maturation like TYR, TYRP1, TRPM1, RPE65, CRALBP, MITF, ALDH1A3, and BEST1 were found to be highly up-regulated by qPCR that is consistent with the immunostaining data (Fig. 1L, S1D). Ultrastructural features of a cell type play an important role in their functionality. Scanning electron microscopy (SEM) showed densely packed monolayer of RPE cells with apical microvilli (Fig. 1M), whereas transmission electron microscopy (TEM) showed the presence of numerous stage III/IV melanosomes (Fig. 1N). These characteristics validate the maturity and functionality of the de novo generated RPE cells.
Lastly, to verify the long-term stability of Eyecyte-RPE, cells were thawed and examined for cell viability and expression of positive markers at 3, 6 and 12 months post cryopreservation. Cells showed optimal viability ( > 70%) at all the time points checked (Fig. 2A) and the percentage of cells expressing RPE65 and TYRP1 were consistent ( > 70%) at all time points analyzed, confirming that the final product is indeed stable up to 12 months (Fig. 2B). In addition, karyotyping analysis at 18 months post cryopreservation demonstrated that the final product is cytogenetically stable and devoid of any structural abnormalities (Fig. 2C).
A–C Stability testing of RPE cells. A Viability assessment (PI staining) of RPE cells at different time points show that viability is >70% across time points. B Expression of RPE65 and TYRP1 markers ( > 70%) at different day points analyzed by FACS showing the stability of product after cryopreservation. C Karyotype analysis of Eyecyte-RPE (18 months post cryopreservation) showing 46XY and no cytogenetic abnormalities. D Principal component analysis (PCA) of RPE samples generated using GMP, GLP and Research protocols. E Volcano plots of differentially expressed RPE genes across different protocols. A significant number of genes are differentially regulated across batches. F Gene ontology analysis of up-regulated and down-regulated genes. Eye development genes are the most up-regulated and cell cycle genes are the most down-regulated. G RPE markers are clustered and plotted as heatmaps to visualize gene expression of early, committed and late stage markers between protocols. Research protocol has higher expression of early RPE markers while GMP protocol expresses most of the mature RPE markers. H Leading edge analysis results highlight differentially expressed gene sets that play a critical role in eye development and visual system development across all batches. A p-value < 0.05 is considered statistically significant.
Transcriptomic profiling reveals a unique molecular signature of Eyecyte-RPE
Bulk RNA sequencing by next generation sequencing (NGS) was employed to understand the detailed molecular signature of the RPE cells generated across different protocols. Principle component Analysis (PCA) performed to explore the similarity of biological samples represented the most relevant sources of variance in the data visualized as a scatter plot. Hierarchical clustering of these datasets also revealed that research and GLP protocols were grouped together whereas, the GMP protocol is distinct from other two protocols (Fig. 2D).
Differential gene expression between the protocols was determined using volcano plots, and a significant number of genes were differentially regulated in the GMP protocol when compared to the research protocol (Fig. 2E). GO terms enrichment analysis revealed that the majority of the up-regulated genes are related to eye development and functions of the retina, while down-regulated genes are related to cell cycle (Fig. 2F). To distinguish between the molecular signature of the RPE cells generated by three different protocols, the gene sets were grouped based on their spatiotemporal expression during retinogenesis. Early, committed, and late-stage RPE genes were clustered according to previously published single cell-transcriptomics report19 and plotted as a heatmap (Fig. 2G). When compared to the GLP and GMP protocols, the research protocol showed a substantial up-regulation of genes related to early RPE development indicating the product comprised of a heterogeneous population of committed, non-committed and mature RPE cells. Similarly, the GLP protocol showed increased expression of genes associated with both committed and mature RPEs, while the GMP protocol had higher levels of late-stage and mature RPE genes than the other two protocols (Fig. 2G). To validate these findings, qPCR was performed for representative genes from cell cycle, neurogenesis and mature RPE groups that displayed a similar pattern of gene expression. Cell cycle related genes such as BCL2 and CNND1 were down-regulated in GMP protocol as compared to research and GLP protocols (p-value < 0.05). Expression of some early neuronal markers like OTX2, GBX2, PAX5, SOX1, NEUROD1, and TBX revealed a drastic down-regulation in GMP protocol when compared to GLP and research protocols (p-value < 0.05). On the other hand, RPE markers TYR and RPE65 showed a higher expression in GMP protocol than other two protocols (Fig. S2A). These results validated the transcriptomic profile of RPE cells generated by GMP protocol.
Further, gene sets that are differentially regulated between the protocols were examined using GSEA and leading-edge analysis. Gene sets related to eye development, neurogenesis, visual system development and epithelium development were up-regulated, while those related to cell cycle, apoptosis and gliogenesis were down-regulated in GMP protocol compared to the research and GLP protocols (Fig. 2H, S2B). This clustering also demonstrated that the enrichment step of the GMP protocol produced mature RPE cells in majority. Thus, molecular profiling by RNA sequencing established that RPE cells generated by the GMP protocol is superior to our research and GLP protocols making it suitable for preclinical studies.
Assessment of proliferating cells and non-RPE cells as a critical measure of in vitro safety
A major concern regarding pluripotent stem cell-based therapy is the risk of having undifferentiated cells in the final product which could cause tumor formation after transplantation. To understand proliferation rate and phenotype of the dividing cells, we examined the levels of Ki67 and pHH3 staining. Ki67 marks actively dividing cells from the G2/M phase while pHH3 expression is limited to cells undergoing mitosis. Flow cytometry analysis revealed that the RPE cells generated from GMP protocol had the lowest percentage ( < 5%) of proliferating cells when compared to GLP and research protocols (Fig. 3A, S3A). Immunofluorescence results provided additional evidence that the final product contained very few Ki67 and pHH3 positive cells in the GMP protocol (Fig. 3A, S3B). This was further supported by our transcriptome data, showing down-regulation of cell cycle and proliferation related genes in the GMP protocol compared to research protocol (Fig. 3B).
A Cell proliferation status examined through FACS analysis indicates proliferation is decreased w.r.t. increased pigmentation across batches. Results show low or negligible levels of Ki67 (<5%) and pHH3 (<3%) in GMP protocol. Cell count post staining for Ki67 and pHH3 markers w.r.t DAPI indicate similar results. B Heatmaps created for cell cycle and proliferation related genes showing lower expression in GMP protocol compared to research and GLP protocols. C–E Gene expression of OCT4 across different protocols by qPCR ( < 0.01), and FACS analyses ( < 1%) indicate absence of undifferentiated pluripotent cells as an impurity in Eyecyte-RPE. RNA seq analysis confirms absence of expression of pluripotent genes across all batches. F-G qPCR and FACS analysis of OCT4 level in RPE cultures spiked with 1% and 10% iPSC (0 hr and 15 day). Absence of OCT4 points out that iPSCs did not survive in RPE cultures. H-I qPCR analyses of trilineage markers (TH, AFP, HAND2) in RPE cultures alone and when spiked with iPSC, demonstrate absence of non-RPE lineage (off-target) markers. J Heatmaps created to compare differentially expressed genes involved in EMT and neural lineage across the three protocols. Significant down-regulation is seen in GMP protocol compared to GLP and research protocols. Scale bars indicate 100 μm. A p-value < 0.05 is considered statistically significant. (* indicates p < 0.05; ** indicates p < 0.01; *** indicates p < 0.001; **** indicates p < 0.0001).
Next, we demonstrated very low levels of OCT4 expression across all protocols (fold change <0.01 with respect to iPSCs) confirming the absence of undifferentiated stem cells (Fig. 3C, S3C). Further, only 0.1% of the total cell population was positive for OCT4 by flow cytometry (Fig. 3D). Transcriptome data also confirmed that the expression level of pluripotency related gene set was drastically reduced in all our protocols, when compared to iPSCs (Fig. 3E). In order to validate this observation, we performed a spiking experiment where, RPE cells were mixed with either 10% or 1% iPSCs and cultured in RMM for about 15 days. qPCR analysis was carried out with RPE cells immediately after spiking with iPSCs at 0 hr and after 15 days in culture. Drastic reduction (p < 0.0001) of OCT4 expression indicated that iPSCs did not survive in samples spiked with 10% and 1% iPSC (Fig. 3F). Likewise, flow cytometry corroborated that less than 1% of the cells were undifferentiated in nature (Fig. 3G). These findings suggest that iPSCs were not able to survive in the culture conditions conducive to RPE differentiation. These results endorse that our GMP product has negligible number of dividing cells thus minimizing the chance of tumor formation.
The presence of cells from other lineages as impurity in the final product could pose another challenge for PSC-based cell therapy. Analysis of lineage specific markers showed a drastic reduction in the ectoderm, endoderm, and mesoderm genes represented by TH, AFP, and HAND2 respectively when compared with positive controls (Fig. 3H, S3C). The same set of markers were also tested in the iPSC spiked samples confirming that iPSCs cannot differentiate into any other lineage under RPE differentiation conditions (Fig. 3I, S3D). Transcriptome data exhibiting down-regulation of non-RPE lineage markers such as EMT and neural lineage related genes corroborated these findings (Fig. 3J). Down-regulated genes included NR2E1, NEUROD1, NOTCH1, HES1, HES5, and GFAP, OLIG2 which are responsible for the generation of other retinal fate neurons and astroglial cells respectively. These findings support the in vitro safety of RPE cells generated using GMP protocol.
GLP toxicology studies showed no serious adverse effects
GLP toxicology studies were conducted in New Zealand rabbits and SD rats (Fig. S4A). No mortality or morbidity was observed in either animal groups throughout the study period. Statistically significant changes were not observed in any of the parameters such as body weight and temperature, food consumption, blood serum levels, and urine profiles across all animals analyzed. Except for local inflammatory changes noted in some eyes at the site of the injection, histopathology showed no sign of systemic or ocular toxicity in any of the animals (Fig. S4B). Further, safety and tumorigenicity studies were conducted in SCID mice where iPSCs and RPE cells were injected subretinally into the eyes. iPSC injected animals showed disruption in the retinal layer due to cell proliferation when compared to vehicle control whereas, RPE transplanted animals did not show major adverse events (Fig. S4E).
Eyecyte-RPE were non-tumorigenic 9 months post transplantation in RNU rats
To investigate the long-term safety profile of our product, we transplanted RPE cells into RNU rats which lack T cells and used iPSCs as a positive control (Fig. S4C). The iPSC injected animals developed teratomas as evidenced by the enlargement of the eye both externally and internally. H&E staining confirmed teratoma formation with the presence of ectoderm, endoderm and mesoderm lineage cells as early as 8–10 weeks (Fig. 4A). RPE cells from both GLP and GMP batches were transplanted and animals were analysed at 4 and 9 months post-transplantation. There was no serious concern about the general health of the animals and the cells were well tolerated. HNM staining of retina sections 9 months after subretinal injection demonstrated presence of human cells indicating graft survival. No abnormal or ectopic growth was observed (Fig. 4B). These experiments using RNU rats strongly supported the non-tumorigenic nature of our RPE cell product.
A Representative images of H&E staining of retinal cross-sections from RNU rats injected with iPSC to demonstrate tumor formation in 2 months (positive control). Formation of cell/tissue types representing ectoderm, endoderm, and mesoderm are evident. B Images of fundus, H&E staining, and corresponding HNM/DAPI staining of retinal cross-sections from RNU rats injected with Eyecyte-RPE generated using the different protocols (4 and 9 months post transplantation). Cells survived upto 9 months without any growth or tumor formation. Scale bars indicate 1000 μm and 100 μm respectively.
Biodistribution studies ruled out off-target effects
The possibility of cellular impurities migrating and forming ectopic growth beyond the target region is a critical risk associated with cell-based therapies. To demonstrate the safety of Eyecyte-RPE for clinical studies, we conducted biodistribution analysis in SCID mice and RNU rats. Major organs were collected from SCID mice (4 months) and RNU rats (9 months) post transplantation. Histological or morphological changes in liver, kidney, lung, heart, spleen, and brain tissues were not seen thus indicating that cells are restricted to the transplant region (Fig. S4D, F).
Eyecyte-RPE integrated in the host retina, provided neuroprotection and helped improve visual acuity in RCS rats
Efficacy studies were conducted in RCS rats with Eyecyte-RPE cells at different doses and harvested at P90 (Fig. S4C). Representative H&E images showed retention of the ONL layer for each dose group (Fig. 5A). Retinal cross-sections stained with HNM/ STEM121 (human specific marker) and PMEL17 demonstrated the survival and integration of transplanted cells across all doses (Fig. 5B). Cryopreserved RPE cells post freeze-thaw often exhibit an intermediate level of differentiation resulting in inconsistent expression of PMEL17. Post transplantation, cells survived as a bolus instead of spreading into a single cell layer. These eye sections also showed low levels of Ki67 which could be attributed to the immature phenotype of some cells (Fig. 5B). However, no abnormal growth was observed in any of the injected eyes. Although some tissue detachment was observed directly above the cell bolus due to cell transplantation, adjacent areas showed no disruption (Fig. 5A). Further, it is known that the retina has a tendency to separate at the sub-retinal bleb, especially with frozen sections.
A, B Representative fundus, H&E, and immunofluorescence images of retinal cross-sections from RCS rats transplanted with 50 k, 100 k and 150 k doses of Eyecyte-RPE and vehicle control demonstrate cell survival and engraftment (HNM/PMEL17) at P90 post transplantation. Surviving cells showed minimal proliferation (STEM121/Ki67) as evident from enlarged images. C ONL thickness, and cone counts data show ONL preservation. D Retinal cross-section from RCS rat post-transplantation stained with HNM, Cone Arrestin, and DAPI is a representative image of photoreceptor protection at P90. E Behavioral analysis by OKT at P60 and P90 indicated rescue of visual acuity as a result of RPE transplantation. Scale bars indicate 50 μm. A p-value < 0.05 is considered statistically significant. (* indicates p < 0.05; ** indicates p < 0.01; *** indicates p < 0.001; **** indicates p < 0.0001).
Photoreceptor rescue was measured histologically by comparing DAPI stained outer nuclear layer counts and cone arrestin positive cone nuclei counts within the temporal injected region versus the nasal untreated region for each eye. At P90, 10 out of 10 eyes injected with the 150 K dose showed highly significant ONL preservation in the temporal region ( ~ 6 nuclei) compared to the untreated nasal region ( ~ 2 nuclei) (p-value < 0.0001). Similar results were observed for the 100 K dose and 50 K dose (Fig. 5C). For the cone counts, the number of cones was significantly higher in the treated temporal region (average 33–39 cones) compared to untreated nasal regions (7–15 cones) (p-value < 0.0001) (Fig. 5C). Confocal imaging of HNM and Cone Arrestin staining confirms ONL preservation in the 150 k dose (Fig. 5D).
Visual performance results as measured by optokinetic thresholds (OKT) showed improved responses in both the 100 k and 150 k dose groups compared to BSS injected vehicle controls at both P60 and P90. At P60, the vehicle group averaged 0.332 cycles/degree while the 100 k and 150 k dose groups averaged 0.396 and 0.398 respectively (p-value < 0.005). At P90, 100 k and 150 k dose groups were still significantly different from the vehicle averaging 0.291 for BSS, 0.362 for the 100k dose, and 0.393 for the 150 k dose (p-value < 0.05). The 50k dose group averaged 0.352 at P60 and 0.353 at P90 which was not significantly different from either the vehicle or other dose groups at either timepoint (Fig. 5E). These results show preservation of visual performance for both the 100 k and 150 k dose group with the 150k dose group maintaining better preservation over time compared to the 100k dose group.
Overcoming variation in surgical techniques and complications of subretinal administration of cell suspension in monkeys
Prior to injections in the NHP eyes, we performed needle validation experiments to ensure that the injected cells are viable and potent. Briefly, required number of RPE cells were resuspended in BSS and passed through MedOne micro dose injector manually, and by connecting it to the vitrectomy device. The cells were aspirated into the injector attached to a Vasofix which was replaced with MedOne 38 G needle with vitrectomy pressure set at 20–30 psi for injection. The viability was assessed after passing the cells through above mentioned conditions and noted that manual method showed lesser viability ( ~ 65% viability; Fig. S5A) than vitrectomy where, more than 75% of the cells were viable (Fig. S5B, C).
A summary of NHPs, injected cell density, and details of various injection parameter combinations is provided in Fig. S6A. All animals received successful delivery of Eyecyte-RPE into the subretinal space. None of the injected eyes were excluded due to severe cataracts, excessive bleeding, excessive swelling and/or inflammation. All animals recovered well post-surgery and were able to tolerate the immunosuppression drugs throughout the whole study. The reflux of cells after subretinal delivery was video recorded during the surgery (data not shown). 3 out of 6 eyes developed epiretinal membrane (ERM) due to cell reflux (Fig. S6A), and OCT images showed ERM formation (Fig. S6B). After attempting multiple injections techniques, we determined that Perfluorocarbon liquid (PFCL) does not increase control in the bleb formation, nor does it reduce cell reflux. Although the utilization of both manual and vitrectomy machine (foot pedal activated) had similar success rates and safety profiles to raise the bleb, using MedOne microinjector syringe connected to vitrectomy console was found to be easier20. The intraocular pressure control was crucial for inducing bleb formation. With reduced intraocular pressure (setting on vitrectomy infusion set at 2–4 mm/Hg) at the time of injection, the bleb could be formed with minimal volume of injection21. We found that the finalized injection technique was able to reduce surgical variation for administering the product.
Transplanted Eyecyte-RPE was well tolerated in NHP eyes
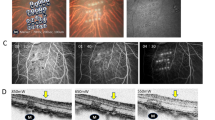
In vivo live imaging snapshots were captured to monitor the localization and behavior of transplanted suspension of Eyecyte-RPE cells within the NHP retina. Comprehensive safety assessments, conducted through multi-modal imaging, demonstrated the absence of tumor formation and acute or chronic immune rejection post-transplantation (Fig. 6A, B). The color fundus images (Fig. 6Ai–Bi) represents the posterior chamber, showcasing optical clarity within the vitreous cavity. Notably, both optic disc and retinal vascular architecture were normal (Fig. 6Aii-Bii), and fundus autofluorescence revealed no observable abnormalities in native RPE (Fig. 6Aiii-Biii). Furthermore, cross-sectional OCT images highlighted the structural integrity of the retina. The presence of an ERM was observed on top of the retinal layers most likely due to cell efflux (Fig. 6iv-Biv, Fig. S6B) and was confirmed by H&E staining (Fig. 6Av-Bv). In 3 out of 6 eyes with ERM formation within the 1 to 3 month timeframe, there was a moderate loss (30%–50% amplitude loss) in b-wave amplitude for both DA 0.01 and 30 Hz flicker (Fig. S6C). Though ERM formation was not severe in most cases, still it remains a complication that warrants careful consideration, particularly in patient safety and clinical translation. However, in cases without ERM formation, full-field ERG demonstrated consistent waveforms and amplitudes. These combined results underscore the safety and structural resilience of the transplanted Eyecyte-RPE cells within the NHP retina.
Cases #1-OD and #1-OS, color fundus pictures show clear view of the posterior chamber (Ai–Bi), and optically clear in the vitreous cavity. Optic disc and retinal vascular architecture appear normal (Aii–Bii). The white lines (Aiii–Biii) indicate the position at which SD-OCT images (Aiv–Biv) taken. ERM was observed on the retinal surface under H&E staining (Av–Bv). C Case #2-OS, the white arrows indicating the ‘cells lump’ at 1 week, 1 month and 3 months. The white doted lines indicate the position of OCT line scan. D Immunohistochemical analysis within injection sites of case #2-OS. Multi-layer transplanted cells were observed at all three locations (positive for Tra-1-85, negative for RPE65, with pigmentation) on top of the host RPE cells (negative for Tra-1-85, positive for RPE65). Scale bars indicate 200 μm in A(iv), B(iv) and (C); 100 μm in A(v) and B(v), 50 μm in (D).
In an illustrative case, notable observations included the recurrent identification of cell clumps at identical positions on OCT line scans over various follow-up timepoints on the OCT reference mode (Fig. 6C). These cell clumps consistently maintained their presence over time, suggesting the transplanted cells survived under NHP retina throughout the study. Transplanted cells were positive for Tra-1-85, a human specific marker, and simultaneously displayed pigmentation when visualized through the Differential Interference Contrast (DIC) channel. Tra-1-85 positive cells were situated on the apical side of the native RPE cells expressing RPE65 (Fig. 6D). Lack of RPE65 expression in the Tra-1-85 positive cells could be attributed to dedifferentiation of cryopreserved RPE post transplantation22. However, we detected the presence of OTX2 and PMEL17 costained with Tra-1-85 indicating that the surviving cells are of retinal lineage (Fig. S7). Taken together, transplanted suspension of Eyecyte-RPE within the NHP retina has provided insights into their safety, structural resilience, and tolerability within the ocular microenvironment.
Eyecyte-RPE CMC
In this study, we used a clinical-grade human iPSC line (TC-1133) from NINDS/NIH with in-depth characterization data along with a robust certificate of analysis23 (https://hpscreg.eu/cell-line/RUCDRi002-A). The master cell bank vial was received at passage 14 and the working cell bank (WCB) was created ( ~ 200 vials at passage 20) inside the GMP facility. RPE differentiation was carried out with WCB iPSC (passage 22–25) in GLP and GMP facilities for preclinical studies and clinical trials respectively.
In 2021, Surendran et al. demonstrated preliminary safety and efficacy using research grade RPE cells generated from the negative fraction after rosette selection15. Multiple batches were made under same specifications to establish repeatability and predictability of the protocol. Since this protocol includes a manual selection step, it was prone to variability in the final product composition and hence attempts were made to replace this step. We introduced an enzymatic selection step using TrypLE Select in the GLP protocol and conducted animal experiments to understand its impact on safety and efficacy in vivo. Though RPE cells from this protocol had <10% proliferating cells (Ki67), co-staining with MITF suggested that the proliferating cells are of retinal origin (data not shown). Further, we ensured that the expression of pluripotency (OCT4), non-lineage markers (TH, AFP, HAND2), and neural markers (TBX, SOX1, PAX5) were absent by qPCR when compared with respective positive controls.
We conjectured that terminally differentiated cells would find it difficult to integrate inside the unfriendly host microenvironment due to prevailing disease condition. Therefore, we focused on developing a unique product with majority of fate committed RPE cells ( > 90±5%) along with a small subpopulation of supporting retinal cells ( < 10±2%). Results from our preclinical studies using GLP grade RPE cells indicated improved ONL thickness and rescue of visual acuity in transplanted animals as key indicators of in vivo functionality16.
However, an additional purification and scale up step was introduced in the GMP protocol which has been elaborately discussed in this manuscript. This latest protocol significantly enhanced the purity of the RPE population (Fig. 1C) and further reduced the percentage of proliferating cells to below 5% (Fig. 3A). The in-house specifications thus finalized had <5% proliferating cells. Both tumorigenicity studies performed in SCID mice for 120 days and RNU rats for 180 days proved to be largely safe with these GMP grade RPE cells.
Finally, we performed large animal study with NHP to simulate injection modalities and arrive at the optimal dosage before initiating human trials. The formulation was well tolerated at 300k dose upto 120 days post injection, with no major safety concerns and needed no surgical intervention. The combination of robust preclinical safety data from immune suppressed RNU rats, toxicology data from GLP tox studies in rats and rabbits and multiple iterations of efficacy data from the RCS rat model provided the blueprint for Eyecyte-RPE to go into human clinical trials (Table S1, Fig. 7).
A Workflow of GMP grade manufacturing of Eyecyte-RPE. B List of QC tests done on Eyecyte-RPE product. C Stability studies performed at various time points. D Summary of pre-clinical safety and efficacy studies done in different animal models starting from rats to rabbits to NHP. E Validation of injection device for optimal delivery of Eyecyte-RPE in sub-retinal space. F Study design of the Phase 1/2a clinical trial of Eyecyte-RPE product submitted as IND application to the Indian regulatory authority. Created with BioRender.com.
Discussion
With the recent advancement in regenerative therapies, several groups are focusing on using PSC derived RPE for treating AMD because of the relative immune privilege within the subretinal space, the small number of cells required, and relative ease of administration24,25,26,27.
Refinement of strategies towards finding a safe and efficacious product include but are not limited to spontaneous differentiation, addition of small molecules, overexpression of transcription factors28, freezing the progenitors during intermediate stages for effective differentiation and scaling up18,24,26,27,29,30,31,32. Here, the GMP protocol presents an improvement from our previously published reports by including enrichment steps to improve purity and scalability. Eyecyte-RPE is a new product with unique cellular composition and its batch release criteria was finalized based on extensive in-house CMC studies. We focused on eliminating the presence of dividing cells in the final product. Although we observed a small number of dividing cells in our previous protocols, a significant reduction of Ki67/pHH3 positive cells (p-value < 0.0001) in the GMP protocol suggested that the additional purification step is necessary and effective. These observations are consistent with the transcriptome data, showing that genes related to retinal development, RPE maturation and retinoid metabolism were highly expressed whereas genes related to cell cycle, mitosis, cell proliferation and epithelial to mesenchymal transition (EMT) were down-regulated. Upon correlating the expression of a candidate set of key genes expressed in human fetal and adult RPE with iPSC-RPE, we found that RPE65, BEST1, MYRIP, DCT, CHRNA3, SERPINF1, TYRP1 are highly up-regulated. This indicated that the in vitro generated RPE cells retain the native RPE gene signature33,34. Besides, the scale of RPE production that was achieved by inclusion of the enrichment steps allowed us to easily obtain sufficient number of the final product (Eyecyte-RPE) for preclinical and clinical studies.
In addition to choosing the most suited cell type in adequate amount, ability to correctly deliver the desired cell population is one key contributing factor to augment the chances of engraftment and tissue regeneration. RPE administration has largely relied on two main approaches: injection of cell suspensions in the subretinal space and RPE patch transplant. However, limited persistence of RPE cell suspension and efficiency of the regeneration process has been questioned in comparison to RPE sheet. Although researchers have postulated different hypotheses to explain this, there is no conclusive and compelling evidence yet. Be it patch or cell suspension, RPE cell survival primarily relies on the adherence and the bonds that transplanted cells are able to establish with the underlying collagenous matrix (Bruch’s membrane).
We found that although RPE cells survived up to 10–12 weeks in the subretinal space, a relatively small number RPE cells integrated into the correct layer. RCS rats exhibit severe photoreceptor loss, but the RPE cell layer remains mostly intact35. While the engraftment is low in presence of an intact host RPE layer, it still provides endogenous photoreceptor protection which is indicative of their neuroprotective properties. Recent studies indicate that transplantation of cell suspensions into retinas lacking photoreceptors might be a better model to study photoreceptor engraftment and synaptic connectivity with host bipolar cells36,37. Histological analyses of eyes transplanted with RPE helped in preservation of retinal anatomy and rescue of photoreceptors from the ONL layer. Strong functional outcomes outweighed occasional side effects seen in form of retinal tissue distortion. The fact that the area of photoreceptor rescue extended beyond the limits of the injection site suggests a non-cell-autonomous trophic effect of transplanted cells on the survival of host photoreceptors rather than their replacement. This was also observed by others38,39 but the neuroprotective mechanism is still unknown.
To date, several groups leading the first clinical trials with hPSC-RPE cells have proven the safety of their products in rodents, pigs or minipigs as preclinical models for teratoma studies24,27,31,32,40. Likewise, the RNU data presented here confirms that Eyecyte-RPE do not form any tumorigenic growths. Simultaneously, biodistribution studies helped to demonstrate off-target effects. Further, through in vitro iPSC-spiking experiments we established that Eyecyte-RPE contains no residual undifferentiated cells or cells from other lineages. Since karyotypically variant PSCs are thought to be associated with persistence of undifferentiated cells, we routinely tested the cytogenetic stability of our product by karyotype analysis. In addition, we demonstrated that the frozen cell product was stable until 18 months from the date of cryopreservation and have included data from transport validation studies.
Delivery of a suspension of RPE cells into the subretinal space continues to be a surgical challenge. Implantation of these cells into the subretinal space via a parsplana vitrectomy is a simple and traditional method. Going forward, cellular efflux into the vitreous cavity and subsequent epiretinal membrane formation are two important obstacles to tackle. Lineage cell therapeutics has employed a unique method to deliver their cells subretinally via the suprachoroidal approach through a microinjector using the Orbit Subretinal Delivery System developed by Gyroscope Therapeutics (formerly Orbit Biomedical, Ltd.). While this avoids the need for retinotomy and associated complications, it is a complex procedure that requires extensive surgical training and poses a risk of hemorrhage leading to damage of retina.
Based on our learnings from RPE cell delivery into the NHP eyes, we have fine-tuned our delivery mechanism in the human eyes. In place of attempting a manual retinotomy following a parsplana vitrectomy, utilizing a microdose injector connected to the vitrectomy console and injecting the cells subretinally under low pressures seems to be an optimal way of cell delivery. This method is relatively easier to adopt, and can be performed by most retina surgeons while reducing variability due to differences in surgical expertize. Epiretinal membrane or schisis noted as a result of cell efflux from the injection site is an expected observation, and may not be severe, but it remains an adverse event that in some cases may require surgery to correct. However, the degree to which these events may occur is hard to predict given the disease state of the human retina versus the normal health of the NHP retina.
These preclinical results led to IND approval titled “A Phase 1/2a multi-center, dose-escalation, dose-expansion study to evaluate the safety and efficacy of Eyecyte-RPE when administered as a single-dose subretinal injection in subjects with Geographic Atrophy (GA) secondary to Dry Age-related Macular Degeneration (d-AMD)” [File No.:CELL-11015(11)/4/2024-e office (E. Comp. No.:2736)] by the Central Drugs Standard Control Organization (CDSCO), Directorate General of Health Services, Ministry of Health & Family Welfare, Government of India. The trial design is in similar lines to that of the Lineage (Genentech) trial (ClinicalTrials.gov number, NCT05626114) and is based on lessons learned from previous RPE cell transplantation trials, with preclinical data and rationales discussed previously9,14,41. It is a traditional open-label study, wherein subjects are enrolled into 3 sequential, ascending dose-level (DL) cohorts with systemic immunosuppressants: (1) DL1-Cohort 1: Eyecyte-RPE 100k cells; (2) DL2-Cohort 2: Eyecyte-RPE 200k cells; and (3) DL3-Cohort 3: Eyecyte-RPE 300 k cells. As part of our ongoing Phase 1 clinical trial, the first six patients with advanced GA (Cohort 1 & 2) have been dosed with Eyecyte-RPE. The surgeries were largely uneventful and patients are safe without any serious adverse event reported so far.
It is noteworthy that this study was designed considering the regulatory environment in India. Phase 1 clinical trial is ongoing in three reputed ophthalmology centres across the country, however, there are some limitations that must be addressed before an IND application can be filed with the USFDA. One key concern is the use of matrigel in our manufacturing protocol. To mitigate the risk involved, rigorous pathogen testing for adventitious viruses was performed to ensure that the animal components did not introduce any infectious agents in the final product. Next, we plan to perform single cell RNA analysis that will provide precise information about the different cell (including non-RPE) populations present in our final product. Further, to map out the fate of these cells in vivo, spatial transcriptomics of the transplanted grafts has been suggested. Nevertheless, we presume that these are supporting cells of retinal lineage that secrete neurotrophic or neuroprotective factors potentially augmenting efficacy in animals.
Taken together, key aspects discussed included details of manufacturing, characterization of cell banks, CMC studies, details of each animal study - cell numbers injected, method of delivery, duration of observations, and choice of readouts employed. In conclusion, our study presented a compelling body of work assessing the gene and protein expression profile, the functionality of de novo RPE cells and the subretinal delivery system. Evaluation of these cells in the RCS rats resulted in long-term vision rescue. Additional studies evaluated teratoma formation in rodents, biodistribution/migration in both rodents and rabbits, and safety/tolerability in NHP. We have developed a robust and efficient clinical-grade RPE manufacturing process that will advance the field of stem cell-based therapies for macular degeneration by making these products scalable and accessible. This work provided a complete framework for IND-enabling studies required to initiate a Phase 1 clinical trial using an allogeneic RPE cell suspension, Eyecyte-RPE.
Methods
The study was approved by ACE Independent Ethics Committee (ERPL/Eyecyte-RPE/2018), Institutional Committee for Stem Cell Research (EYESTEM (CCAMP)-ICSCR/09/2019) registered with National Apex Committee for Stem Cell Research and Therapy (NAC-SCRT), Indian Council of Medical Research (ICMR), New Delhi and Institutional Biosafety Committee of Centre for Cellular and Molecular Platforms (BT/BS/17/643/2015-PID), Bangalore. The Phase 1/2a Clinical Trial protocol for Eyecyte-RPE was registered with ClinicalTrials.gov registry on 23rd September, 2024 and the following ClinicalTrials.gov ID NCT06394232 was obtained.
iPSC culture and RPE differentiation
The human iPSC line TC-1133 derived from a healthy individual, procured from RUCDR/NINDS was used as the starting material. iPSCs were cultured on vitronectin-coated (#A27940, Thermo Fisher) plates in mTeSR plus (#100-0274/ 100-0275, Stem Cell Technologies) medium until they attained optimal confluency (70–80%) with well-defined borders. Subsequently, these iPSCs were seeded in ultra low-attachment plates (#CLS3471, Corning) to form forced aggregates known as embryoid bodies (EBs), which were further guided to differentiate into retinal pigment epithelium (RPE) cells using a previously described method from our laboratory16. The EBs were grown in mTeSR plus medium for 2 days, followed by differentiation induction medium (DIM) for 4 days to initiate the retinal specification. After 6 days, EBs were plated on matrigel-coated (#354277, Corning) tissue culture plates (#140675, Nunc). Two days post-plating, cultures were exposed to differentiation propagation medium (DPM) to promote rapid cell multiplication. The retinal progenitor cells in culture were then transitioned to RPE maturation medium (RMM) on day 22, facilitating further differentiation into melanin pigment-producing RPE cells. Research protocol comprised of a rosette selection step on day 20–22 and the non-rosette fraction was cultured in RMM for about 70 days followed by cryopreservation. In the GLP protocol we eliminated the rosette selection step, instead, around day 40–45 an enrichment step was performed using TrypLE Select (#A1285901, Gibco) and the cells were cultured for about 90 days and then cryopreserved. Further, GMP protocol has an additional scaling up step over the GLP protocol at around day 80–90 followed by expansion for another 30–40 days. RPE cells were frozen in Cryostor CS10 cryoprotectant (#07930, Stem Cell Technologies) by slow freezing method and stored in LN2 container.
Flow cytometry
Cells were fixed and permeabilized with ice cold methanol for 20 min at 4°C. Cells were then stained with primary antibody diluted in FACS buffer made with 2% FBS (#SH30071.03, Hyclone) and 0.25% Triton-X-100 (#85111, Thermo Fisher) in PBS for 30 min followed by secondary antibody diluted in FACS buffer for 15–20 min. Analysis was done on CytoFlex (Beckman Coulter). Details of antibodies used have been provided in Table-S2.
Immunocytochemistry
Cells were treated with 4% paraformaldehyde (PFA) (#28906, Thermo Fisher) for 10 min, followed by permeabilization and blocking using 0.1% Triton-X-100 in 4% fetal bovine serum (FBS) for 30 min. Primary antibodies, diluted in 4% FBS, were added to the fixed cells and incubated overnight at 4°C. Subsequently, secondary antibodies, diluted in 2% FBS, were added to the cells and incubated for 45 min. DAPI was used as the nuclear stain. The imaging was done using EVOS M5000 microscope. Details of antibodies used have been provided in Table-S2.
Gene expression analysis by quantitative real time PCR (qPCR)
Cells were harvested in Trizol (#15596-018, Thermo Scientific) and stored at -80°C until RNA isolation. RNA isolation, cDNA synthesis and qPCR were carried out according to the manufacturer’s instructions. RNA was isolated using the RNA extraction kit (74104, Qiagen) and 1 μg of RNA was converted to cDNA (#AB1453/B, Thermo Scientific). qPCR was performed either with SYBR green (#A25742, Applied Biosystems) or Taqman reagents (#4304437, Applied Biosystems) in triplicates and normalized to β-actin and GAPDH respectively. Fold change was calculated by 2^-ddCt method. Details of primers and probes used have been provided in Table-S2.
Spiking of iPSC with RPE cells
In vitro spiking experiments were performed by mixing iPSCs and RPE cells where, RPE cells were spiked with iPSCs at different ratios (1% iPSC+ 99% RPE; 10% iPSC+90% RPE), seeded on matrigel-coated plates, and cultured in RMM for a duration of 15 days. The mixed cultures were then harvested for gene expression analysis for iPSC and lineage specific markers by qPCR and flow cytometry analysis with 100% iPSCs and 100% RPE cells as controls.
ELISA to determine PEDF and VEGF secretion
PEDF (#ab246535, Abcam) and VEGF (DY293B-5, R&D Systems) were quantitatively measured from cell supernatant by ELISA method by following manufacturer’s instructions. Cell culture supernatant was collected after culturing the cells in RPE maturation medium for 48 h. RPE supernatant and serially diluted standards (provided in the kits) were used for the analysis. Spectrophotometric readings were taken and quantification was done by standard curve method.
RNA sequencing and data analysis
RPE cells generated using different differentiation protocols were subjected to transcriptomic profiling. The global RNA sequencing data was evaluated for the quality of the reads by removing unwanted sequences, particularly non-polyA tailed RNA from all the samples. HISAT2 (2.1.0) was used to align the paired-end reads to the reference human genome (hg19). DESeq2 was used to normalize read count data after alignment were used to estimate gene expression. The ratio of normalized read counts for GLP and GMP protocol over the research protocol was taken as a fold change. The log2 fold change values were calculated for all the annotated genes between control and test samples, and p-value was calculated for each of the genes individually. In order to consider any genes to be up- or down-regulated, the following two criteria were followed, (i) gene should be -1 < = log2 (fold change) > = +1 and (ii) p-value < = 0.05 based on DeSeq2 analysis. Further, the significant genes filtered based on above two criteria were represented as heatmap and as well as volcano plot. Principle component Analysis (PCA) was used to investigate the similarity of biological samples from the RNA-seq datasets. Differential gene expression analysis, Gene set enrichment analysis (GSEA) and leading edge analysis were carried out using R software packages. mRNA sequencing data has been made available on gene expression omnibus database (GSE264545).
Transmission Electron Microscopy
Following enrichment, RPE cells were plated onto 0.4 μm transwell dishes to establish monolayer formation and polarization. The cells were allowed to mature until day 120, then fixed and subjected to stepwise ethanol dehydration. TEM images of ultrathin sections were captured using a JEOL JEM-1010 transmission electron microscope (JEOL, Peabody, MA).
Scanning electron microscopy
RPE monolayers were detached from the transwell membrane, fixed and appropriately trimmed to fit in the sample holder, and dehydrated via sequential ethanol dehydration. Subsequently, samples were subjected to critical point drying (CPD) to achieve further dehydration compatible with the high vacuum necessary for scanning electron microscopy. Imaging was performed using a Zeiss EVO MA 10 SEM.
Rabbit surgery, Eyecyte-RPE injection and GLP toxicological studies
Good Laboratory Practices (GLP) studies are conducted to evaluate the safety and toxicology of a drug product as per recommendations by the concerned regulatory agency for IND filing. The study was performed at Dabur Research Foundation (DRF), Faridabad, India in compliance with the OECD principles of Good Laboratory Practice. Institutional Animal Ethics Committee (IAEC) approved these studies conducted using New Zealand white rabbits (IAEC/76/1476) and Sprague Dawley rats (IAEC/76/1476). A total of 26 New Zealand white rabbits (13 male+13 female) and 70 rats (35 male+35 female), were used in the study. The animals were distributed into seven groups; 3 groups received either 50 k, 100 k or 150 k of RPE cells, 3 groups (positive control) received either 50 k, 100 k or 150 k of iPSCs while the vehicle control group received BSS only (Fig. S4A). Animals were anesthetized and received a subretinal injection of (3-5ul) cells/BSS using a 33-guage Hamilton syringe. Each animal underwent an optical coherence tomography (OCT) prior to the injection. Animals were monitored twice daily for mortality and morbidity and once daily for clinical signs. Apart from the systemic examination, each animal underwent a detailed eye examination, including slit lamp assessment, measurements of the corneal thickness and intraocular pressure. The rats were observed for a period of 28 days and the rabbits for a period of 180 days, at the end of which the animals were euthanized and subjected to histopathology. Hematological and biochemical examination and urine analysis was conducted at the end of the study.
Rats for safety and efficacy studies
Royal College of Surgeon (RCS) rat breeders (Cat # 315) were obtained from The Rat Resource & Research Center (Columbia, MO, USA). All (forty) RCS rats used for the study were bred at the Casey Eye Institute, OHSU. Rat pups of both sexes were weaned at postnatal day (PD)21. Rowett Nude (RNU) rats used in the study were obtained from Charles River Labs (Wilmington, MA, USA). Eighty RNU rats were housed in standard conditions under a 12/12 h light-dark cycle. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at OHSU, and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Experiments were approved under IACUC protocol TR02_IP00001387.
Non-human primates for tolerability studies
Three male Macaca fascicularis animals, aged 4–6 years and weighing 3–5 kg at the study’s commencement, were procured from the Singhealth Experimental Medicine Centre (SEMC) in Singapore, which is approved by the Association for Assessment and Accreditation of Lab Animal Care, prioritized animal welfare, ensuring appropriate housing conditions, lighting, and temperature. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Singhealth (Ref: 2022/SHS/1750), and accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. This ensures that all animal experimentation complies with standards as per the National Advisory Committee for Laboratory Animal Research guidelines set out by the Agri-Food and Veterinary Authority of Singapore. Adhering to stringent scientific norms, the animals were uniquely identified with tattoo numbers on their chests, and comprehensive details were provided on cage cards, encompassing study number, IACUC number, species, group, individual number, sex, drug dose level (if applicable), and contact information. Following a 2-week period of quarantine and acclimatization, a veterinarian conducted a thorough evaluation of their general health. Housed individually, the animals received a certified primate diet (5048) and had unrestricted access to water. The study, authorized by the Institutional Animal Care and Use Committee (IACUC) of Singhealth (2022/SHS/1750), prioritized animal welfare, ensuring appropriate housing conditions, lighting, and temperature. All procedures aimed to minimize pain, distress, and discomfort. The utilization of NHPs was justified based on the similarity of their ocular anatomy to humans and their immune system’s compatibility with human RPE xenografts. Overall, the study’s design and practices were in strict adherence to recognized standards, guaranteeing both the welfare of the animals and the reliability of the study’s outcomes. All data from the experimental animals were reported and there were no exclusions in this study.
All animals underwent immunosuppression induction 7–10 days before surgery, maintained throughout the experiment. Immunosuppression comprised sirolimus, doxycycline, and minocycline. Sirolimus was orally administered, starting with 2 tablets of 1 mg/tab on the first day and then 1 tablet once a day until enucleation. Doxycycline and minocycline, 15 mg/kg each, were given orally twice daily. Due to challenges in oral sirolimus administration, it was delivered under light sedation with intramuscular ketamine (5 mg/kg BW) to prevent identification by the animals. Monitoring closely for any impact on general well-being, the animals’ body weight was carefully tracked during daily sedation.
Rat surgery, Eyecyte-RPE injection
One day prior to injections RCS animals were placed on ad libidum Cyclosporine water (210 mg/L) to begin immunosuppression, RNU animals were not placed on an immunosuppressive regimen. One hour prior to injections, pupils were dilated with a combination of 2.5% phenylephrine and 1% atropine. Animals were anesthetized with an intraperitoneal injection of 100/10 mg/kg ketamine/xylazine. Animals eyes were numbed using 0.5% proparacaine HCl immediately prior to injection. An incision in the corner of the eyelid allowed for visualization of the posterior retina. A small scleral/choroidal incision was made using the tip of a 27 gauge needle to facilitate placement of the injection cannula into the subretinal space. A corneal puncture was then made using a 30 gauge needle to limit the increase of intraocular pressure. Injection of 2 μL of cell dose (RPE in the RCS animals, iPSCs and RPE for the RNUs) or control media was then performed in the scleral/choroidal incision using a glass cannula attached by microtubing to a 10 μL Hamilton syringe. After injection a glass coverslip was used to visualize the subretinal bleb to check for injection related issues. Post operative treatment consisted of 0.5% erythromycin ointment applied to the eyes while animals recovered from anesthesia on a hot pad. For the RCS animals dexamethasone (1.0 mg/kg) was administered intraperitoneally on the day of injections as well as every other day for 2 weeks post-op along with the continuation of ad libidum cyclosporine until harvest.
Ophthalmic imaging of RCS and RNU rats
Post-operative imaging was performed to assess retinal health and observe transplanted cells. RCS rats were imaged at P90, RNU rats were imaged at harvest (4 and 9 months post injection). Pupils were dilated with a combination of 1% atropine and 2.5% phenylephrine 1 h prior to imaging. Animals were then anesthetized by intraperitoneal injection of ketamine/xylazine (100/10 mg/kg). During imaging, eyes were kept lubricated using 0.3% Hypromellose gel. Bright field fundus images of the retina were captured using a Micron IV (Phoenix-Micron, Inc.). Images were captured in the far periphery-temporal retina to best visualize treated retina.
Optokinetic tracking on RCS rats
Optokinetic tracking (OKT) was performed on RCS rats at P60 and P90 to monitor changes in visual acuity. Optokinetic thresholds were assessed using a virtual optomotor system (VOS; CerebralMechanics). Animals were placed on the central pedestal and allowed to acclimate for 2–3 min before testing began. Once the animal was acclimated a simple staircase testing method at 100% contrast was used to examine each eye and determine the spatial frequency threshold in cycles/degree. Animals were tested for a maximum of 30 min per day; if results were not acquired in that time the animal was retested the following day. All OKT testing was performed by the same individual to maintain consistency of results.
NHP surgery, Eyecyte-RPE injection
The 20–30 min procedure involved a 25 G vitrectomy performed under general anesthesia. Sedation before induction of anesthesia was performed with IM injection of ketamine (10 mg kg−1 BW) and SC injection of atropine (0.05 mg kg−1 BW). IM injection of buprenorphine (0.005-0.03 mg kg−1 BW) was administered for pain relief 30 min before surgery. This dose was repeated 6 h after surgery. Intubation was performed by a qualified veterinarian using an appropriately sized endotracheal tube and general anesthesia induced using 2% isofluorane and maintained using 0.5-2% isofluorane. Pupils were dilated with 1% tropicamide and 2.5% phenylephrine. A three-port 25 G core vitrectomy removed the posterior vitreous, inducing detachment with triamcinolone. Cells were injected into the subretinal space through a 38 G needle (MedOne Surgical Inc., USA). Sclerotomies and conjunctiva wounds were sutured with 7–0 vicryl. Tobradex® eye ointment (tobramycin and dexamethasone) was applied twice daily for post-operative inflammation reduction. A 6 week gap separated surgeries on the right and left eyes.
Ophthalmic imaging of Non-Human Primates (NHP)
Post-operative follow-ups were carried out under general anesthesia on day 1, 7, 14, and 30, with subsequent monthly check-ups. General anesthesia induction utilized atropine (0.05 mg kg-1) and ketamine (10 mg kg-1), maintained with 2% isoflurane initially and reduced to 0.5-2% during the procedure. Intraocular pressure was measured using a TonoPen XL tonometer (Reichert Ophthalmic Instruments). Color fundus images were captured using a Topcon TRC-50DX camera (Topcon Corp, Tokyo, Japan) in posterior mode with a 50o angle, controlling flash intensity within 12–18 wx range. Retinal layers were examined in real-time using a Spectralis® HRA/OCT device (Heidelberg engineering, Heidelberg, Germany), providing ultrasound-based in vivo depth images for structural assessment. Longitudinal and transversal line scans were performed at injection sites. Fluorescein Fundus Angiography (FFA) images were obtained with a Heidelberg Spectralis device (Heidelberg Engineering Inc., Germany) equipped with a 55oC lens, following intravenous administration of 10% sodium fluorescein solution (Curatis AG; dose: 10 mg/kg). Images captured early (0–2 min) and late (5–7 min onwards) post-injection revealed dynamic changes.
NHPs underwent dark adaptation for 20 min before full-field electroretinography (ERG). ERGs were recorded using the Espion system (Diagnosis LLC, USA), following protocols recommended by the International Society for Clinical Electrophysiology of Vision (ISCEV) for human subjects. ERG components were named based on the adaptive state (dark-adapted [DA] or scotopic; light-adapted [LA] or photopic) and stimulus strength (measured in cd.s/m2). For instance, DA 0.01 indicates a 0.01 cd.s/m2 flash under dark adaptation, while LA 3 indicates a 3 cd.s/m2 flash under photopic conditions.
Histopathological examination of rats and NHP
Rats were euthanized following IACUC guidelines by CO2 gas with a bilateral thoracotomy as secondary method. Eyes were then enucleated and placed into cold 4% paraformaldehyde (PFA). After 10–60 min of fixation, the anterior chamber and lens were removed and discarded, the posterior eyecup was then placed back into 4% PFA in a 4 °C fridge overnight. Eyecups were then treated with a sucrose gradient of 10, 20, and 30% sucrose in PBS for 24 h at each stage. Eyecups were then dried gently on kimwipes and placed into labeled cryomolds filled with OCT compound. We oriented eyecups based on injection site, with the eyecup facing the front of the mold injection site placed at 2 o’clock for OS and 10 o’clock for OD. Cryomolds were then frozen on a steel plate cooled with liquid nitrogen. Eyes were sectioned at 12 μm collecting representative sections through the injection site totaling ~40 slides with 4 sections each. We selected several representative slides from the group which were then stained with H&E. Representative RNU sections were stained for human nuclear marker (HNM) and Ki67 to measure safety. Representative slides of RCS eyes were also selected for immunofluorescence staining. Sections were blocked with 4% horse serum, 1% bovine serum albumin (BSA), 0.5% triton-x-100 in phosphate buffered saline (PBS) for 45 min. Slides were incubated with anti-cone arrestin (generously provided by W. Clay Smith, PhD, University of Florida) overnight at 4°C followed by secondary antibody incubation (1:300) for 45 min at room temp in the dark. Slides were then washed and stained with DAPI. After a final wash, slides were mounted with 100 μL Fluoromount G.
NHPs were humanely euthanized following IACUC ethical guidelines with an intracardiac injection of Phenobarbital. Perfusion fixation was carried out via the carotid artery with 10% neutral buffered formalin solution. Both eyes of each animal were enucleated and fixed overnight. After anterior segment removal, full-thickness samples (retina to sclera) were cut and paraffin-embedded. Sections of 10 µm were obtained, with ~30 slides per block. Slides 1, 10, 20, and 30 were H&E stained to assess retinal structure changes. Remaining slides were used for immunohistochemistry; sections were deparaffinized, rehydrated, and antigen retrieval performed. Tissues were blocked, incubated with primary antibodies (Tra-1-85, BD Bioscience Cat# 563020, 1:50 and RPE65, Abcam Cat# ab231786, 1:500) overnight, followed by secondary antibody incubation. Confocal microscopy imaging was conducted using the Olympus Fluoview FV1000 system.
Quantification of photoreceptors in RCS rats
The outer nuclear layer thickness (ONL) was measured using immunohistochemical staining and imaging with scanning laser confocal microscopy (Leica SP5 using LAS AF software; Leica) as the primary indicator of photoreceptor rescue. Z-stack images were taken at 10 and 20x with a step size of 1 μm. Laser gain was kept consistent for each emission wavelength. Z-stack images were then flattened into a single tiff file. ONL cell bodies were counted within rows of ONL in both injected and uninjected regions to obtain retinal thickness values. Cones were counted in both injected (temporal) and uninjected (nasal) regions to obtain cone per image values. ONL and cone count values were averaged for each group and region.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 8® (GraphPad, La Jolla, CA). For comparisons of more than two groups, one-way ANOVA was done with a Brown-Forsythe and Welch ANOVA tests followed by Tukey’s multiple comparisons test and Sidak’s multiple comparisons tests. p-value of <0.05 was considered statistically significant. For animal studies, the number of animals needed per group was determined by a power calculator. Based on our previous data, a samples size of 10, with an alpha level set to 0.05, provides power of 0.92–1.0 for visual performance, retinal thickness and cone counts. All statistical analyses were performed using one way or two way ANOVA post hoc Bonferroni tests. Multi-parametric comparisons between groups were tested with two tailed t-tests. p-value <0.05 were considered significant. Graphs illustrating the data were generated using GraphPad Prism 8®. All in vitro experiments were conducted in biological triplicates.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. Those datasets used and/or analyzed during the current study available from the corresponding author on reasonable request. mRNA sequencing data has been made available on gene expression omnibus database (GSE264545).
References
Ambati, J., Ambati, B. K., Yoo, S. H., Ianchulev, S. & Adamis, A. P. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv. Ophthalmol. 48, 257–293 (2003).
Chen, Y., Bedell, M. & Zhang, K. Age-related macular degeneration: genetic and environmental factors of disease. Mol. Interventions 10, 271–281 (2010).
Bhutto, I. & Lutty, G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Asp. Med. 33, 295–317 (2012).
Bird, A. C., Phillips, R. L. & Hageman, G. S. Geographic Atrophy: a histopathological assessment. JAMA Ophthalmol. 132, 338–345 (2014).
Kaiser, S. M., Arepalli, S. & Ehlers, J. P. <p>Current and future anti-VEGF agents for neovascular age-related macular degeneration</p>. J. Exp. Pharmacol. 13, 905–912 (2021).
Heier, J. S. et al. Pegcetacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): two multicentre, randomised, double-masked, sham-controlled, phase 3 trials. Lancet 402, 1434–1448 (2023).
Pharmaceuticals, I. A. Pegcetacoplan (syfovre) for geographic atrophy in age-related macular degeneration. Med. Lett. Drugs Ther. 65, 49–50 (2023).
Duarri, A. et al. Autologous transplantation of induced pluripotent stem cell-derived retinal pigment epithelium for geographic atrophy. Int. J. Mol. Sci. 22, 10497 (2021).
Roche, H.-L. Safety and Efficacy Study of OpRegen for Treatment of Advanced Dry-Form Age-Related Macular Degeneration. https://hpscreg.eu/browse/trial/30 (2024).
Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 85, 845–881 (2005).
Horimoto, Y. et al. Analysis of circulating tumour cell and the epithelial mesenchymal transition (EMT) status during eribulin-based treatment in 22 patients with metastatic breast cancer: a pilot study. J. Transl. Med. 16, 287 (2018).
McGill, T. J. et al. Allogeneic iPSC-Derived RPE cell graft failure following transplantation into the subretinal space in nonhuman primates. Invest. Ophthalmol. Vis. Sci. 59, 1374–1383 (2018).
Sohn, E. H. et al. Allogenic iPSC-derived RPE cell transplants induce immune response in pigs: a pilot study. Sci. Rep. 5, 11791 (2015).
Ho, A. C. et al. Safety and efficacy of a phase 1/2a clinical trial of transplanted allogeneic retinal pigmented epithelium (RPE, OpRegen) cells in advanced dry age-related macular degeneration (AMD). Invest. Ophthalmol. Vis. Sci. 63, 1862–1862 (2022).
Surendran, H. et al. Transplantation of retinal pigment epithelium and photoreceptors generated concomitantly via small molecule-mediated differentiation rescues visual function in rodent models of retinal degeneration. Stem Cell Res. Ther. 12, 70 (2021).
Surendran, H. et al. An improved protocol for generation and characterization of human-induced pluripotent stem cell-derived retinal pigment epithelium cells. STAR Protocols 3, 101803 (2022).
Sonoda, S. et al. Attainment of polarity promotes growth factor secretion by retinal pigment epithelial cells: relevance to age-related macular degeneration. Aging (Albany NY) 2, 28 (2010).
McGill, T. J. et al. Long-term efficacy of GMP grade hESC-free hesc-derived RPE cells following transplantation. Transl. Vis. Sci. Technol. 6, 17–17 (2017).
Petrus-Reurer, S. et al. Molecular profiling of stem cell-derived retinal pigment epithelial cell differentiation established for clinical translation. Stem Cell Rep. 17, 1458 (2022).
Liu, Z. et al. Surgical Transplantation of Human RPE Stem Cell-Derived RPE Monolayers into Non-Human Primates with Immunosuppression. Stem Cell Rep. 16, 237–251 (2021).
Tan, G. S. W. et al. Hints for gentle submacular injection in non-human primates based on intraoperative oct guidance. Transl. Vis. Sci. Technol. 10, 1–13 (2021).
Baqué-Vidal, L. et al. Clinically compliant cryopreservation of differentiated retinal pigment epithelial cells. Cytotherapy 26, 340–350 (2024).
Baghbaderani, B. A. et al. CGMP-manufactured human induced pluripotent stem cells are available for pre-clinical and clinical applications. Stem Cell Rep. 5, 647–659 (2015).
Sharma, R. et al. Clinical-grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci. Transl. Med. 11, eaat5580 (2019).
Nair, D. S. R. et al. Long-term transplant effects of ipsc-rpe monolayer in immunodeficient rcs rats. Cells 10, 2951 (2021).
Zhang, H. et al. Transplantation of GMP-grade human iPSC-derived retinal pigment epithelial cells in rodent model: the first pre-clinical study for safety and efficacy in China. Ann. Transl. Med. 9, 245–245 (2021).
Mandai, M. et al. Autologous induced stem-cell–derived retinal cells for macular degeneration. N. Engl. J. Med. 376, 1038–1046 (2017).
Dewell, T. E. et al. Transcription factor overexpression drives reliable differentiation of retinal pigment epithelium from human induced pluripotent stem cells. Stem cell Res. 53, 102368 (2021).
Petrus-Reurer, S. et al. Preclinical safety studies of human embryonic stem cell-derived retinal pigment epithelial cells for the treatment of age-related macular degeneration. Stem Cells Transl. Med. 9, 936–953 (2020).
Maruotti, J. et al. Small-molecule-directed, efficient generation of retinal pigment epithelium from human pluripotent stem cells. Proc. Natl Acad. Sci. USA 112, 10950–10955 (2015).
Cruz, L. D. et al. Phase 1 clinical study of an embryonic stem cell–derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018 36:4 36, 328–337 (2018).
Kanemura, H. et al. Tumorigenicity studies of induced pluripotent stem cell (iPSC)-derived retinal pigment epithelium (RPE) for the treatment of age-related macular degeneration. PLoS ONE 9, e85336 (2014).
Kamao, H. et al. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem cell Rep. 2, 205–218 (2014).
Strunnikova, N. V. et al. Transcriptome analysis and molecular signature of human retinal pigment epithelium. Hum. Mol. Genet. 19, 2468–2486 (2010).
Salas, A. et al. Cell therapy with hiPSC-derived RPE cells and RPCs prevents visual function loss in a rat model of retinal degeneration. Mol. Ther. Methods Clin. Dev. 20, 688–702 (2021).
Collin, J. et al. CRX Expression in Pluripotent Stem Cell-Derived Photoreceptors Marks a Transplantable Subpopulation of Early ConesHuman PISC-derived neural progenitors preserve vision in an AMD-like mode. Stem Cells 37, 609–622 (2019).
Eiraku, M. et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nat. 2011 472:7341 472, 51–56 (2011).
Cuenca, N. et al. Phagocytosis of photoreceptor outer segments by transplanted human neural stem cells as a neuroprotective mechanism in retinal degeneration. Invest. Ophthalmol. Vis. Sci. 54, 6745–6756 (2013).
Tsai, Y. et al. Human PISC-derived neural progenitors preserve vision in an AMD-like model. Stem Cells 33, 2537–2549 (2015).
Gropp, M. et al. Standardization of the teratoma assay for analysis of pluripotency of human ES cells and biosafety of their differentiated progeny. PLoS ONE 7, e45532 (2012).
Kashani, A. H. et al. One-year follow-up in a phase 1/2a clinical trial of an allogeneic REP cell bioengineered implant for advanced dry age-related macular degeneration. Transl. vision Sci. Technology 10, 13 (2021).
Acknowledgements
This work was supported in part by the Biotechnology Industry Partnership Programme (BIPP) under Biotechnology Industry Research Assistance Council (BIRAC), Department of Biotechnology (DBT), Govt. of India (Ref No. BT/BIPP/1128/44/18). Cellular and Molecular Platforms (C-CAMP), Bengaluru is acknowledged for support in form of incubation and core facilities. The authors are thankful to Dabur Research Foundation (DRF), Faridabad; MedGenome Labs, Bengaluru and Institute of Bioinformatics and Applied Biotechnology (IBAB), Bengaluru for GLP toxicology studies, RNA sequencing and genomics data analysis services respectively. The authors are grateful to Jair Montford, National Eye Institute, NIH, Bethesda for Electron Microscopy and Bhav Harshad Parikh, Institute of Molecular and Cell Biology, Singapore for help with imaging of NHP IHC slides. The authors acknowledge Center for Ocular Regeneration (CORE), LV Prasad Eye Institute, Hyderabad as our GMP manufacturing partner. Drs. Jogin Desai, Dhruv Sareen and Mahendra Rao are duly acknowledged for helpful suggestions during the course of this study and preparing the manuscript.
Author information
Authors and Affiliations
Contributions
R.P., R.B., L.S. and H.S. conceptualized and designed the overall study. L.S., H.S. and N.P. conducted the in vitro experiments, performed data analyses, drafted figures and manuscript. J.S., S.A. and R.C.R. conducted the experiments on rat models and performed data analyses. L.Z., L.G. and X.S. performed the experiments on NHPs and performed data analyses. R.P. supervised all experiments and data analyses; R.P., R.B., R.C.R. and X.S. revised figures and manuscript. The manuscript was written through contributions from all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Soundararajan, L., Surendran, H., Patlolla, N. et al. Allogeneic RPE cell suspension manufactured at scale demonstrating preclinical safety and efficacy led to IND approval. npj Regen Med 10, 19 (2025). https://doi.org/10.1038/s41536-025-00407-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41536-025-00407-0