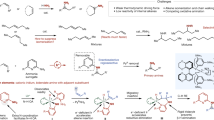
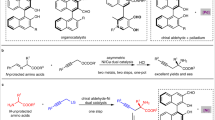
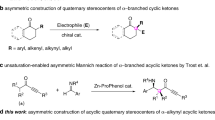
Abstract
Asymmetric catalysis enables the synthesis of optically active compounds, often requiring the differentiation between two substituents on prochiral substrates1. Despite decades of development of mainly noble metal catalysts, achieving differentiation between substituents with similar steric and electronic properties remains a notable challenge2,3. Here we introduce a class of Earth-abundant manganese catalysts for the asymmetric hydrogenation of dialkyl ketimines to give a range of chiral amine products. These catalysts distinguish between pairs of minimally differentiated alkyl groups bound to the ketimine, such as methyl and ethyl, and even subtler distinctions, such as ethyl and n-propyl. The degree of enantioselectivity can be adjusted by modifying the components of the chiral manganese catalyst. This reaction demonstrates a wide substrate scope and achieves a turnover number of up to 107,800. Our mechanistic studies indicate that exceptional stereoselectivity arises from the modular assembly of confined chiral catalysts and cooperative non-covalent interactions between the catalyst and the substrate.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available in the main text or the supplementary materials.
References
Walsh, P. J. & Kozlowski, M. C. Fundamentals of Asymmetric Catalysis (Univ. Science Books, 2010).
Zhang, F.-H., Zhang, F.-J., Li, M.-L., Xie, J.-H. & Zhou, Q.-L. Enantioselective hydrogenation of dialkyl ketones. Nat. Catal. 3, 621–627 (2020).
Zhou, H. et al. Organocatalytic stereoselective cyanosilylation of small ketones. Nature 605, 84–89 (2022).
McDaniel, D. H. & Brown, H. C. An extended table of Hammett substitutent constants based on the ionization of substituted benzoic acids. J. Org. Chem. 23, 420–427 (1958).
Tolman, C. A. Steric effects of phosphorus ligands in organometallic chemistry and homogeneous catalysis. Chem. Rev. 77, 313–348 (1977).
McFord, A. W., Butts, C. P., Fey, N. & Alder, R. W. 3× Axial vs 3× equatorial: the ΔGGA value is a robust computational measure of substituent steric effects. J. Am. Chem. Soc. 143, 13573–13578 (2021).
Bell, S. et al. Asymmetric hydrogenation of unfunctionalized, purely alkyl-substituted olefins. Science 311, 642–644 (2006).
Li, C., Wang, C., Villa-Marcos, B. & Xiao, J. Chiral counteranion-aided asymmetric hydrogenation of acyclic imines. J. Am. Chem. Soc. 130, 14450–14451 (2008).
Li, C., Villa-Marcos, B. & Xiao, J. Metal−Brønsted acid cooperative catalysis for asymmetric reductive amination. J. Am. Chem. Soc. 131, 6967–6969 (2009).
Tang, W. et al. Cooperative catalysis: combining an achiral metal catalyst with a chiral Brønsted acid enables highly enantioselective hydrogenation of imines. Chem. Eur. J. 19, 14187–14193 (2013).
Schramm, Y., Barrios-Landeros, F. & Pfaltz, A. Discovery of an iridacycle catalyst with improved reactivity and enantioselectivity in the hydrogenation of dialkyl ketimines. Chem. Sci. 4, 2760–2766 (2013).
Bae, H. Y. et al. Approaching sub-ppm-level asymmetric organocatalysis of a highly challenging and scalable carbon-carbon bond forming reaction. Nat. Chem. 10, 888–894 (2018).
Wang, Z., Yang, X.-Y., Xu, Y. & Zhou, Q.-L. Iridium-catalyzed asymmetric hydrogenation of dialkyl imines. CCS Chem. 6, 905–911 (2024).
Yasukawa, T., Masuda, R. & Kobayashi, S. Development of heterogeneous catalyst systems for the continuous synthesis of chiral amines via asymmetric hydrogenation. Nat. Catal. 2, 1088–1092 (2019).
Bahamonde, A., Al Rifaie, B., Martín-Heras, V., Allen, J. R. & Sigman, M. S. Enantioselective Markovnikov addition of carbamates to allylic alcohols for the construction of α-secondary and α-tertiary amines. J. Am. Chem. Soc. 141, 8708–8711 (2019).
Xi, Y. et al. Application of Trimethylgermanyl-substituted bisphosphine ligands with enhanced dispersion interactions to copper-catalyzed hydroboration of disubstituted alkenes. J. Am. Chem. Soc. 142, 18213–18222 (2020).
Knowles, R. R. & Jacobsen, E. N. Attractive noncovalent interactions in asymmetric catalysis: links between enzymes and small molecule catalysts. Proc. Natl Acad. Sci. USA 107, 20678–20685 (2010).
Drauz, K., Gröger, H. & May, O. (eds) Enzyme Catalysis in Organic Synthesis (Wiley, 2012).
Savile, C. K., & Janey, J. M. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to Sitagliptin manufacture. Science 329, 305–309 (2010).
Reetz, M. T. Laboratory evolution of stereoselective enzymes: a prolific source of catalysts for asymmetric reactions. Angew. Chem. Int. Ed. 50, 138–174 (2011).
Renata, H., Wang, Z. J. & Arnold, F. H. Expanding the enzyme universe: accessing non-natural reactions by mechanism-guided directed evolution. Angew. Chem. Int. Ed. 54, 3351–3367 (2015).
Wang, Q.-Q. et al. Self-assembled nanospheres with multiple endohedral binding sites pre-organize catalysts and substrates for highly efficient reactions. Nat. Chem. 8, 225–230 (2016).
Leenders, S. H. A. M., Gramage-Doria, R., de Bruin, B. & Reek, J. N. H. Transition metal catalysis in confined spaces. Chem. Soc. Rev. 44, 433–448 (2015).
Mitschke, B., Turberg, M. & List, B. Confinement as a unifying element in selective catalysis. Chem 6, 2515–2532 (2020).
Hou, G. et al. Enantioselective hydrogenation of N−H imines. J. Am. Chem. Soc. 131, 9882–9883 (2009).
Zuo, W., Lough, A. J., Li, Y. F. & Morris, R. H. Amine(imine)diphosphine iron catalysts for asymmetric transfer hydrogenation of ketones and imines. Science 342, 1080–1083 (2013).
Li, B., Chen, J., Liu, D., Gridnev, I. D. & Zhang, W. Nickel-catalysed asymmetric hydrogenation of oximes. Nat. Chem. 14, 920–927 (2022).
Mas-Roselló, J., Smejkal, T. & Cramer, N. Iridium-catalyzed acid-assisted asymmetric hydrogenation of oximes to hydroxylamines. Science 368, 1098–1102 (2020).
Oates, C. L., Goodfellow, A. S., Bühl, M. & Clarke, M. L. Manganese catalysed enantioselective hydrogenation of in situ-synthesised imines: efficient asymmetric synthesis of amino-indane derivatives. Green Chem. 25, 3864–3868 (2023).
Chen, J.-J. et al. Enantioconvergent Cu-catalysed N-alkylation of aliphatic amines. Nature 618, 294–300 (2023).
Xi, Y., Ma, S. & Hartwig, J. F. Catalytic asymmetric addition of an amine N-H bond across internal alkenes. Nature 588, 254–260 (2020).
Yang, Y., Shi, S.-L., Niu, D., Liu, P. & Buchwald, S. L. Catalytic asymmetric hydroamination of unactivated internal olefins to aliphatic amines. Science 349, 62–66 (2015).
Friedfeld, M. R., Zhong, H., Ruck, R. T., Shevlin, M. & Chirik, P. J. Cobalt-catalyzed asymmetric hydrogenation of enamides enabled by single-electron reduction. Science 360, 888–893 (2018).
Wang, Y. et al. Structure, reactivity and catalytic properties of manganese-hydride amidate complexes. Nat. Chem. 14, 1233–1241 (2022).
Freitag, F., Irrgang, T. & Kempe, R. Mechanistic studies of hydride transfer to imines from a highly active and chemoselective manganate catalyst. J. Am. Chem. Soc. 141, 11677–11685 (2019).
Wen, J., Wang, F. & Zhang, X. Asymmetric hydrogenation catalyzed by first-row transition metal complexes. Chem. Soc. Rev. 50, 3211–3237 (2021).
Garbe, M. et al. Manganese(I)-catalyzed enantioselective hydrogenation of ketones using a defined chiral PNP pincer ligand. Angew. Chem. Int. Ed. 56, 11237–11241 (2017).
Widegren, M. B., Harkness, G. J., Slawin, A. M. Z., Cordes, D. B. & Clarke, M. L. A highly active manganese catalyst for enantioselective ketone and ester hydrogenation. Angew. Chem. Int. Ed. 56, 5825–5828 (2017).
Liu, C., Liu, X. & Liu, Q. Stereodivergent asymmetric hydrogenation of quinoxalines. Chem 9, 2585–2600 (2023).
Yamashita, Y., Noguchi, A., Fushimi, S., Hatanaka, M. & Kobayashi, S. Chiral metal salts as ligands for catalytic asymmetric Mannich reactions with simple amides. J. Am. Chem. Soc. 143, 5598–5604 (2021).
Huang, H., Liu, X., Zhou, L., Chang, M. & Zhang, X. Direct Asymmetric reductive amination for the synthesis of chiral β-arylamines. Angew. Chem. Int. Ed. 55, 5309–5312 (2016).
Murakami, M., Takahashi, K., Mase, T., Murase, K. & Ida, H. α-Aminomethylbenzyl alcohol derivatives. US patent 3,994,974 (1976).
Kim, M.-S. et al. Heteroaryl compounds and their use as therapeutic drugs. Patent WO2017039331 (2017).
Acknowledgements
We thank Y. Xi from the National University of Singapore and J. Xiao from the University of Liverpool for helpful discussions and valuable revisions. Financial support from the National Key R&D Program of China (2021YFF0701600) and the National Natural Science Foundation of China (22225103 and 22171159) is greatly appreciated.
Author information
Authors and Affiliations
Contributions
M.W., Y.L. and Q.L. did the conceptualization of the study. M.W., S.L. and H.L. contributed to the methodology. M.W., S.L. and H.L. did the investigation. Y.L. and Q.L. were involved in funding acquisition. Y.L. and Q.L. did the project administration. Y.L. and Q.L. supervised the work. M.W., S.L., Y.W. and Q.L. wrote the original draft. M.W., Y.L. and Q.L. contributed to the writing, review and editing of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Rhett Kempe and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Comparison of free energy profiles.
Free energy profiles for the hydride transfer of 1a promoted by fac-Int4 and fac-Int5. Ar = o-bromophenyl.
Extended Data Fig. 2 Catalytic cycle.
Proposed mechanism for the Mn-Catalyzed asymmetric hydrogenation of ketimines.
Extended Data Fig. 3 Substrate scope of imines with primary/secondary/tertiary alkyl and methyl groups.
aReaction conditions: 1 (0.25 mmol), Mn-17 (2 mol%) and NaHMDS (5 mol%) in PhF (0.5 mL) at 5 °C for 20 h. Isolated yields (%) were given and enantioselectivities (%ee) were determined by chiral-phase HPLC. bMn-15 was used instead of Mn-17 at 25 °C. cMn-6 was used instead of Mn-17 at 25 °C. dMn-1 (2 mol%) and NaOtBu (10 mol%) in 1,4-dioxane (0.8 mL) at 60 °C for 20 h. eMn-1 (2 mol%) and KOtBu (20 mol%) in diethylene glycol dimethyl ether (0.8 mL) at 25 °C.
Extended Data Fig. 4 Synthetic applications.
a, Test of catalytic activity, TON experiment on a scale of grams. b, Deprotection of N-PG for the synthesis of primary amine. c, Preparation of (S)-Levdobutamine. d, Preparation of bioactive MER tyrosine kinase inhibitor (TCCA = trichloroisocyanuric acid; HATU = 2-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate).
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, M., Liu, S., Liu, H. et al. Asymmetric hydrogenation of ketimines with minimally different alkyl groups. Nature 631, 556–562 (2024). https://doi.org/10.1038/s41586-024-07581-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07581-z