Abstract
Extracellular matrix remodelling of cardiac tissue is a key contributor to age-related cardiovascular disease and dysfunction. Such remodelling is multifaceted including changes to the biochemical composition, architecture and mechanics, clouding our understanding of how and which extracellular matrix properties contribute to a dysfunctional state. Here we describe a decellularized extracellular matrix–synthetic hydrogel hybrid scaffold that independently confers two distinct matrix properties—ligand presentation and stiffness—to cultured cells in vitro, allowing for the identification of their specific roles in cardiac ageing. The hybrid scaffold maintains native matrix composition and organization of young or aged murine cardiac tissue, whereas its mechanical properties can be independently tuned to mimic young or aged tissue stiffness. Seeding these scaffolds with murine primary cardiac fibroblasts, we identify distinct age- and matrix-dependent mechanisms of cardiac fibroblast activation, matrix remodelling and senescence. Importantly, we show that the ligand presentation of a young extracellular matrix can outweigh the profibrotic stiffness cues typically present in an aged extracellular matrix in maintaining or driving cardiac fibroblast quiescence. Ultimately, these tunable scaffolds can enable the discovery of specific extracellular targets to prevent ageing dysfunction and promote rejuvenation.
Similar content being viewed by others
Main
Age-related alterations in tissue properties have been recognized as key drivers of organ dysfunction and disease. Although much remains unknown about what happens to the extracellular matrix (ECM) at the micro and nano length scales during these large-scale remodelling events, it is widely acknowledged that the mechanics, organization and composition of the ECM vary with age. For instance, it has been shown that the cardiac muscle stiffens1, whereas ECM composition and organization undergo age-dependent modifications2,3,4. Cardiac fibroblasts (CFs) are the resident cells largely responsible for the remodelling of heart tissue and are known to be mechanosensitive from both in vitro and in vivo studies5,6.
In healthy tissue, CFs largely remain in a quiescent state, but external stimuli, including biochemical, structural and mechanical cues5,6,7, are able to activate quiescent CFs, leading to their differentiation into a proto-myofibroblast phenotype and subsequently into a mature myofibroblast phenotype when these stimuli are impactful and persistent6,8. The process of CF activation and proper myofibroblast maturation are essential for ECM deposition and the maintenance of matrix homeostasis but can also lead to fibrosis and result in functional consequences9. This is important in ageing tissues, as alterations in the ECM can be vast and multifaceted, thereby leading to the activation of CFs and subsequent aberrant tissue remodelling6,10.
Indeed, it has been shown that myofibroblasts are more abundant in aged versus young hearts and directly induce changes to the tissue geometry10,11,12. Although in vitro material systems have identified individual properties of the ECM that play distinct roles in CF function, it remains a challenge to vary these properties independently. In most scaffold platforms, tuning the mechanical properties will alter the ligands and/or architecture. A handful of novel material systems have been described that are capable of independent tunability13,14,15, yet the incorporation of native ECM properties is still lacking. Thus, our understanding of the specific contributions stemming from ECM cues is currently limited. We, therefore, sought to develop a native ECM-based scaffold in which we could individually tune the mechanics and faithfully mimic the in vivo cardiac environment—both composition and architecture—allowing for the identification of ECM-specific roles in age-related CF activation, mechanosensing, matrix remodelling and senescence.
To do this, we developed a novel approach called DECellularized In situ Polyacrylamide Hydrogel–ECM hybRid (DECIPHER) that integrates polyacrylamide (PA)-hydrogel-linked cardiac tissue slices and in situ decellularization of ECM. We demonstrate that our decellularization technique is not only able to maintain the ECM composition and fibre architecture but that the samples can be tuned to exhibit young or aged stiffness, namely, ~10 kPa or 40 kPa, respectively. Furthermore, we demonstrate the ability of DECIPHER scaffolds to exhibit physiologically relevant viscoelastic properties due to the interpenetrating network. Seeding young and aged CFs onto combinations of young or aged matrix with young or aged stiffness, we demonstrate distinct age-dependent CF activation, mechanosensing, ligand interaction, ECM remodelling and senescence. Although we observe that such phenotypes can be driven by multiple matrix cues, ECM ligand presentation can outweigh the ECM mechanics, with young ECM promoting CF quiescence despite the profibrotic stiffness cue that is characteristic of aged cardiac tissue. These findings provide a unique insight into the distinct roles of ECM in regulating age-related cardiac dysfunction, which have important implications in matrix-based treatment strategies.
DECIPHER maintains native ECM ligands
The DECIPHER method (Fig. 1a) was inspired by a previously reported method for tissue clearing16, in which N-methylolacrylamide, formed by prereacting acrylamide hydrogel solution with formaldehyde, binds to the amine groups of tissue proteins. After crosslinking the PA hydrogel with ultraviolet (UV) light, the tissue proteins are stabilized onto the PA mesh, thereby making an interpenetrating and interconnected hybrid hydrogel, and then decellularized in place. We modified the original method in three ways: (1) by linking the PA gel to methacrylated coverslips, enabling stable sample handling; (2) by modifying the PA hydrogel solutions to dictate the scaffold stiffness; and (3) by applying an optimized decellularization method to maintain the ECM composition and architecture.
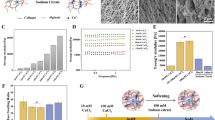
a, Murine cardiac tissue (young and aged) was sliced with a vibratome, placed on a hydrophobic glass slide (i) and incubated in a hydrogel stabilization solution consisting of acrylamide pretreated with formaldehyde, bis-acrylamide and Irgacure 2959 of soft (~10 kPa) or stiff (~40 kPa) formulations (ii). Crosslinking of the hydrogel mesh was carried out with UV (iii). At this step, the tissue is stabilized in the hydrogel mesh (as indicated in the box below) and samples are referred to as ‘pre-DECIPHER’. Subsequent decellularization is carried out using SDC and DNase (iv) and samples are referred to as ‘DECIPHER’. The scaffolds are seeded with young or aged CFs (v). b, DECIPHER maintains the native tissue architecture and mechanics, compared with conventional dECM hybrid materials. c, Images of pre-DECIPHER and DECIPHER samples with a tissue/ECM scaffold indicated by the dashed lines, surrounded by a coverslip/pure PA gel. d, SEM images of native tissue and DECIPHER samples with arrows indicating the ECM fibres versus the hydrogel mesh. Scale bars, 200 nm. e, Three-dimensional confocal reconstruction of DECIPHER samples with Nile Blue-tagged acrylamide and labelled collagen. Scale bars, 50 µm.
With DECIPHER, we created four sample combinations of young (1–2 months) and aged (18–24 months) ECM, from isolated native murine tissue sections, and young and aged stiffness, dictated by the hydrogel component; in all these cases, the native tissue architecture was maintained (Fig. 1b). These samples are referred to as follows: ‘SoftY’ for soft, young ECM; ‘StiffY’ for stiff, young ECM; ‘SoftA’ for soft, aged ECM; and ‘StiffA’ for stiff, aged ECM (Fig. 1 and Extended Data Fig. 1a). Decellularization increases the transparency of the tissue, and the DECIPHER sample is stabilized within the PA hydrogel on a coverslip (Fig. 1c). Scanning electron microscopy (SEM) of the DECIPHER samples show a crosslinked fibrous structure on the ECM due to PA stabilization, which is not present surrounding the collagen fibrils in native tissue (Fig. 1d). Confocal imaging of Nile Blue-tagged acrylamide and collagen (CNA35) reveals the penetration of PA into the tissue and surrounding ECM fibres, leaving the ECM exposed on the surface for cell binding (unmodified PA hydrogels repel cell adhesion17) due to the placement on a hydrophobic glass slide at the surface during polymerization (Fig. 1e).
Immunohistochemistry (IHC) of the nuclei and actin in young and aged tissues confirmed the complete removal of cellular structures and the preservation of ECM composition and organization (collagen, fibronectin, hyaluronan and laminin) (Fig. 2a,b). PicoGreen dsDNA assay (Extended Data Fig. 1c) further verified full decellularization18, whereas collagen and sulfated glycosoaminoglycan (sGAG) quantifications showed minimal loss post-DECIPHER (>95.8% of collagen and >52.0% of sGAG were preserved; Extended Data Fig. 1d,e). The loss of sGAGs was ascribed to their removal on the plasma membrane and inside cells19. After optimizing the decellularization approach to be minimally damaging using sodium deoxycholate (SDC) and deoxyribonuclease (DNase), we confirmed that collagen fibrils remained intact through the staining and quantification of collagen hybridizing peptide (Extended Data Fig. 1g,h). We compared our decellularization method with a widely used decellularization protocol (sodium dodecyl sulfate (SDS) + Triton X-100) and found denatured collagen in both PA-stabilized and unstabilized samples (Extended Data Fig. 1g,h), although hydrogel stabilization was effective in reducing collagen denaturation.
a,b, IHC of pre-DECIPHER (top set) and DECIPHER (bottom set) samples from young (a) and aged (b) murine cardiac tissue for nucleus, actin, collagen, fibronectin, hyaluronan and laminin show the removal of cellular structures in DECIPHER versus pre-DECIPHER samples, as well as the preservation of ECM components. c,d, Collagen architecture of young or aged tissue (N = 3 biological replicates for each condition) in pre-DECIPHER and DECIPHER scaffolds were quantified using TWOMBLI (c) followed by a principal component analysis (d). HGU, hyphal growth unit. e,f, Nanoindentation of native cardiac tissue from young and aged mice (e; N = 3 biological replicates for each condition) was used to dictate the PA hydrogel compositions for soft and stiff samples (f; Soft (N = 5 gels) and Stiff (N = 7 gels)). g–i, Stiffness mapping of DECIPHER samples showed mechanical tunability to maintain stiffness in SoftY, increase stiffness in StiffY, decrease stiffness in SoftA and maintain stiffness in StiffA (g; N = 3 biological replicates for each condition). Viscoelastic properties were measured using dynamic mechanical analysis for plain PA hydrogel (h; N = 3 gels) and DECIPHER samples (i; N = 3 biological replicates for each condition). j,k, Correlative confocal–AFM was performed on DECIPHER samples for collagen fibres and the surrounding hydrogel (j and k; N = 2 biological replicates for each condition). Data are mean ± s.d. (e–g and k) or mean ± s.e.m. (h and i). Unpaired two-tailed t-test (e and f) or one-way analysis of variance (ANOVA) with Dunn’s multiple comparisons (g). NS, not significant (P > 0.05). Scale bars, 100 µm (a–c); 10 µm (j).
We next quantified the ECM architecture from collagen IHC images using the TWOMBLI Fiji plug-in20. A principal component analysis shows no difference between the pre- versus post-DECIPHER or soft versus stiff samples across multiple geometric parameters (branch points, fractal dimension, alignment, endpoints, hyphal growth unit and lacunarity) (Fig. 2c,d). Importantly, we quantitatively demonstrate that young versus aged ECM exhibits distinguishable architectural differences across all architectural readouts, where endpoints per unit length (hyphal growth unit) was identified with the greatest principal component contribution (Extended Data Fig. 1f), further emphasizing the importance of ECM architecture in ageing (Fig. 2d).
DECIPHER decouples ECM ligands and stiffness
One of the major improvements that DECIPHER brings to current decellularized ECM (dECM)-based material systems is in situ hydrogel–tissue stabilization. Hydrogel stabilization not only prevents tissue architecture disruption and damage during decellularization but also confers tunable mechanical properties to the scaffold. Using nanoindentation, we first measured the stiffness (Young’s modulus E) of the native tissue obtained from young or aged murine hearts (Fig. 2e). Young and aged tissues were found to have E = 13.1 ± 5.2 kPa and 38.6 ± 7.9 kPa, respectively, consistent with previous reports1. Subsequently, we optimized the PA hydrogel compositions to mimic the measured tissue stiffnesses, E = 11.5 ± 0.9 kPa and 39.6 ± 4.0 kPa for young and aged tissue stiffnesses, respectively (Fig. 2f). Stiffness mapping of pre- and post-DECIPHER samples was carried out and demonstrated the ability to obtain decoupled stiffness tunability from native tissue stiffness (Fig. 2g and Extended Data Fig. 2a).
We next sought to quantify the viscoelastic properties of the DECIPHER scaffolds. Interpenetrating network materials have been shown to exhibit time-dependent mechanics14, which is important for biomaterials design due to the inherent viscoelasticity of most tissues, including the heart21,22. Plain PA hydrogel recipes used in this study were shown to be purely elastic (Fig. 2h), whereas DECIPHER scaffolds exhibited viscoelastic properties, with loss moduli ranging from ~3.5 kPa to 4.8 kPa for SoftY scaffolds and ~5.4 kPa to 7.3 kPa for StiffY scaffolds (Fig. 2i and Extended Data Fig. 2b,c). These ranges match the reported tissue viscoelasticity values and are an improvement over the reconstituted dECM materials21, further emphasizing the ability of our scaffolds to mimic native tissue mechanical properties. We also demonstrate that the covalent linking between the hydrogel and ECM fibres enhances the viscoelastic properties of the scaffolds (Extended Data Fig. 2f,g), and plain PA hydrogel mechanics were not influenced by the linking agent (Extended Data Fig. 2d,e).
The nanoindentation experiments described above use a 50-µm-radius spherical cantilever, which is unable to distinguish nanoscale ECM fibre mechanics. Thus, we sought to map the mechanical properties at higher resolution by applying correlative confocal–atomic force microscopy (AFM). To do this, we live stained collagen fibres to identify specific regions of interest with confocal microscopy and then moved to the same position in the AFM setup23. By scanning the surface of DECIPHER samples with a nanometre-sized pyramidal-shaped cantilever, we found that the DECIPHER process does not impact the ECM fibre stiffness across all the sample conditions compared with the native tissue control samples, although collagen bundle size could contribute to stiffness variance (Fig. 2j,k and Extended Data Fig. 2h). When measuring the mechanics in regions adjacent to the ECM fibres, we measured the expected values based on the hydrogel formulations used (for example, ~12.6 kPa in SoftY or SoftA gel regions and ~37.8 kPa in StiffY or StiffA gel regions; Fig. 2k). This means that the hydrogel component provides the indentation resistance of the entire hybrid scaffold, as dECM alone has been shown to exhibit reduced compressive modulus and spontaneous shrinkage when unrestrained24,25. Taken together, DECIPHER allows for the mechanical tunability of decellularized tissue that is decoupled from native ECM composition and architecture.
Ageing alters intra- and extracellular cardiac phenotypes
CFs are largely responsible for cardiac ECM remodelling3,26. To characterize age-related changes in both CFs and ECM of young (1 month) and aged (24 months) mice, we performed RNA sequencing (RNA-seq) on isolated primary CFs and tandem-mass-tag-based quantitative mass spectrometry on dECM from cardiac tissue. For RNA-seq, isolated cells were examined for cell-type-specific markers for Vim (encodes vimentin in fibroblasts), Pecam1 (encodes CD31 in endothelial cells) and Tnni3 (encodes Troponin I in cardiomyocytes), confirming a pure fibroblast population in both age groups (Extended Data Fig. 3n). Differentially expressed gene (DEG) analysis of aged versus young CFs found 490 upregulated genes and 597 downregulated genes (Fig. 3a), with heat maps showing global differential expression patterns between cell populations (Fig. 3b). Those upregulated included multiple senescence markers (for example, cellular communication network factor 1 (Ccn1), Cdkn1a and Cdkn2) and fibroblast-associated profibrotic markers (for example, Ankrd1, Tnc, Ccr2 and Adamts1). The majority of significantly downregulated genes encode ECM proteins (for example, Col1a1, Col1a2, Col3a1, Fn1 and Eln), which have been observed in aged rodent models3, as well as matricellular protein gene Ccn5, cyclin family genes (for example, Ccnb1 and Ccnb2) and cyclin-dependent kinase-encoding genes (for example, Cdk1 and Cdk6), indicating both increased potential of aged CFs to be activated27 and abnormal proliferative ability that could suggest a senescent-like phenotype28,29 (Fig. 3a). KEGG enrichment analysis of the DEGs in aged versus young CFs corroborated these findings as multiple significantly upregulated pathways indicated their greater potential towards profibrotic ECM remodelling, a senescent-like phenotype, and ECM deregulation as found in the aged heart3 (Fig. 3c).
a, DEG analysis of RNA-seq data of primary aged versus young CFs show fold-change (FC) expression, with the specific upregulated and downregulated genes highlighted. b, Heat map of clustered DEGs with three sample replicates of young and aged CFs. c, KEGG enrichment analysis of aged versus young CF DEGs shows the activated and suppressed pathways. The bubble size indicates the number of DEGs in each pathway set and the colour bar indicates the adjusted significance by Q value. Significantly regulated pathways are marked with asterisks on the y axis: *Q < 0.05, **Q < 0.01, ***Q < 0.001. d,e, DEPs in aged versus young cardiac ECM shown as the clustered heat map (d) and volcano plot (e), with the upregulated and downregulated expressions indicated. f, Ligand–receptor interaction map for the top 25 CF receptors and top 20 ECM ligands, where the bar width indicates the FC expression in aged versus young and node colours indicate the correlation of two ends. g–j, DECIPHER comparisons of ‘aged heart’ (aged CFs on StiffA) versus ‘young heart’ (young CFs on SoftY) (g and h), with the DEGs shown in the volcano plot (i) and heat map (j). N = 3 biological replicates for each condition, two-sided Benjamini–Hochberg test (a–e, i and j). DEGs are FC > 2 and P < 0.05 in a and b; FC > 1.5 and P < 0.05 in i and j; DEPs are FC > 1.2 and P < 0.05 in d–f. The colour bars in b, d and j indicate the row-standardized expression. Scale bar, 100 μm (h).
Quantitative mass spectrometry found more than 3,000 proteins in decellularized young and aged cardiac tissues, with the extracted proteome highly enriched in ECM proteins based on gene ontology enrichment analysis (Extended Data Fig. 1m). Differentially expressed protein (DEP) analysis identified 117 upregulated and 58 downregulated proteins (Fig. 3d,e). Due to a larger CF population and altered ECM turnover in the aged heart11,12,30, many ECM proteins were found to be significantly upregulated in cardiac ageing (Fig. 3e and Extended Data Fig. 1o) including collagen VI, laminin α2, laminin β2, fibronectin, vitronectin, versican, decorin, periostin, collagen XII and perlecan, whereas elastin, collagen III and aggrecan were found to be downregulated (Extended Data Fig. 1o). Matrix-immobilized molecules were also identified, with greater TGF-βI, CXCL12 and LOXL1 found in aged ECM (Extended Data Fig. 1n). By analysing the protein–protein interaction (PPI) network using the STRING database, we found that structural ECM proteins, matricellular proteins, matrix-immobilized molecules, membrane-associated proteins and other functional proteins are closely associated, with fibronectin, vitronectin and nidogen having the largest contributions (Extended Data Fig. 1p).
We next characterized the ligand–receptor interactome using these two datasets31. By comparing the top 20 up- or downregulated extracellular proteins of the ECM with the top 25 lowly or highly expressed cell surface molecules, we can understand the age-specific regulation of matrix-mediated signalling processes10,32. We find enhanced integrin expression in aged versus young CFs (for example, genes encoding integrins αv/4/6/9/10 and β1/3/6) corresponds to upregulated ECM components in aged tissue (for example, laminins α2/α4/β1, fibronectin, versican, periostin and perlecan; Fig. 3f). Genes encoding non-integrin receptors were also identified in the top regulated CF receptors with ageing (for example, Egfr and Cd44 are upregulated; Sdc3 is downregulated) and correlate with ECM abundance.
CF gene expression is regulated by ECM as a function of age
We next sought to understand how young and aged CFs perceive and respond to ECM stiffness and ligands, both individually and cooperatively, using our DECIPHER scaffolds (all combinations are detailed in Extended Data Fig. 1b). We first compare our most aged state (that is, aged CFs cultured on a scaffold representing the aged heart, StiffA) to our most young state (that is, young CFs cultured on a scaffold representing the young heart, SoftY) (Fig. 3g,h). After culturing primary CFs on DECIPHER samples, we identified global DEGs (>1,100 genes) in the aged versus the young state (Fig. 3i). Upregulated genes include those that encode matrix remodellers (for example, Timp3 and Lox), specific ECM proteins (for example, Col12a1 and Col8a1) and activators of fibroblasts (for example, Ccn4 and Ccnd2), whereas downregulated genes include those that encode matrix degraders (for example, Mmp2 and Mmp12), matrix adhesions (for example, Dpt and Itga8) and specific ECM proteins (for example, Eln and Col13a1).
When comparing aged CF gene expression on all other DECIPHER samples versus StiffA, the cells exhibited a rejuvenation-like effect, shifting expression towards a quiescent phenotype, particularly on the young ECM samples (Fig. 3j; original clustering data are shown in Extended Data Fig. 4a). The opposite trend was observed for young CFs on all other DECIPHER samples versus SoftY, with the expression indicating an ageing-like shift and enhanced activation (Fig. 3j). Interestingly, ligand presentation had a larger impact compared with stiffness in driving aged cells towards a young state.
We further applied comprehensive pairwise KEGG enrichment analyses on all DECIPHER samples to identify specific pathways that were activated or suppressed by stiffness cues as a function of age (Extended Data Fig. 5a) or by ECM as a function of age (Extended Data Fig. 5b). We found that stiffness activates nucleocytoplasmic transport, whereas ligand presentation promotes arginine and proline metabolism, which regulates collagen synthesis and has been associated with ECM-driven fibrotic remodelling in other tissues33,34. In summary, primary aged CFs exhibit both senescent and activated phenotypes, and young ECM is able to shift expression, regardless of stiffness, towards a rejuvenated-like state. Conversely, both higher stiffness and aged ECM can shift young CFs towards a more activated and aged-like state. These matrix-property-specific differences between young and aged CFs underscore the interplay of cellular and extracellular ageing, which DECIPHER is capable of unravelling.
Age regulates ligand interactions and mechanosignalling
Cellular mechanotransduction is triggered after interaction with the ECM; thus, we sorted our RNA-seq data for membrane receptors and well-known mechanosensors (Fig. 4a; Extended Data Figs. 3 and 6 show both RNA-seq and reverse-transcription (RT) quantitative polymerase chain reaction (qPCR)). Overall, young CFs upregulated mechanosensitive gene expression on stiff substrates regardless of the ECM, whereas aged CFs showed mechanosensing impairments, as previously reported10. Furthermore, age-specific receptor expression was conserved on DECIPHER samples, correlating with data from freshly isolated cells (Figs. 4a and 3f). Delving deeper into age- and ECM-specific ligand–receptor responses after culturing CFs on DECIPHER samples, we found the expression of Ddr2 (encoding discoidin ___domain receptor 2 (DDR2)), which is associated with CF activation and ECM remodelling in the pathological heart35, to be inversely related to age—young CFs on aged ECM have reduced Ddr2 expression, whereas aged CFs on young ECM have enhanced expression (Fig. 4d).
a–n, RNA-seq data analysis and ICC for ECM ligand–receptor interactions and mechanosensing (a–i) and activation (j–n) are shown. a, RNA-seq analysis of ECM receptors and mechanosensitive proteins of young and aged CFs on DECIPHER samples. d–g, Normalized gene expression for highlighted membrane receptors (Ddr2, Itga11 and Itgb3) and intracellular mechanosensors (Zyx). b,c,h–k, Highlighted ICC groups of young CFs on SoftY or StiffY DECIPHER samples stained for nucleus, F-actin and α5 integrin (b), YAP (c) or α-SMA (j and k), and quantified in h and i. SF, stress fibres (j). N = 3 samples for each condition in each ICC (b, c and h–k). l, CF activation genes from the RNA-seq data of young and aged CFs on DECIPHER samples. m,n, Normalized gene expression for CF activation markers (Acta2 and Ccn2) for young and aged CFs on DECIPHER samples. The colour bar indicates the row-standardized expression (a and l). Data are mean ± s.e.m. (d–g, m and n). One-way ANOVA with Fisher’s preplanned multiple comparisons (each pair with one variable, ECM or stiffness) was used for d–i, m and n. Scale bars, 10 µm (inset of α5 integrin in b); 50 µm (b, c, j and k).
Among integrins, α11 is an important collagen receptor that modulates CF activation and ECM protein synthesis in disease models, although its mechanosensitive function remains unclear29,36. Using DECIPHER, we observed a significant increase in α11 integrin expression on aged ECM (Fig. 4e), whereas stiffness did not play a major role. Fibronectin-binding integrins (for example, α5β1 and αvβ3) are regulated by age and fibronectin tensional state, which has been shown to alter biochemical and mechanosignalling via focal adhesion recruitment4. The immunostaining of α5 integrin confirmed its mechanosensitive nature37,38, with larger α5 clusters on stiff substrates regardless of ECM (Fig. 4b,h and Extended Data Fig. 7a,b). By contrast, RNA-seq shows β3 integrin gene expression is regulated by ECM age (Fig. 4f), where we observed reduced expression on aged ECM for both young and aged CFs. These results highlight that specific receptors exhibit unique responses to the extracellular environment.
We next focused on intracellular mechanosensors from our RNA-seq data (Fig. 4a). Specifically, young CFs upregulated focal adhesion, serum response factor/myocardin-related transcription factor A (Mrtfa) and Yap1 gene expression on stiff and/or aged substrates (StiffY, SoftA and StiffA). Aged CFs, on the other hand, generally required both aged ECM and high stiffness (StiffA samples) to upregulate mechanosensitive genes. At the focal adhesion level, zyxin (Zyx)—responsible for actin regulation—was significantly upregulated in young CFs when exposed to either aged ECM and/or a stiff environment, whereas aged CFs required both cues presented together to increase expression (Fig. 4g). Immunocytochemistry (ICC) staining of focal adhesion sites (paxillin) showed a reduction in their formation on soft and/or young ECM, suggesting a less activated phenotype (Extended Data Fig. 7c–h).
Despite altered mechanosignalling, we found that Actr2 and Actr3 were upregulated on StiffA samples (Fig. 4a), suggesting that aged CFs favour facilitating ECM remodelling through actin-mediated force exertion32. Although YAP translocation has classically been shown to depend on substrate stiffness, we found that ECM ligand cues contribute to the process as well, with young ECM preventing nuclear translocation in both young and aged CFs regardless of stiffness (Fig. 4c,i and Extended Data Fig. 8i,j). Our proteomics data found a lower collagen I/III ratio in young ECM (Extended Data Fig. 1q), which has been shown to promote the degradation of translocated YAP3,10,39. On aged ECM, young CFs enhanced YAP translocation on stiff substrates unlike aged CFs, which exhibit mechanosensing dysregulation. Furthermore, the activation of transient receptor potential vanilloid 4 (Trpv4), which regulates the integration of multiple extracellular cues during fibroblast activation and myofibroblast differentiation, was upregulated in aged CFs on StiffA samples (Fig. 4a), suggesting their profibrotic potential10,29.
ECM ligand presentation outweighs stiffness in CF activation
The activation of CFs towards a myofibroblast phenotype was assessed after seeding young CFs and aged CFs on DECIPHER samples using ICC for F-actin and α-smooth muscle actin (α-SMA). Cell and nuclear morphological metrics were quantified, and CFs generally exhibited an increase in cell area and filopodia on stiffer substrates (Extended Data Fig. 9a–j), whereas ECM age did not affect the morphology. Furthermore, we observed an enhanced expression of α-SMA and stress fibre formation as a function of stiffness regardless of ECM or cell age, although to differing levels (Fig. 4j,k and Extended Data Fig. 8a–d), corresponding to previous reports that stiffness alone can activate CFs6,8,40. Importantly, when comparing ECM age, α-SMA was significantly reduced when CFs were plated on young ECM versus aged ECM, indicating that the composition and architecture of young ECM can prevent CF activation, overriding the stiffness cue. This is particularly evident when young CFs are cultured on StiffY samples, as well as when aged CFs are cultured on SoftY samples. Although young ECM alone cannot prevent the partial upregulation of α-SMA in aged CFs (~40% stress fibre formation on StiffY versus ~29% on SoftY), there is a clear reduction compared with aged ECM samples, highlighting the distinct mechanosensitive differences between young and aged cells. Aged ECM, on the other hand, enhanced α-SMA regardless of stiffness, with StiffA samples resulting in >64% stress fibre formation (Fig. 4j,k).
RNA-seq uncovered multiple genes that regulate CF activation in young and aged CFs as a function of ECM properties (Fig. 4l). Corresponding to the ICC data, both young and aged CFs upregulated Acta2 (encodes α-SMA) when on aged ECM and/or high stiffness except in the case of aged CFs on StiffY (Fig. 4m; Extended Data Fig. 6a,b compares RNA-seq and RT-qPCR data). Similar correlations were observed for Ccn2 (encodes cellular communication network factor 2), which is another known CF activation marker (Fig. 4n). Myl9 (encodes myosin light chain 9), a downstream myofibroblast maturation marker, was upregulated in aged CFs on both SoftA and StiffA, pointing to the crucial role of ECM ligands in persistent CF activation independent of stiffness (Extended Data Fig. 3a). Taken together, we find that ECM ligand presentation can dictate myofibroblast activation state, outweighing the matrix stiffness cue.
Native ECM architecture is crucial for cellular function
ECM architecture alone has been shown to play distinct roles in driving fibroblast fate and function7,41. To understand the impact of native ECM architecture on fibroblast adhesion and activation, we compared DECIPHER and reconstituted young and aged dECM film substrates (Extended Data Fig. 10a). Reconstituted dECM films are conventionally utilized in ECM-related studies, but we show the complete loss of native ECM architecture due to the milling and solubilization steps (Extended Data Fig. 10b). CFs seeded on the reconstituted dECM-coated coverslips had higher α-SMA expression, stress fibre formation and focal adhesion assembly than cells on DECIPHER samples, which can be ascribed to the loss of native ECM architecture and mechanics (Extended Data Fig. 10c–i). Furthermore, young dECM alone was not sufficient to reduce or prevent the fibroblast activation of aged CFs, indicating that native architecture plays a crucial role (Extended Data Fig. 10g,i). These results underscore the importance of maintaining native ECM architecture in biomaterial approaches42,43.
Cell and matrix age determine ECM regulation
In the healthy heart, CFs maintain matrix homeostasis by balancing protein degradation, synthesis and modification29. In ageing, intra- and extracellular perturbations cause dysregulation of the ECM, leading CFs to aberrantly remodel the matrix (Figs. 2c,d and 3d,e). For example, in native cardiac tissues, lysyl oxidase (LOX), which is responsible for crosslinking ECM proteins, increases with age (Extended Data Fig. 1k), with ~23% co-localized with ECM in aged tissues versus ~8% in young (Extended Data Fig. 1l). DECIPHER is capable of maintaining this age-related difference in matrix-immobilized molecules (Fig. 5b and Extended Data Fig. 1k), whereas intracellular molecules (for example, NF-κB) are completely removed due to cell removal (Extended Data Fig. 1i,j). We further confirmed the presence of accessible ligands on collagen I fibres with DECIPHER using immunogold labelling followed by SEM imaging (Fig. 5d), indicating that cells are capable of attaching to and remodelling the ECM in the scaffolds44.
a, RNA-seq data of genes for ECM degradation, crosslinking and secretion of young and aged CFs on DECIPHER samples, with ECM protein secretion-related genes and matrix crosslinking or degradation-related genes. The colour bar indicates the row-standardized expression. b, DECIPHER preserves matrix-immobilized LOX in aged ECM as shown in the immunostained images (collagen, nucleus, F-actin and LOX) (N = 3 biological replicates for each condition). c, ICC of young or aged CFs on DECIPHER samples stained for nucleus, F-actin and LOX (N = 3 samples for each condition). d, DECIPHER maintains functional ligands as shown by immunogold SEM. AuNP, gold nanoparticle. 2° Ab control, immunogold 2° antibody with no primary antibody. e–h, Normalized gene expression for Lox, Mmp2 and Col12a1 given as mean ± s.e.m. One-way ANOVA with Fisher’s preplanned multiple comparisons (each pair with one variable, ECM or stiffness) was used for e–h. h, Quantified LOX from c (N = 3 samples for each condition). Scale bars, 50 μm (b and c); 200 nm (d).
We sorted our RNA-seq data for the ECM-regulating genes. Aged CFs on StiffA scaffolds showed an upregulation of genes encoding matrix modifiers including LOX family enzymes and a downregulation of genes encoding matrix degraders including matrix metalloproteases, opposite from what is observed for young CFs on SoftY scaffolds (Fig. 5a). Enhanced LOX was also observed in aged CFs on SoftA scaffolds on both transcriptome and proteome levels (Fig. 5a,c,g,h), suggesting that both cell and matrix age increase the profibrotic potential of CFs. These results agree with our data on CF activation (Fig. 4j,k), where young ECM can override the stiffness cue in aged CFs. Profibrotic CFs increase the crosslinking of ECM, which not only directly alters the cellular behaviour through architecture-mediated mechanisms7,45 but also exacerbates myocardial stiffening3. In addition, these samples showed significantly downregulated Mmp2 and Mmp3, which are responsible for normal tissue ECM turnover and degradation, respectively46 (Fig. 5e and Extended Data Fig. 3h).
In addition to ECM remodelling, the gene expression of multiple fibrillar and fibril-associated ECM proteins (for example, Col1a1, Col5a1, Col12a1 and Fn1) and non-fibrillar proteins (for example, Col8a1 and Lama4) were significantly upregulated in aged CFs on SoftA and StiffA samples (Fig. 5a,f and Extended Data Fig. 3i–k; Extended Data Fig. 6c–f shows a comparison of RNA-seq and RT-qPCR data), which corresponds to our mass spectrometry data (Fig. 3e and Extended Data Fig. 1o). The glycoprotein secreted protein acidic and rich in cysteine (Sparc), which is essential for ECM fibril formation and age-related tissue stiffening3, was also significantly upregulated in aged CFs on SoftA and StiffA (Extended Data Fig. 3l).
Interestingly, the synthesis of ECM proteins did not display a universal increase in response to aged ECM and high stiffness. Specifically, stiffness reduced collagen III expression, which could contribute to a positive feedback loop through an increasing collagen I/III ratio. Indeed, our proteomics data identified an ~9.4% increase in collagen I/III with age, leading to the altered tissue mechanics found in other aged tissue models (Extended Data Fig. 1q,o)3,40. Genes encoding ECM components associated with maintaining healthy mechanics and regulating fibrotic tissue size such as elastin (Eln)47 and collagen V (Col5a1)5 were also downregulated (Fig. 5a), which could explain the reduction in both proteins identified in the aged cardiac ECM (Extended Data Fig. 1o). These results indicate specific combinations of matrix mechanics and the ECM age could affect the extent of matrix regulation, yet the CF age and ECM age play dominant roles.
ECM and mechanics dictate CF senescence and rejuvenation
In the ageing heart, senescence is an inevitable cellular phenotype that directly impacts organ physiology, leading to and/or accelerating cardiovascular diseases28. Due to the irreplaceable role of CFs in cardiac ECM deposition and remodelling, their acquisition of senescence is believed to cause an ECM imbalance, especially during the wound healing process post-acute myocardial infarction28. By comparing DEGs from DECIPHER groups seeded with young or aged CFs, we showed a shift of expression indicating in vitro ‘cardiac ageing’ of young CFs from SoftY to StiffA, as well as ‘cardiac rejuvenation’ of aged CFs from StiffA to SoftY (Fig. 6a,b). For young CFs, as the stiffness increases (SoftY to StiffY) or ECM ages (SoftY to SoftA), gene expression shifted towards a senescent-like phenotype that is seen on the StiffA substrate mimicking the aged heart (Fig. 6a; the unaveraged heat maps are shown in Extended Data Fig. 4b). For aged CFs, young ECM and reduced stiffness induced rejuvenation-like behaviour, with ECM playing a greater role than stiffness, as we observed for myofibroblast activation (Fig. 6b; unaveraged heat maps are shown in Extended Data Fig. 4c).
a,b, Heat maps of RNA-seq-identified DEGs of young CFs (a) and aged CFs (b) on StiffA versus SoftY substrates. c, Schematic of the ECM property regulating young and aged CF functions as elucidated via DECIPHER scaffolds. d, Heat map of specific CF senescence regulators. For the DEGs in a and b, FC > 1.5 and P < 0.05. e, Normalized log2[FC] values of all DEGs (957) identified in the DECIPHER samples driven by stiffness or ECM for young and aged CFs. Probability density of stiffness- and ECM-driven genes is shown on the respective axis for young and aged CFs. f–i, Normalized gene expression for Trp53 (f), Cdkn1a (g), Ccn1 (h) and Timp3 (i) are mean ± s.e.m. j–n, ICC of CFs on DECIPHER samples for nucleus, F-actin and TIMP3 (j and k) or p53 (l and m) and quantified in n. The arrows in l indicate the example nuclei that are above the p53+ intensity threshold. N = 3 samples for each condition (j and n). In a, b and d, the colour bar indicates the row-standardized expression. One-way ANOVA with Fisher’s preplanned multiple comparisons (each pair with one variable, ECM or stiffness) was used in f–j and n. Scale bar, 100 μm (k–m).
In our global KEGG analysis (Extended Data Fig. 5), DECIPHER revealed the roles of ligands and substrate stiffness in activating the cellular senescence pathways, especially for the p53 signalling pathway that has been identified as one of the most well-characterized pathways in CF senescence28,48. Many key senescence-associated pathways were activated concurrently with p53/p21 (encoded by Trp53/Cdkn1a), including p16 (encoded by Cdkn2a) and p38 mitogen-activated protein kinase, although CFs on StiffA DECIPHER samples do not demonstrate a terminally senescent phenotype in terms of collagen expression (reduced) and proinflammatory cytokines expression (enhanced) probably due to the short culture time28,49 (Fig. 6d,f,g). On the protein level, the activation of the p53/p21 pathway was also observed, where an increased population of p53-positive cells was found on aged and stiff ECMs (Fig. 6l–n and Extended Data Fig. 8e–h). Downstream gene expression encoding senescence-associated secretory phenotype proteins that have been shown in senescent fibroblasts28,50, including tissue inhibitor of metalloproteinase 3 (TIMP3), Ccn1 and interleukin 6, were also enhanced (Fig. 6h–k and Extended Data Figs. 3m, 6o,p and 8k,l). Overall, these results suggest the critical roles of aged ECM and myocardial stiffness in CF activation, senescence and ‘rejuvenation’, with the ECM age outweighing mechanics in aged cells (Fig. 6c).
DECIPHER also uncovered the importance of cell age in the perception of ECM cues. Although we focus on specific players throughout the manuscript, we sought to understand the global regulation of age-specific cellular function by extracellular cues and, thus, analysed all DEGs identified in our RNA-seq data as a function of stiffness (comparisons: StiffY versus SoftY and StiffA versus SoftA) or ECM (comparisons: SoftA versus SoftY and StiffA versus StiffY). We found that young CFs integrate both stiffness and ECM cues to a similar extent, whereas aged CFs are less mechanosensitive but more influenced by ECM (Fig. 6e and Supplementary Note 1). In aged CFs, genes related to cardiac ageing/disease and ECM remodelling (for example, Ccn3, Sned1, Col27a1, Fgfr2 and Timp3) were found to be regulated by ECM versus stiffness, whereas Cmpk2 is mechanosensitive. In young CFs, multiple genes were found to be driven by both stiffness and ECM ligands (for example, Apol9a, Adamts15, Pygo1 and Saa3). These findings reveal compelling prospects for rejuvenation therapies as well as underscoring the importance of matrix mechanism-targeted approaches.
Outlook
Important studies have recently uncovered the essential roles of ECM composition, architecture and mechanics in driving cellular function2,6,45,51. To describe the impact of ECM-specific properties, new tunable dECM-based biomaterial systems have been designed for a variety of applications including two- and three-dimensional in vitro bulk hydrogel systems, injectable in vivo hydrogel formulations and complex bioprinted scaffolds2,52,53,54. Although dECM-based biomaterials can provide faithful recapitulation of native ECM composition to some degree, preserving mechanical and architectural aspects of the native tissue remain a challenge. This is crucial because the ECM architecture has been shown to regulate cellular phenotypes and organ physiology in recent studies7,43,45,51,55, whereas ECM mechanics have become increasingly appreciated in the field of cell biology over the past two decades56,57,58.
While some dECM-based biomaterial systems can provide tunable mechanics, although within a limited range and typically much lower than the original tissue stiffness and viscoelasticity21, the tunability depends on other ECM properties. For instance, when modifying the mechanics of lyophilized dECM, the ECM structure and composition will be influenced. On the other hand, emerging strategies in engineered heart tissues and three-dimensional dECM hydrogel models are capable of reproducing some aspects of tissue dimensionality and complexity59, yet face challenges in throughput and reproducibility43. Thus, our new DECIPHER approach not only faithfully mimics the native ECM composition and architecture but also provides independent mechanical tunability spanning a greater range of physiologically relevant stiffnesses due to the fact that the hydrogel component can be synthesized from ~1 kPa to 100 kPa (refs. 17,57). Furthermore, the interpenetrating network structure of the hydrogel–ECM hybrid enhances the viscoelastic properties of the scaffolds to more accurately mimic native tissues, which improves on conventional elastic materials or reconstituted dECM materials21.
Here we show that we can decouple the contributions of age-specific ECM ligand presentation and age-specific stiffness on cell behaviour using DECIPHER (Fig. 1). Importantly, we find that signals from the ECM can override stiffness-dependent mechanosignalling in age-related CF activation, matrix remodelling and the acquisition of senescent-like phenotypes (Fig. 6c), finding key roles of ligand-specific cues that have been shown to dictate other cellular behaviours, including collective migration and stem cell fate60,61. We further show that young ECM contributes to the rejuvenation potential of aged CFs through distinct signalling pathways, overriding substrate mechanics (Fig. 6e).
The ultimate application of this DECIPHER platform is to identify new targets for matrix-based rejuvenation strategies, and we found that young, soft ECM could enhance the rejuvenation potential of aged CFs. How CFs integrate biochemical, architectural and mechanobiological signalling cues to determine their phenotypical transition towards an aged, dysfunction state still requires further investigation with this system, and future studies will focus on the dynamics of this process, as previous reports have highlighted the importance of time dependence in cardiac function62,63. Taken together, we describe here a novel material platform that has widespread applicability to other tissues and/or disease types for which cell–ECM interactions play a crucial role.
Methods
Preparation of coverslips and hydrogel solutions
Coverslips (ϕ = 15 mm) were cleaned via sonication during progressive washes with acetone, ethanol and MilliQ water for 10 min each and then air dried. For hydrogel attachment, coverslips were treated with a UV/ozone cleaner (Bioforce ProCleaner Plus) for 5 min followed by immersion in a solution containing 20 ml of ethanol, 600 μl of 10% acetic acid and 200 μl of 3-trimethoxysilylpropylmethacrylate for 5 min. Coverslips were then washed twice in ethanol and air dried before placing onto the tissue slice and hydrogel solution. The PA-based hydrogels used in this study mimicking the young (~10 kPa) and aged (~40 kPa) cardiac tissue stiffness were labelled as ‘soft’ and ‘stiff’ for easy reference, with their composition detailed in Supplementary Table 1. Prepared solutions of acrylamide and bis-acrylamide were prereacted with formaldehyde at 4 °C for 3 h at a stoichiometric ratio of >10:1 to ensure no excess formaldehyde in the solution16. Before hydrogel formation, Irgacure 2959 (2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone, Sigma-Aldrich) was added for UV photoinitiation. For hydrogel visualization experiments, separate samples of 10-kPa solution were made with Nile Blue-tagged acrylamide (Polysciences no. 25395-100) at 1:1,000.
Tissue handling, slicing and stabilization
Young (1–2 months) and aged (18–24 months) hearts were extracted from C57BL/6J strain female mice (obtained via NUS Animal Tissue Sharing Programme under Institutional Animal Care and Use Committee, National University of Singapore) after euthanasia and quickly transferred to sterile 1× phosphate-buffered saline (PBS) to minimize blood clot formation. The major vascular tissues superiorly attached to the hearts were then carefully removed with sterile surgical instruments. Before slicing the heart using a vibratome (Leica VT 1200S), the heart was embedded in a 4% low-melting-point agarose (Invitrogen) solution in sterile 1× PBS, with the orientation adjusted such that coronal slices are obtained during slicing. Agarose was kept on ice until solidified, attached to the slicing stage with superglue and loaded onto the vibratome. Following the initial trimmings of the agarose cube, the slicing speed was set to 0.18 mm s–1 and 150-μm slice thickness.
After tissue sectioning, vibratome slices were carefully transferred to a hydrophobic glass slide (dichlorodimethylsilane-treated) in a humid condensation chamber. The agarose surrounding the cardiac tissue was gently removed, as well as any excess liquid on the cardiac slices, by wicking away at the edges with a Kimwipe. Then, 21 ± 2 μl of the hydrogel solutions were added to each 150-μm-thick cardiac slice (solution volume was adjusted based on the volume of the tissue to fill the coverslip area) and incubated at 4 °C for 60 min in the dark. Crosslinking was then initiated using the same parameters as the plain hydrogel samples (35 mW cm–2 for 3 min). The samples were then transferred to a 12-well plate and kept in sterile MilliQ water at 4 °C before decellularization.
Decellularization of PA-stabilized cardiac tissue sections
All solutions were aseptically prepared, and all the experiment areas were sterilized frequently with 70% ethanol to prevent microbial contamination. SDC (Sigma-Aldrich) was dissolved in MilliQ water at 5% (w/v). The PA-stabilized tissue samples were exchanged with 2 ml per well of SDC solution and kept on a gentle shaker at room temperature for 2.5 days with one exchange at 24 h. The samples were then washed with MilliQ water and 10,000 U penicillin/streptomycin (pen/strep, Gibco) for 2 h. Subsequently, 2 ml of 300 kU ml–1 DNase-I solution (Sigma-Aldrich; dissolved in 0.15-M NaCl supplemented with 5-mM CaCl2 and 1% pen/strep) was added to each sample after discarding the pen/strep and gently shaking for 3.5 days followed by a final MilliQ water wash for 2 h.
Mass spectrometry
Freshly harvested and sliced cardiac tissues from young (2 months) or aged (24 months) mice were decellularized using the same protocol as described in the previous section using SDC and DNase-I. Then, dECM samples were snap frozen using liquid nitrogen and shipped on dry ice to BGI Genomics for further extraction steps followed by tandem-mass-tag-based quantitative mass spectrometry. Samples were ultrasonicated in an extraction cocktail containing EDTA and SDS-lysate buffer (7-M urea, 2-M thiourea, 20-mM Tris-HCl, pH 8.0) for 5 min on ice and centrifuged at 25,000g for 15 min at 4 °C. The supernatant was incubated in dithiothreitol (10 mM) and incubated at 37 °C for 30 min, followed by iodoacetamide (55 mM) treatment for 45 min. Proteins were precipitated using cold acetone at –20 °C and collected by a centrifuge at 25,000g for 15 min at 4 °C. Protein pellets were resuspended in a lysis buffer without SDS-lysate and centrifuged again to remove any insoluble residues. Quality control was performed after each round of protein extraction by Bradford quantification and sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Then, 0.5-M tetraethylammonium bromide was used to collect the protein by centrifuging three rounds at 12,000g and 20 °C, followed by trypsin digestion. Subsequently, peptides were labelled with a tandem mass tag for 2 h at room temperature, fractionized using Shimadzu LC-20AB liquid-phase system and freeze dried. The dried peptide samples were then injected into a Thermo Scientific UltiMate 3000 UHPLC connected to a mass spectrometer (Thermo Scientific Q-Exactive HF X). The ion source voltage was set to 1.9 kV; the MS1 scanning range was 350–1,500m/z and the resolution was set to 60,000; the starting m/z of MS2 was fixed at 100 and the resolution was 15,000.
Reconstituted solubilized dECM substrates
Young (1–2 months) or aged (18–24 months) murine hearts were sliced and decellularized using the same protocol as described in the previous section with SDC and DNase-I. The dECM samples were then snap frozen in liquid nitrogen and lyophilized overnight. Lyophilized dECM was then pulverized and solubilized2. Briefly, 1 mg ml–1 pepsin (Roche) was dissolved in 0.1-M HCl and added to milled dECM at a 10:1 ratio (w/w, dECM:pepsin) followed by constant stirring at room temperature. When a homogeneous solution was obtained, 1-M NaOH was used to neutralize the supernatant after removing insoluble residues. A Pierce BCA Protein Assay Kit (Thermo Scientific) was then used to quantify the total ECM protein amount. Solubilized dECM was diluted to 1 mg ml–1 with 1× PBS, coated onto 15-mm coverslips and incubated for 30 min in a condensation chamber at 37 °C.
ECM quantitative compositional assays
Quantitative compositional analyses of samples were performed for DNA, sGAG and collagen content. The sample’s DNA was extracted using phenol–chloroform and kept at −20 °C until applying the PicoGreen dsDNA assay kit (Invitrogen). Total sGAGs of the samples were obtained via papain (Sigma-Aldrich) extraction followed by Blyscan sGAG assay (Biocolor). For collagen quantification, the samples were first homogenized and hydrolysed in concentrated HCl at 120 °C for 3 h before being quantified using a hydroxyproline assay kit (Sigma-Aldrich). A microplate reader (Promega GloMax) was used for all the assays. Duplicate technical replicates with N = 3 biological replicates were measured along with standard curves for quantification, according to the suppliers’ instructions.
IHC
IHC of the native tissue sections and DECIPHER samples was carried out for ECM components and cellular structures (antibodies and dilutions are detailed in Supplementary Table 2). Briefly, samples were fixed with 4% formaldehyde (Sigma-Aldrich) and rinsed with 1× PBS before blocking and permeabilizing with 2% bovine serum albumin (BSA; Sigma-Aldrich) in 0.2% Triton X-100 (Sigma-Aldrich) for 30 min. The samples were then incubated with primary antibodies in 2% BSA on a gentle shaker at room temperature for 3 days followed by three times of 1× PBS washing and incubation of secondary antibodies and CF Phalloidin (Biotium) staining for 1 h at room temperature in the dark. To label any denatured collagen, Cy3-conjugated collagen hybridizing peptide (R-CHP, 3Helix) was diluted to 5 μM and used to stain the samples after CNA35 labelling according to the product’s instructions64,65. The samples were further stained with Hoechst 33342 (Invitrogen) and mounted using Fluoromount-G (Invitrogen). For hydrogel visualization experiments using Nile Blue-tagged acrylamide, the samples were co-stained with CNA35.
Immunogold labelling and SEM
SEM was used to visualize the nanoarchitecture of PA-hydrogel-stabilized cardiac tissue after decellularization (DECIPHER samples) and native cardiac tissue cryosections cut at 10 µm (Leica CM1950) from snap-frozen fresh young hearts embedded in OCT (Tissue-Tek, Sakura Finetek). For immunogold labelling, the samples were first fixed with 4% formaldehyde and rinsed with 1× PBS before blocking with 3% goat serum and 1% BSA for 30 min. Endogenous mouse IgG was blocked followed by incubation with a site-specific primary antibody. Samples were then rinsed with PBS + 0.1% Tween 20 and incubated with secondary antibody conjugated with 10-nm gold (Invitrogen) according to the supplier’s instructions. Immunogold-labelled samples were then rinsed and enhanced using Nanoprobes GoldEnhance EM Plus kit to improve the SEM visualization. Before SEM, all the samples were fixed with glutaraldehyde (Sigma-Aldrich) followed by dehydration using a series of ethanol exchanges of increasing concentration and dried using a Critical Point Dryer (Tousimis Autosamdri-815). The samples were coated with 8-nm platinum (for native tissue samples and unstained DECIPHER samples) or carbon (for immunogold samples) before being imaged using a Hitachi Regulus 8230 FE-SEM at 1–3-kV acceleration voltage and 8-mm working distance.
Nanoindentation
To obtain the Young modulus of PA hydrogels and PA-hydrogel-stabilized cardiac tissue slices pre- and post-DECIPHER, the samples were attached to glass-bottom Petri dishes with superglue. For native tissues, vibratome slices were prepared at 500 μm and attached to dishes with GLUture (World Precision Instruments, 503763). All the samples were then indented with the Optics11 Life Chiaro Nanoindenter using a spherical probe (k = 0.5 N m–1; tip radius, 50 μm). To minimize sample–probe adhesion, 1% Pluronic (Sigma-Aldrich) solution was used to coat the probe before the experiments. Calibration was carried out against a glass dish. Quasi-static matrix scans were performed with a 2-μm indentation depth and step sizes of 500 μm (native tissue, PA-stabilized tissue and DECIPHER samples) or 800 μm (plain PA hydrogel samples). The Young modulus of each data point was obtained from the Hertzian contact model with Poisson’s ratio ν = 0.5 in the Optics11 DataViewer software (v.2.7.0).
Dynamic mechanical analysis was used to measure the storage and loss moduli with a 2-μm indentation depth and 300-nm oscillation amplitude. The maximum indentation depth in the dynamic mechanical analysis was reached at 0.1 s, followed by 20-s relaxation, and then an oscillatory frequency sweep at 1, 2, 4 and 10 Hz was performed. Five periods of oscillation were carried out for all frequencies, except in the 10-Hz experiment, where ten periods were used to ensure a minimum oscillation time of 1 s. The storage modulus (E′) and loss modulus (E″) were calculated using the Optics11 DataViewer software.
Correlative confocal–AFM
AFM calibrations and measurements were performed after confocal imaging23. Briefly, a JPK NanoWizard 4 XP Bioscience AFM system with a BioMAT Workstation (Bruker) were used in conjunction with an upright Stellaris 8 laser scanning confocal microscope (Leica) equipped with a customized BioMAT shuttle stage, which enabled the precise positioning and alignment of the measured areas for the sequential confocal–AFM scans. A triangular cantilever (Nanoworld, Pyrex-Nitride Probe) with a nominal spring constant of 0.32 N m–1 with a pyramidal tip of 35° face half-angle were used. Calibration for deflection sensitivity and the spring constant was done under water with the contact-based calibration mode on a glass slide. The manufacturer’s calibration slide (Bruker) was used to optically align the confocal and AFM scan areas using the DirectOverlay function of the JPK NanoWizard data processing software (v.8.0.144), and minor alignment corrections were performed manually in post-processing.
The DECIPHER and native tissue samples were first stained with CNA35 without fixation (to provide live visualization of the collagen ECM) and imaged under the confocal microscope to obtain a three-dimensional stack and locate the regions of interest of the exposed ECM (collagen) fibres and the surrounding hydrogel (for DECIPHER). The samples were then transferred to the AFM system on the shuttle stage to perform surface topographic scans using the cells in the liquid quantitative imaging mode (force setpoint, 15–25 µN; indentation depth, 0.5–2 µm; Z speed, 25–40 µm; Z length, 4–5 µm; scan rate, 25–40 kHz; pixel time, 20 ms; x–y resolution: –312.50 nm per pixel). The scan sizes ranged in 25 × 25 µm2 to 50 × 50 µm2 with a resolution from 32 × 32 pixels to 80 × 80 pixels, respectively. After scanning, all the quantitative imaging images and force–distance curves were analysed in the JPK NanoWizard data processing software (v.8.0.144). The force–distance curves underwent baseline correction and smoothing before contact-point determination and fitting for Young’s moduli. The apparent Young’s modulus for each pixel in the quantitative imaging image was obtained by fitting a Sneddon contact model for the whole approach curve with ν = 0.5 to obtain the modulus maps. For quantification, three to five regions of interest with sizes of 4–6.25 µm2 were selected on the fibre and on the surrounding gel (for DECIPHER) to derive the regional average moduli.
Primary CF isolation and in vitro culture
The use of animals was approved by the Institutional Animal Care and Use Committee (protocol no. R22-0579), National University of Singapore. Mice are housed in individually ventilated cages with sex-matched littermates, 12-h light–dark cycles, ambient temperature (20–24 °C) and humidity (30%–70%), and ad libitum food and water supply. Primary young and aged CFs were extracted66 from female young (1–2 months) and aged (24 months) C57BL/6J murine hearts, respectively. Briefly, mouse hearts were perfused with cardiac digestion buffers by intraventricular injection. Three rounds of gravity settling were applied to separate cardiomyocyte and non-cardiomyocyte cells. Non-cardiomyocyte cells were seeded onto untreated tissue culture plastic and washed after 1 h to enrich for CFs. CFs were expanded in CF growth medium (Dulbecco’s modified Eagle’s medium/high glucose (Cytiva) supplemented with 15% fetal bovine serum (Gibco)), penicillin (100 U ml–1), streptomycin (100 µg ml–1, Nacalai Tesque), FGF2 (10 ng ml–1, Stemcell Technologies) and SB-431542 (5 µM, Targetmol).
All cells used in the in vitro studies were from passages 2 or 3. Before cell seeding, all the DECIPHER samples were immersed in sterile 1× PBS overnight, UV sterilized in a biosafety cabinet for 60 min and equilibrated in full maintenance media for 30 min. The isolated cells were seeded at 10,000 cells per well of a 12-well plate in 1 ml of culture media (Dulbecco’s modified Eagle’s medium + 1% fetal bovine serum + 1% pen/strep) onto DECIPHER samples and kept in a 37 °C, 5% CO2, humidified incubator for a culture period of 2 days. Cell culture supernatant was regularly tested negative for mycoplasma contamination using InvivoGen’s MycoStrip.
ICC
After culturing, cells were rinsed in sterile 1× PBS three times and then fixed with 4% formaldehyde for 15 min, permeabilized with 0.1% Triton X-100 for 15 min and blocked with 2% BSA for 30 min for ICC. For mouse-anti-mouse primary antibodies, 2% goat serum with 1% BSA was used instead as the blocking and staining buffer, with an extra step of blocking endogenous mouse IgG for 1 h at room temperature to reduce the background noise from the ECM. Samples were stained with primary antibodies (Supplementary Table 2) overnight at 4 °C in 2% BSA, rinsed three times in 1× PBS + 0.1% Tween 20, followed by secondary antibodies and CF Phalloidin for 1 h at room temperature diluted in 2% BSA. The samples were washed with PBS + 0.1% Tween 20 three times (5 min each) and stained with Hoechst 33342 in H2O for 10 min at room temperature before three rounds of water rinses and mounted with Fluoromount-G.
Low-input bulk RNA-seq
All the reagents are DNase/RNase/protease free and diluted with HyPure Molecular Biology Grade Water (Cytiva). Cells attached to the DECIPHER samples were lysed using TRIzol (Ambion) after scraping away the excess hydrogel region and kept under −80 °C pending RNA extraction. To further provide baseline (in vivo) control groups, freshly isolated young/aged primary CFs were directly lysed with TRIzol after extraction. Total RNA of the samples was extracted using a chloroform–isopropanol approach according to the supplier’s instructions. Briefly, chloroform was added to the lysed cell samples after thawing and vigorously mixed and centrifuged at 14,000g for 15 min before the supernatant was collected. RNA was then precipitated using cold isopropanol, collected by centrifugation at 14,000g for 15 min and washed twice with precooled 75% ethanol followed by centrifugation at 14,000g for 10 min. The RNA samples were air dried for 5–10 min before being dissolved in HyPure water and processed for quality control with TapeStation High Sensitivity RNA ScreenTape Analysis. Sample RNA with an RNA integrity number (RINe) larger than 8.5 (high quality) was applied to NEBNext Single Cell/Low Input RNA Library Prep Kit for Illumina for cDNA synthesis and sequence-ready library preparation according to the supplier’s instructions. Specifically, 20 ng of total RNA from each sample was used for cDNA synthesis and eight PCR cycles were used for cDNA amplification. The libraries were indexed with NEBNext Multiplex Oligos for Illumina (96 Unique Dual Index Primer Pairs Set 2) and submitted to NovogeneAIT Genomics (Singapore) for sequencing on the Illumina NovaSeq 6000 system.
Multiplex RT-qPCR
RNA extraction and quality control were performed as described in the RNA-seq section. Subsequently, a Reliance One-Step Multiplex RT-qPCR Supermix (Bio-Rad) was used to perform reverse transcription and qPCR by PrimePCR Probe Assays built in a Bio-Rad CFX96 Real-Time PCR Detection System. The thermal cycling protocol was set to the Bio-Rad product sheet default: 50 °C for 10 min (RT), 95 °C for 10 min (DNA polymerase activation and template denaturation) and 40 cycles of 95 °C for 10 s followed by 60 °C for 30 s (amplification). All the PrimePCR Probes used in this study were purchased from Bio-Rad (Supplementary Table 3), which had been optimally designed and validated for the multiplex qPCR supermix and experiments.
Imaging and analysis
The IHC and ICC samples were observed using a Nikon A1R scanning laser confocal microscope (×40 and ×60 water-immersion objectives, NIS Elements v. 5.30.05) or Oxford Instruments BC43 spinning-disc confocal microscope (×20 and ×40 air objectives, Fusion v. 2.3) and subsequently imported to Fiji. A Fiji macro, TWOMBLI, was used to quantify the ECM architectural changes post-decellularization and in ageing with the default parameters20. Confocal images from pre- and post-decellularized samples were analysed for lacunarity, endpoints, branch points, fractal dimension, alignment and hyphal growth unit, with a principal component analysis performed in GraphPad Prism v. 10. Confocal imaging was performed on Nile Blue-tagged samples from the sample surface and reconstructed using Imaris. LOX and NF-κB staining on DECIPHER and native tissue samples were quantified for their co-localization with collagen (CNA35) using Fiji JaCoP macro67. ICC samples stained with α-SMA, integrin α5, LOX, p53, TIMP3 and F-actin were analysed with CellProfiler for intensity and morphological metrics. Nucleus/cytosol localization ratio of YAP was quantified using CellProfiler (v.4.2.5) by identifying the fluorescence from a 30-pixel perinuclear ring as the cytosolic localization. Paxillin quantification was performed using a custom Fiji script in which focal adhesion sites were identified using auto local threshold (Bernsen) followed by particle analysis.
Bioinformatics analysis
For mass spectrometry data, the raw reads were obtained with FDR ≤ 1% and quantitative analysis was performed based on the peak intensity, peak area and liquid chromatography retention time of the samples, followed by a series of statistical analysis and quality control. The protein identification results were based on UniProt Protein Database (Mus musculus). Subsequent bioinformatics analysis and data visualization were processed using BGI’s Dr. Tom online analysis platform. Additional data visualization was performed using OriginPro 2024 and TBtools68.
RNA-seq reads were aligned to the mouse genome assembly mm39 (BioProject no. PRJNA20689) with STAR (v.2.7.8a). The aligned reads were quantified using HTSeq (0.11.0) with Ensembl transcripts release 105. The above computation was performed within the PartekFlow cloud environment with default parameters (v.10.0.23.0425). DEG analysis was done with the DESeq2 package69 (Bioconductor 3.17) and clusterProfiler package70,71 (Bioconductor 3.17) was used to perform the functional enrichment analysis. Cellinker31 was used to correlate the ligand–receptor interaction between ECM proteomics and CF RNA. Data visualization was performed using OriginPro 2024, GraphPad Prism 9, MATLAB R2023a and TBtools68.
Statistics and reproducibility
No statistical method was used to predetermine the sample size. Sample sizes were determined based on previous studies in similar fields. Unless otherwise noted, all the experiments were performed as triplicate independent experiments (N ≥ 3). Detailed sample sizes can be found in the respective figure captions. The number of cells analysed are reported in each figure with individual data points as well as in Supplementary Table 4. Data are expressed as the mean ± standard deviation (s.d.) or standard error of the mean (s.e.m.), with the sample size and applied statistical analysis specified in each figure caption. Statistical analyses were performed using GraphPad Prism v. 9 and v. 10. All the box graphs shown in the figures have the box range showing the interquartile data, centre of the box represents the median, ‘+’ mark represents the mean, and whiskers represent the minimum and maximum values. Representative IHC and ICC images shown in this study were successfully reproduced with similar results in three independent attempts. Other representative micrographs, such as Figs. 1c and 2a,b, were successfully reproduced with similar results for more than ten times throughout this study. No data were excluded from the analyses. In vitro experiments and samples used were randomly allocated. The investigators were blinded during data collection but not blinded during outcome assessment because the analyses were done objectively with automated scripts and codes.
Heat maps demonstrating the DEG analysis in the RNA-seq data were graphed by averaging the replicates, with the original (unaveraged) readings and dendrograms shown in Extended Data Fig. 4 (except in Fig. 3b where the original data are shown). The heat map in Fig. 3j is shown following the order of the other DEG heat maps, with the original clustering dendrogram shown in Extended Data Fig. 4a. The gene expression results for the DECIPHER samples are normalized to the young and aged groups of their respective in vivo mimic, that is, young CFs are normalized to the young ECM in a soft matrix (young CFs on SoftY), whereas aged CFs are normalized to the aged ECM in a stiff matrix (aged CFs on StiffA). The Ct (cycle threshold) values of RT-qPCR results were obtained via Bio-Rad CFX Maestro software (v.2.3) and analysed by the 2−ΔΔCt method72 against glyceraldehyde 3-phosphate dehydrogenase and the aforementioned in vivo mimic groups.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information. Raw images are available from the corresponding author upon request due to large file sizes. The mass spectrometry proteomics data have been deposited in the ProteomeXchange Consortium in the PRIDE repository with dataset identifier PXD060864. The RNA-seq data have been deposited in the NCBI GEO repository with accession code GSE289885. Source data are provided with this paper.
Code availability
All codes and scripts newly written in this study can be accessed via Zenodo at https://doi.org/10.5281/zenodo.15080541 (ref. 73) and via GitHub at https://github.com/onghuiting/focal_adhesion_analysis.
References
Nance, M. E. et al. Attenuated sarcomere lengthening of the aged murine left ventricle observed using two-photon fluorescence microscopy. Am. J. Physiol. Circ. Physiol. 309, H918–H925 (2015).
Ozcebe, S. G., Bahcecioglu, G., Yue, X. S. & Zorlutuna, P. Effect of cellular and ECM aging on human iPSC-derived cardiomyocyte performance, maturity and senescence. Biomaterials 268, 120554 (2021).
Meschiari, C. A., Ero, O. K., Pan, H., Finkel, T. & Lindsey, M. L. The impact of aging on cardiac extracellular matrix. Geroscience 39, 7–18 (2017).
Antia, M., Baneyx, G., Kubow, K. E. & Vogel, V. Fibronectin in aging extracellular matrix fibrils is progressively unfolded by cells and elicits an enhanced rigidity response. Faraday Discuss. 139, 229–249 (2008).
Yokota, T. et al. Type V collagen in scar tissue regulates the size of scar after heart injury. Cell 182, 545–562 (2020).
Herum, K. M., Choppe, J., Kumar, A., Engler, A. J. & McCulloch, A. D. Mechanical regulation of cardiac fibroblast profibrotic phenotypes. Mol. Biol. Cell 28, 1871–1882 (2017).
Wang, Z. et al. Snake venom-defined fibrin architecture dictates fibroblast survival and differentiation. Nat. Commun. 14, 1029 (2023).
Tomasek, J. J., Gabbiani, G., Hinz, B., Chaponnier, C. & Brown, R. A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Bio. 3, 349–363 (2002).
Talman, V. & Ruskoaho, H. Cardiac fibrosis in myocardial infarction—from repair and remodeling to regeneration. Cell Tissue Res. 365, 563–581 (2016).
Angelini, A., Trial, J., Ortiz-Urbina, J. & Cieslik, K. A. Mechanosensing dysregulation in the fibroblast: a hallmark of the aging heart. Ageing Res. Rev. 63, 101150 (2020).
Weber, K. T., Sun, Y., Bhattacharya, S. K., Ahokas, R. A. & Gerling, I. C. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat. Rev. Cardiol. 10, 15–26 (2013).
Cieslik, K. A., Trial, J., Carlson, S., Taffet, G. E. & Entman, M. L. Aberrant differentiation of fibroblast progenitors contributes to fibrosis in the aged murine heart: role of elevated circulating insulin levels. Faseb J. 27, 1761–1771 (2013).
Chen, Z. et al. Intrafibrillar crosslinking enables decoupling of mechanical properties and structure of a composite fibrous hydrogel. Adv. Mater. 36, e2305964 (2023).
Cunha, C. Bda et al. Influence of the stiffness of three-dimensional alginate/collagen-I interpenetrating networks on fibroblast biology. Biomaterials 35, 8927–8936 (2014).
Berger, A. J., Linsmeier, K. M., Kreeger, P. K. & Masters, K. S. Decoupling the effects of stiffness and fiber density on cellular behaviors via an interpenetrating network of gelatin-methacrylate and collagen. Biomaterials 141, 125–135 (2017).
Chung, K. et al. Structural and molecular interrogation of intact biological systems. Nature 497, 332–337 (2013).
Tse, J. R. & Engler, A. J. Preparation of hydrogel substrates with tunable mechanical properties. Curr. Protoc. Cell Biol. 47, 10.16.1–10.16.16 (2010).
Crapo, P. M., Gilbert, T. W. & Badylak, S. F. An overview of tissue and whole organ decellularization processes. Biomaterials 32, 3233–3243 (2011).
Großkopf, H. et al. Identification of intracellular glycosaminoglycan-interacting proteins by affinity purification mass spectrometry. Biol. Chem. 402, 1427–1440 (2021).
Wershof, E. et al. A FIJI macro for quantifying pattern in extracellular matrix. Life Sci. Alliance 4, e202000880 (2021).
Chaudhuri, O., Cooper-White, J., Janmey, P. A., Mooney, D. J. & Shenoy, V. B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546 (2020).
Ma, Y. et al. Viscoelastic cell microenvironment: hydrogel‐based strategy for recapitulating dynamic ECM mechanics. Adv. Funct. Mater. 31, 2100848 (2021).
Bovio, S., Long, Y. & Monéger, F. Use of atomic force microscopy to measure mechanical properties and turgor pressure of plant cells and plant tissues. J. Vis. Exp. https://doi.org/10.3791/59674 (2019).
Nakayama, K. H., Batchelder, C. A., Lee, C. I. & Tarantal, A. F. Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Eng. A 16, 2207–2216 (2010).
Fathi, I. et al. Decellularized whole-organ pre-vascularization: a novel approach for organogenesis. Front. Bioeng. Biotechnol. 9, 756755 (2021).
Sun, A. R., Hengst, R. M. & Young, J. L. All the small things: nanoscale matrix alterations in aging tissues. Curr. Opin. Cell Biol. 87, 102322 (2024).
Jeong, D. et al. Matricellular protein CCN5 reverses established cardiac fibrosis. J. Am. Coll. Cardiol. 67, 1556–1568 (2016).
Chen, M. S., Lee, R. T. & Garbern, J. C. Senescence mechanisms and targets in the heart. Cardiovasc. Res. 118, 1173–1187 (2021).
Pesce, M. et al. Cardiac fibroblasts and mechanosensation in heart development, health and disease. Nat. Rev. Cardiol. 20, 309–324 (2022).
Sun, S.-N. et al. G-MDSCs promote aging-related cardiac fibrosis by activating myofibroblasts and preventing senescence. Cell Death Dis. 12, 594 (2021).
Zhang, Y. et al. Cellinker: a platform of ligand–receptor interactions for intercellular communication analysis. Bioinformatics 37, 2025–2032 (2021).
Kanchanawong, P. & Calderwood, D. A. Organization, dynamics and mechanoregulation of integrin-mediated cell–ECM adhesions. Nat. Rev. Mol. Cell Biol. 24, 142–161 (2022).
He, J., Fang, B., Shan, S. & Li, Q. Mechanical stiffness promotes skin fibrosis through piezo1-mediated arginine and proline metabolism. Cell Death Discov. 9, 354 (2023).
Rao, L.-Z. et al. IL-24 deficiency protects mice against bleomycin-induced pulmonary fibrosis by repressing IL-4-induced M2 program in macrophages. Cell Death Differ. 28, 1270–1283 (2021).
Goldsmith, E. C., Bradshaw, A. D., Zile, M. R. & Spinale, F. G. Myocardial fibroblast–matrix interactions and potential therapeutic targets. J. Mol. Cell. Cardiol. 70, 92–99 (2014).
Li, R. & Frangogiannis, N. G. Integrins in cardiac fibrosis. J. Mol. Cell. Cardiol. 172, 1–13 (2022).
Roca-Cusachs, P., Gauthier, N. C., Rio, Adel & Sheetz, M. P. Clustering of α5β1 integrins determines adhesion strength whereas αvβ3 and talin enable mechanotransduction. Proc. Natl Acad. Sci. USA 106, 16245–16250 (2009).
Schiller, H. B. et al. β1- and αv-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat. Cell Biol. 15, 625–636 (2013).
Stanton, A. E., Tong, X. & Yang, F. Extracellular matrix type modulates mechanotransduction of stem cells. Acta Biomater. 96, 310–320 (2019).
Ma, Y., Iyer, R. P., Jung, M., Czubryt, M. P. & Lindsey, M. L. Cardiac fibroblast activation post-myocardial infarction: current knowledge gaps. Trends Pharm. Sci. 38, 448–458 (2017).
Bugg, D. et al. Infarct collagen topography regulates fibroblast fate via p38-yes-associated protein transcriptional enhanced associate ___domain signals. Circ. Res. 127, 1306–1322 (2020).
Ozcebe, S. G. & Zorlutuna, P. In need of age‐appropriate cardiac models: impact of cell age on extracellular matrix therapy outcomes. Aging Cell 22, e13966 (2023).
Cho, S., Discher, D. E., Leong, K. W., Vunjak-Novakovic, G. & Wu, J. C. Challenges and opportunities for the next generation of cardiovascular tissue engineering. Nat. Methods 19, 1064–1071 (2022).
Klein, J. et al. Proteasix: a tool for automated and large‐scale prediction of proteases involved in naturally occurring peptide generation. Proteomics 13, 1077–1082 (2013).
Devarasou, S., Kang, M., Kwon, T. Y., Cho, Y. & Shin, J. H. Fibrous matrix architecture-dependent activation of fibroblasts with a cancer-associated fibroblast-like phenotype. ACS Biomater. Sci. Eng. 9, 280–291 (2023).
DeLeon-Pennell, K. Y., Meschiari, C. A., Jung, M. & Lindsey, M. L. Chapter two—matrix metalloproteinases in myocardial infarction and heart failure. Prog. Mol. Biol. Transl. Sci. 147, 75–100 (2017).
Mizuno, T., Yau, T. M., Weisel, R. D., Kiani, C. G. & Li, R.-K. Elastin stabilizes an infarct and preserves ventricular function. Circulation 112, I81–I88 (2005).
Meyer, K., Hodwin, B., Ramanujam, D., Engelhardt, S. & Sarikas, A. Essential role for premature senescence of myofibroblasts in myocardial fibrosis. J. Am. Coll. Cardiol. 67, 2018–2028 (2016).
Zhu, F. et al. Senescent cardiac fibroblast is critical for cardiac fibrosis after myocardial infarction. PLoS ONE 8, e74535 (2013).
Coppé, J.-P., Desprez, P.-Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
DePalma, S. J. et al. Matrix architecture and mechanics regulate myofibril organization, costamere assembly, and contractility in engineered myocardial microtissues. Adv. Sci. 11, 2309740 (2024).
Basara, G., Ozcebe, S. G., Ellis, B. W. & Zorlutuna, P. Tunable human myocardium derived decellularized extracellular matrix for 3D bioprinting and cardiac tissue engineering. Gels 7, 70 (2021).
Kim, H. et al. Light‐activated decellularized extracellular matrix‐based bioinks for volumetric tissue analogs at the centimeter scale. Adv. Funct. Mater. 31, 2011252 (2021).
Spang, M. T. et al. Intravascularly infused extracellular matrix as a biomaterial for targeting and treating inflamed tissues. Nat. Biomed. Eng. 7, 94–109 (2023).
Umehara, T. et al. Female reproductive life span is extended by targeted removal of fibrotic collagen from the mouse ovary. Sci. Adv. 8, eabn4564 (2022).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Hadden, W. J. et al. Stem cell migration and mechanotransduction on linear stiffness gradient hydrogels. Proc. Natl Acad. Sci. USA 114, 5647–5652 (2017).
Iskratsch, T., Wolfenson, H. & Sheetz, M. P. Appreciating force and shape—the rise of mechanotransduction in cell biology. Nat. Rev. Mol. Cell Biol. 15, 825–833 (2014).
Min, S. et al. Versatile human cardiac tissues engineered with perfusable heart extracellular microenvironment for biomedical applications. Nat. Commun. 15, 2564 (2024).
Russo, J. D. et al. Integrin α5β1 nano-presentation regulates collective keratinocyte migration independent of substrate rigidity. eLife 10, e69861 (2021).
Trappmann, B. et al. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 11, 642–649 (2012).
Young, J. L. & Engler, A. J. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials 32, 1002–1009 (2011).
Walker, C. J. et al. Nuclear mechanosensing drives chromatin remodelling in persistently activated fibroblasts. Nat. Biomed. Eng. 5, 1485–1499 (2021).
Hwang, J. et al. Molecular assessment of collagen denaturation in decellularized tissues using a collagen hybridizing peptide. Acta Biomater. 53, 268–278 (2017).
Aper, S. J. A. et al. Colorful protein-based fluorescent probes for collagen imaging. PLoS ONE 9, e114983 (2014).
Ackers-Johnson, M. & Foo, R. S. Langendorff-free isolation and propagation of adult mouse cardiomyocytes. Methods Mol. Biol. 1940, 193–204 (2019).
Bolte, S. & Cordelières, F. P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 (2006).
Chen, C. et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 13, 1194–1202 (2020).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Wu, T. et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation 2, 100141 (2021).
Yu, G., Wang, L.-G., Han, Y. & He, Q.-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001).
Ting, O. H. Hybrid hydrogel-extracellular matrix scaffolds identify biochemical and mechanical signatures of cardiac aging. Zenodo https://doi.org/10.5281/zenodo.15080541 (2025).
Acknowledgements
We thank J. Marlena (Mechanobiology Institute (MBI), National University of Singapore (NUS)) for illustrations; C. J. Chan, V. Prabhakaran and B. H. Ng (MBI, NUS) for providing hyaluronan binding protein and vibratome for usage; R. Li, A. Holle, J. Yan, H. H. Susapto, H. T. Ong, R. D. Mets, R. M. Hengst, X. Gao, Y. Li, J. P. Singh (MBI, NUS), J. J. Zhou (Electrical and Computer Engineering, NUS) and C. Mellace (Optics11 Life) for fruitful discussions and technical advice; H. T. Ong (MBI, NUS) for image analysis codes; X. Zhang (Department of Biological Sciences, NUS) for assistance with optimizing the AFM experiments; and T. Luu (Cardiovascular Research Institute, National University Health System) for assistance with animal handling. We thank the MBI Wet Lab Core for facility and technical support, the Singapore Microscopy and Bioimaging Analysis (SiMBA, MBI) core for microscopy and data processing facilities and the High-throughput Molecular Genetics (HMG, MBI) core for RNA-seq support. This work was supported by the Ministry of Education under the Research Centres of Excellence programme through the Mechanobiology Institute at the National University of Singapore and the Biomedical Engineering Department at the National University of Singapore, as well as the Singapore Ministry of Education Academic Research Fund Tier 3 (MOE grant no. MOET32021-0003) to J.L.Y.
Author information
Authors and Affiliations
Contributions
J.L.Y. and A.R.S. conceptualized the project and wrote the paper. J.L.Y. supervised the data collection and analysis. A.R.S. performed majority of the experiments and data analysis. M.F.H.R., X.S. and D.C. assisted in the bench experiments related to ECM staining, imaging and data analysis. X.S. assisted in tissue harvesting and mice handling. K.K.R. and Y.L. performed the correlative confocal–AFM experiments. Y.H., P.V. and M.A.-J. contributed to the revision experiments (not included in the paper). M.A.-J. performed the primary cell isolations. J.Z. assisted in the RNA-seq experiments and analysis. R.S.F. provided the key reagents and contributed to the discussion.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Feng Zhao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of DECIPHER samples.
Nomenclatures used in this study describing DECIPHER samples before (a) and after cell seeding (b). Assay for dsDNA content in native tissue (‘Native’) vs. DECIPHER samples, with the accepted decellularization threshold of 50 ng/mg indicated by the dashed line (c). Hydroxyproline (collagen) content (d), and sGAG content (e) in Pre-DECIPHER vs. DECIPHER samples. The number of endpoints per micron (hyphal growth factor, HGU) is the most age-regulated ECM architectural factor identified by TWOMBLI (f). IHC images of collagen hybridizing peptide (CHP) and collagen stained samples of Pre-DECIPHER, DECIPHER, SDS + Triton X-100 +/− PA stabilization (g), quantified in (h). IHC staining of intracellular transcription factor NF-kB1 (i) and quantified in (j) as negative control for LOX area (k) and localization (l) quantification. Gene ontology (GO) enrichment analysis of ECM mass spectrometry data, where bubble size indicates number of DEPs in each set and color bar indicates adjusted significance by Q-value (m). Highlighted matrix-immobilized molecules (n) and ECM proteins (o) quantified by mass spectrometry. Protein-protein interaction (PPI) analysis of differentially expressed proteins (p). Collagen I/III ratio increased 9.4% in aged cardiac ECM calculated from mass spectrometry intensity using propagation of uncertainty (q). Color bar indicates row-standardized expression (n, o). Data are mean \(\pm \,\)SD (c-f, h, q). N = 3 biological replicates for each condition in (c-o). One-way ANOVA with Dunn’s multiple comparison (h, j, k), unpaired two-tailed t-test (l). ns, not significant (p > 0.05). Scale bar: 50 µm (g), 100 µm (i).
Extended Data Fig. 2 Stiffness mapping of pre- and post-DECIPHER samples reveals scaffold tunability.
Nanoindentation maps of Pre-DECIPHER and DECIPHER samples of young (Y) and aged (A) tissue with soft or stiff hydrogel component (a). Storage (b) and loss (c) moduli of DECIPHER samples at 1 Hz oscillation measured by dynamic mechanical analysis. Storage modulus, loss modulus, and tanδ of plain soft (d) or stiff PA hydrogel (e), as well as SoftY (f) or StiffY (g) DECIPHER samples fabricated with or without formaldehyde (+FA or -FA, respectively). Examples of correlative confocal-atomic force microscopy maps for tissue (‘Control (cryosection)’) and DECIPHER samples (h). Top row images are maximum intensity projections (MIPs) of the confocal stacks live stained with CNA35 (collagen). Bottom row images are AFM stiffness (E, kPa) maps. Quantified regions of interests (hydrogel vs. collagen regions) from the AFM maps are indicated. N = 3 (b-g) or 2 (h) biological replicates for each condition. StiffY image is a zoomed-in version of Fig. 2j. Scale bar: 5 µm (h).
Extended Data Fig. 3 Additional CF gene expression levels quantified by RNA-seq.
Normalized gene expression on different DECIPHER samples (SoftY, StiffY, SoftA, StiffA). Quantified RNA-seq data for genes regulating young or aged CF activation (a), mechanosensing (b, c, d, e), ECM remodeling (f, g, h, i, j, k, l), and senescence (m). Specificity analysis of primary CF isolation reveals a pure vimentin-expressing (Vim) CF population with negligible CD31-expressing (Pecam1) endothelial cells and troponin-I-expressing (Tnni3) cardiomyocytes (n). N = 3 biological replicates for each condition, except Young CF+SoftY group in RNA-seq (N = 2). Data are mean \(\pm \,\)SEM (a-n). One-way ANOVA with Fisher’s preplanned multiple comparison (each pair with one variable, ECM or stiffness, a-n).
Extended Data Fig. 4 CF activation/quiescence and aging/rejuvenation-like phenotypes shown by original DEG heatmaps with dendrograms.
Extended Data Fig. 5 KEGG enrichment analysis of CFs seeded on DECIPHER samples.
Highlighted pathways of interest in young or aged CFs impacted by mechanical cues (Stiff vs. Soft, a) or ECM aging (Aged ECM vs. Young ECM, b) were graphed in the order of normalized enrichment score (NES) with color bar showing adjusted significance using Q-value (Two-sided Benjamini-Hochberg test). Significantly regulated pathways were marked with asterisks beside the pathway name: *Q < 0.05, **Q < 0.01, ***Q < 0.001. Bubble size indicates the number of genes differentially expressed in each pathway set.
Extended Data Fig. 6 RT-qPCR validation of gene expression compared to RNA-seq.
Normalized gene expression on different DECIPHER samples (SoftY, StiffY, SoftA, StiffA). Fold changes in RT-qPCR results (b, d, f, h, j, l, n, p) were calculated using 2−ΔΔCt method and compared side-by-side with RNA-seq results (a, c, e, g, i, k, m, o). N = 3 biological replicates for each condition, except Young CF+SoftY group in RNA-seq (N = 2). Data are mean \(\pm \,\)SEM (a-p). One-way ANOVA with Fisher’s preplanned multiple comparison (each pair with one variable, ECM or stiffness, a-p).
Extended Data Fig. 7 Integrin and focal adhesion formation on DECIPHER scaffolds.
ICC of F-actin, nucleus, α5 integrin, and paxillin in young CFs (a, c) and aged CFs (b, d) on DECIPHER samples (SoftY, StiffY, SoftA, or StiffA). α5 integrin insets are indicated by the black boxes, paxillin insets are indicated by the white boxes, and white arrows point to focal adhesions. The number and area of focal adhesion sites in young CFs (e, f) and aged CFs (g, h) quantified from paxillin staining. N = 3 biological replicates for each condition (e-h). Data are mean \(\pm \,\)SD (e, g). One-way ANOVA with Fisher’s preplanned multiple comparison (each pair with one variable, ECM or stiffness, e-h). Scale bar: 50 µm.
Extended Data Fig. 8 ECM ligands and mechanics dictate CF activation, mechanosensitive, and senescence phenotypes.
ICC images shown in Fig. 4j and k were quantified for α-SMA intensity and stress fiber assembly for young CFs (a, c) and aged CFs (b, d). ICC images shown in Fig. 6l and m were quantified for p53 integrated intensity and mean intensity for young CFs (e, g) and aged CFs (f, h). ICC images of young (i) and aged CFs (j) on DECIPHER samples stained for nucleus, F-actin, YAP (i, j), and TIMP3 (k, l). SoftY and StiffA TIMP3 images are also shown in Fig. 6k. One-way ANOVA with Fisher’s preplanned multiple comparison (each pair with one variable, ECM or stiffness) was used for (a-h), N = 3 biological replicates for each condition. Scale bar: 50 µm (i, j), 100 µm (k, l).
Extended Data Fig. 9 Morphological changes in CFs are governed by stiffness.
Low magnification images of young and aged CFs cultured on DECIPHER samples immunostained for F-actin, nucleus, and collagen (a, b). Cell morphology (area and circularity) of young CFs (c, d) and aged CFs (e, f), as well as nuclear morphology (area and circularity) of young CFs (g, h) and aged CFs (i, j) on DECIPHER samples. One-way ANOVA with Fisher’s preplanned multiple comparison (each pair with one variable, ECM or stiffness) was used for (e-h), N = 12 biological replicates for each condition. Scale bar: 100 µm.
Extended Data Fig. 10 Conventional reconstituted dECM films do not recapitulate native tissue properties.
Decellularized young or aged ECM were lyophilized, pulverized, and solubilized prior to coverslip coating (a). Immunostaining shows fragmented reconstituted dECM and a destruction of native architecture (b) compared to native tissue or DECIPHER materials (shown in Fig. 2a, b). Young or aged CFs were seeded onto dECM films (with control coating fibronectin, FN) and immunostained for nucleus, F-actin, and α-SMA(c). CF activation and focal adhesion formation were quantified for young (d, f, h) and aged CFs (e, g, i) and compared with DECIPHER data (SoftY and StiffA). N = 3 samples for each condition. One-way ANOVA with Fisher’s preplanned multiple comparison (each pair with one variable, ECM or stiffness, d-i). Scale bar: 50 µm (b), 100 µm (c).
Supplementary information
Supplementary Information
Supplementary Note 1 and Tables 1–4.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, A.R., Ramli, M.F.H., Shen, X. et al. Hybrid hydrogel–extracellular matrix scaffolds identify biochemical and mechanical signatures of cardiac ageing. Nat. Mater. (2025). https://doi.org/10.1038/s41563-025-02234-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41563-025-02234-6