Abstract
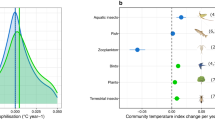
Turnover in species composition through time is a dominant form of biodiversity change, which has profound effects on the functioning of ecological communities1,2,3,4. Turnover rates differ markedly among communities4, but the drivers of this variation across taxa and realms remain unknown. Here we analyse 42,255 time series of species composition from marine, terrestrial and freshwater assemblages, and show that temporal rates of turnover were consistently faster in locations that experienced faster temperature change, including both warming and cooling. In addition, assemblages with limited access to microclimate refugia or that faced stronger human impacts on land were especially responsive to temperature change, with up to 48% of species replaced per decade. These results reveal a widespread signal of vulnerability to continuing climate change and highlight which ecological communities are most sensitive, raising concerns about ecosystem integrity as climate change and other human impacts accelerate.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Species composition data are available from BioTIME (https://biotime.st-andrews.ac.uk/), human impact data from ref. 66 (pan310071-sup-0003-Supinfo2.7z from https://doi.org/10.1002/pan3.10071), sea surface temperature from ERSST v.5 (ftp://ftp.cdc.noaa.gov/Datasets/noaa.ersst.v5/sst.mnmean.nc), land surface temperature from CRU TS v.4.03 (https://dap.ceda.ac.uk/badc/cru/data/cru_ts/cru_ts_4.03/data/tmp/cru_ts4.03.1901.2018.tmp.dat.nc), terrestrial microclimate data from WorldClim 2.0 (wc2.0_bio_30s_01.tif from https://worldclim.org) and marine microclimate data from Bio-ORACLE 2.2 (https://www.bio-oracle.org/). Further details are available at Zenodo (https://doi.org/10.5281/zenodo.13905417)67.
Code availability
Scripts are available at Zenodo (https://doi.org/10.5281/zenodo.13905417)67.
Change history
07 March 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41586-025-08857-8
References
Dornelas, M. et al. Assemblage time series reveal biodiversity change but not systematic loss. Science 344, 296–299 (2014).
Hillebrand, H. et al. Biodiversity change is uncoupled from species richness trends: consequences for conservation and monitoring. J. Appl. Ecol. 55, 169–184 (2018).
Global Assessment Report on Biodiversity and Ecosystem Services https://ipbes.net/global-assessment (IPBES, 2019).
Blowes, S. A. et al. The geography of biodiversity change in marine and terrestrial assemblages. Science 366, 339–345 (2019).
Lewthwaite, J. M. M., Debinski, D. M. & Kerr, J. T. High community turnover and dispersal limitation relative to rapid climate change. Glob. Ecol. Biogeogr. 26, 459–471 (2017).
Sgardeli, V., Zografou, K. & Halley, J. M. Climate change versus ecological drift: assessing 13 years of turnover in a butterfly community. Basic Appl. Ecol. 17, 283–290 (2016).
Daskalova, G. N. et al. Landscape-scale forest loss as a catalyst of population and biodiversity change. Science 368, 1341–1347 (2020).
Nakadai, R. Degrees of compositional shift in tree communities vary along a gradient of temperature change rates over one decade: application of an individual‐based temporal beta‐diversity concept. Ecol. Evol. 10, 13613–13623 (2020).
Lindholm, M., Alahuhta, J., Heino, J. & Toivonen, H. Temporal beta diversity of lake plants is determined by concomitant changes in environmental factors across decades. J. Ecol. 109, 819–832 (2021).
Angilletta Jr, M. J. Thermal Adaptation: A Theoretical and Empirical Synthesis (Oxford Univ. Press, 2009).
Sunday, J. M. et al. Thermal tolerance patterns across latitude and elevation. Philos. Trans. R. Soc. B 374, 20190036 (2019).
Pinsky, M. L., Comte, L. & Sax, D. F. Unifying climate change biology across realms and taxa. Trends Ecol. Evol. 37, 672–682 (2022).
Lenoir, J. et al. Species better track climate warming in the oceans than on land. Nat. Ecol. Evol. 4, 1044–1059 (2020).
Antão, L. H. et al. Temperature-related biodiversity change across temperate marine and terrestrial systems. Nat. Ecol. Evol. 4, 927–933 (2020).
Dornelas, M. et al. BioTIME: a database of biodiversity time series for the Anthropocene. Glob. Ecol. Biogeogr. 27, 760–786 (2018).
Collins, S. L., Micheli, F. & Hartt, L. A method to determine rates and patterns of variability in ecological communities. Oikos 91, 285–293 (2000).
Post, E., Steinman, B. A. & Mann, M. E. Acceleration of phenological advance and warming with latitude over the past century. Sci. Rep. 8, 3927 (2018).
Millar, R. B., Anderson, M. J. & Tolimieri, N. Much ado about nothings: using zero similarity points in distance–decay curves. Ecology 92, 1717–1722 (2011).
Muletz‐Wolz, C. R. et al. Diversity and temporal dynamics of primate milk microbiomes. Am. J. Primatol. 81, e22994 (2019).
Stuart-Smith, R. D., Edgar, G. J. & Bates, A. E. Thermal limits to the geographic distributions of shallow-water marine species. Nat. Ecol. Evol. 1, 1846–1852 (2017).
Stuart-Smith, R. D., Edgar, G. J., Barrett, N. S., Kininmonth, S. J. & Bates, A. E. Thermal biases and vulnerability to warming in the world’s marine fauna. Nature 528, 88–92 (2015).
Bonachela, J. A., Burrows, M. T. & Pinsky, M. L. Shape of species climate response curves affects community response to climate change. Ecol. Lett. 24, 708–718 (2021).
Khaliq, I. et al. Warming underpins community turnover in temperate freshwater and terrestrial communities. Nat. Commun. 15, 1921 (2024).
Gallagher, R. V., Allen, S. & Wright, I. J. Safety margins and adaptive capacity of vegetation to climate change. Sci. Rep. 9, 8241 (2019).
Peng, S. et al. Towards an understanding of the latitudinal patterns in thermal tolerance and vulnerability of woody plants under climate warming. Ecography 44, 1797–1807 (2021).
Pinsky, M. L., Eikeset, A. M., McCauley, D. J., Payne, J. L. & Sunday, J. M. Greater vulnerability to warming of marine versus terrestrial ectotherms. Nature 569, 108–111 (2019).
Bertrand, R. et al. Ecological constraints increase the climatic debt in forests. Nat. Commun. 7, 12643 (2016).
Beaugrand, G., Kirby, R. & Goberville, E. The mathematical influence on global patterns of biodiversity. Ecol. Evol. 10, 6494–6511 (2020).
Suggitt, A. J. et al. Extinction risk from climate change is reduced by microclimatic buffering. Nat. Clim. Change 8, 713 (2018).
Williams, J. J. & Newbold, T. Local climatic changes affect biodiversity responses to land use: a review. Divers. Distrib. 26, 76–92 (2020).
Tóth, A. B. et al. Reorganization of surviving mammal communities after the end-Pleistocene megafaunal extinction. Science 365, 1305–1308 (2019).
Kortz, A. R. et al. Elevated compositional change in plant assemblages linked to invasion. Proc. R. Soc. B 290, 20222450 (2023).
McCauley, D. J. et al. Marine defaunation: animal loss in the global ocean. Science 347, 1255641–1255641 (2015).
Ontiveros, V. J., Capitán, J. A., Casamayor, E. O. & Alonso, D. The characteristic time of ecological communities. Ecology 102, e03247 (2021).
Hortal, J. et al. Seven shortfalls that beset large-scale knowledge of biodiversity. Annu. Rev. Ecol. Evol. Syst. 46, 523–549 (2015).
Zhang, L., Takahashi, D., Hartvig, M. & Andersen, K. H. Food-web dynamics under climate change. Proc. R. Soc. B 284, 20171772 (2017).
Lee, J.-Y. et al. in Climate Change 2021: The Physical Science Basis (eds Masson-Delmotte, V. et al.) 553–672 (Cambridge Univ. Press, 2021).
Svensson, S., Thorner, A. M. & Nyholm, N. E. I. Species trends, turnover and composition of a woodland bird community in southern Sweden during a period of fifty-seven years. Ornis Svec. 20, 31–40 (2010).
Becker, R. A., Wilks, A. R., Brownrigg, R., Minka, T. P. & Deckmyn, A. maps: Draw geographical maps. R package version 3.4.1 https://CRAN.R-project.org/package=maps (2022).
Barnes, R. & Sahr, K. dggridR: Discrete global grids for R. R package version 2.0.4 https://github.com/r-barnes/dggridR/ (2017).
Chao, A. & Jost, L. Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology 93, 2533–2547 (2012).
Gotelli, N. J. & Colwell, R. K. in Biological Diversity: Frontiers in Measurement and Assessment (eds Magurran, A. E. & McGill, B. J.) 39–54 (Oxford Univ. Press, 2011).
Baselga, A. Partitioning the turnover and nestedness components of beta diversity: partitioning beta diversity. Glob. Ecol. Biogeogr. 19, 134–143 (2010).
Baselga, A. et al. betapart: Partitioning beta diversity into turnover and nestedness components. R package version 1.5.2 https://CRAN.R-project.org/package=betapart (2020).
Magurran, A. E. Ecological Diversity and its Measurement (Croom Helm, 1988).
Chao, A. et al. Approach to multiple-community similarity indices. Biometrics 64, 1178–1186 (2008).
Kubinec, R. Ordered beta regression: a parsimonious, well-fitting model for continuous data with lower and upper bounds. Polit. Anal. 31, 519–536 (2023).
Rosenblad, K. C. & Sax, D. F. A new framework for investigating biotic homogenization and exploring future trajectories: oceanic island plant and bird assemblages as a case study. Ecography 40, 1040–1049 (2017).
Harris, I., Osborn, T. J., Jones, P. & Lister, D. Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci. Data 7, 109 (2020).
Huang, B. et al. Extended reconstructed sea surface temperature, version 5 (ERSSTv5): upgrades, validations, and intercomparisons. J. Clim. 30, 8179–8205 (2017).
Theil, H. A rank-invariant method of linear and polynomial regression analysis. I, II, III. Proc. Akadamie Van Wet. Amst. 53, 386–392 (1950).
Sen, P. K. Estimates of the regression coefficient based on Kendall’s Tau. J. Am. Stat. Assoc. 63, 1379–1389 (1968).
Munch, S. B. & Salinas, S. Latitudinal variation in lifespan within species is explained by the metabolic theory of ecology. Proc. Natl Acad. Sci. USA 106, 13860–13864 (2009).
Dillon, M. E., Wang, G. & Huey, R. B. Global metabolic impacts of recent climate warming. Nature 467, 704–6 (2010).
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M. & West, G. B. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004).
Brooks, E. M. et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378 (2017).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2023).
Hartig, F. DHARMa: Residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.4.0 https://CRAN.R-project.org/package=DHARMa (2021).
Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach (Springer, 2002).
North, B. V., Curtis, D. & Sham, P. C. A note on the calculation of empirical P values from Monte Carlo procedures. Am. J. Hum. Genet. 71, 439–441 (2002).
Zellweger, F. et al. Forest microclimate dynamics drive plant responses to warming. Science 368, 772–775 (2020).
Kroeker, K. J. et al. Ecological change in dynamic environments: accounting for temporal environmental variability in studies of ocean change biology. Glob. Change Biol. 26, 54–67 (2020).
Thomas, M. K. et al. Temperature–nutrient interactions exacerbate sensitivity to warming in phytoplankton. Glob. Change Biol. 23, 3269–3280 (2017).
Fick, S. E. & Hijmans, R. J. Worldclim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017).
Assis, J. et al. Bio‐ORACLE v2.0: extending marine data layers for bioclimatic modelling. Glob. Ecol. Biogeogr. 27, 277–284 (2018).
Bowler, D. E. et al. Mapping human pressures on biodiversity across the planet uncovers anthropogenic threat complexes. People Nat. 2, 380–394 (2020).
Pinsky, M. L. et al. Supporting data and code for: Warming and cooling catalyse widespread temporal turnover in biodiversity (v1.0.0). Zenodo https://doi.org/10.5281/zenodo.13905417 (2024).
Acknowledgements
We thank K. Lew and J. Hauser for help with data entry; M. Stein for statistical advice; A. Bates, C. Meyer, R. Remelgado and the Global Change Research Group at Rutgers University and the University of California Santa Cruz for useful discussions; Z. Kitchel, A. Maureaud and P. Morin for feedback on earlier drafts; the BioTIME consortium for their commitment to open science; and the Rutgers School of Environmental and Biological Sciences for access to the Annotate and Annotate2 scientific workstations. Views and opinions expressed are those of the authors only and do not necessarily reflect those of the European Union or the European Research Council. Neither the European Union nor the granting authority can be held responsible for them. The work was supported by the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig, funded by the German Research Foundation grant no. FZT 118 (M.L.P., S.A.B., J.M.C., U.B., M.H., B.G, and B.R.); the Helmholtz Institute for Functional Marine Biodiversity (M.L.P. and H.H.); US National Science Foundation grant no. DEB-1616821 (M.L.P.); US National Science Foundation grant no. CBET-2137701 (M.L.P.); US National Science Foundation grant no. DEB-2129351 (M.L.P.); an ERC Advanced Grant (MetaChange) funded by the European Union (J.M.C. and S.A.B.); German Research Foundation grant nos. Hi848/26 and EXC2077 (H.H.); and Academy of Finland grant no. 340280 (L.H.A.).
Author information
Authors and Affiliations
Contributions
Conceptualization: M.L.P. Data curation: S.A.B., M.L.P., B.G., L.H.A., M.T.B., M.R.H. Formal analysis: M.L.P. Funding acquisition: M.L.P., H.H., J.M.C., U.B. Methodology: M.L.P., S.A.B., J.M.C., M.T.B., H.H., B.R. Project administration: M.L.P. Software: M.L.P., S.A.B., L.H.A. Visualization: M.L.P. Writing—original draft: M.L.P., H.H. Writing—review and editing: M.L.P., L.H.A., J.M.C., B.G., S.A.B., U.B., M.R.H., B.R.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Naia Morueta-Holme, David Warton and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Characteristics of the assemblage time series.
a) Start year. b) End year. c) Number of years between the start and end year. d) Number of annual samples in each time series.
Extended Data Fig. 2 The effect of time series duration on turnover rates (change in dissimilarity/yr) and the statistical challenges when time series are compared.
a) Duration affects turnover rates partly because there is a 0-1 constraint on dissimilarity, such that longer duration time series (blue) are constrained to a shallower slope than shorter duration time series (green). b) Turnover rates show strong heteroskedasticity with higher variance and faster rates among shorter time series. The red line shows mean turnover rate estimated from LOESS smoothing. c) Temperature changes (°C/yr) also showed strong heteroskedasticity with higher variance among shorter time series. The red line shows a fit from LOESS smoothing. d) Slopes calculated from Gaussian white noise time series also show strong heteroskedasticity with higher variance among shorter time series. The durations of the white noise time series matched the durations in the species composition dataset. The red line shows a fit from LOESS smoothing. e) A comparison of Type I (false positive) error rates shows that one-stage (i.e. fit directly to dissimilarities) generalized linear mixed models (GLMMs) with ordered beta errors have an acceptably low false positive rate when time series of different durations are analyzed together, while other common analytical methods (Pearson correlations of time series slopes, meta-analysis of time series slopes, or one-stage mixed effect models with Gaussian errors fit to time series data) have unacceptably high false positive rates if time series differ in duration (range of durations > 0). All methods have low false positive rates when time series are all the same duration (range of durations = 0). Data are presented as means with error bars for the 95% binomial confidence bounds. f) Example of a time series with a negative turnover rate. Data are demersal marine taxa from the Northeast Fisheries Science Center Bottom Trawl Survey. Beta regression trend line is shown with shading for +/− one standard error.
Extended Data Fig. 3 The statistical approach was implemented via one-stage generalized linear mixed models (GLMMs) in which the response variable was species composition dissimilarity among years.
a) The simplest model included the relationship between dissimilarity and temporal distance among observations so that, for example, dissimilarity could increase with time. The slope of this relationship is the turnover rate. Random intercepts and slopes helped account for variation among studies and time series (not shown). b) We tested the hypothesis that faster rates of temperature change (Tchange) were associated with faster accumulation of dissimilarity through time (compare red vs. blue line). This hypothesis was statistically tested as an interaction (Tchange × Years). c) We additionally tested the hypothesis that the influence of temperature change on the turnover rate depended on average baseline temperatures. For example, the slope of dissimilarity over time could be steeper in areas with hotter average temperatures and fast rates of temperature change than in areas with colder average temperatures and fast rates of temperature change (compare dashed red vs. solid red line). Statistically, this was tested as a three-way interaction (Tchange × Tave × Years). d) Turnover rates as a function of temperature change rates, showing an increase in turnover rate with increasing rates of temperature change (i.e., the same relationship as panel b but summarized as rates). The slope of this relationship was termed sensitivity (Δturnover rate/Δtemperature change rate). e) Turnover rates as a function of temperature change rates and average baseline temperatures, showing a faster increase in turnover rate with temperature change at hotter average baseline temperatures (i.e., summarizing the same relationship as panel c). f) Sensitivity as a function of average temperatures, showing an increase in sensitivity at hotter average temperatures (i.e., summarizing the same relationship as in panels c and e). The x-axis could also be other environmental covariates, such as microclimates or non-climate human impacts (as in Fig. 3).
Extended Data Fig. 4 Association of turnover rate with taxonomic group and uncertainty of the association with temperature change and average temperature.
a) Turnover rate [proportion of species per year] for studies organized by taxonomic group. Dashed lines are the averages across studies within taxa, and the top horizontal lines indicate the 95% confidence intervals on the averages. The x- and y-axes have been square-root transformed to facilitate visualization. b) Uncertainty in the marginal effects of temperature change on the turnover rate, calculated by downsampling each time series of dissimilarities (see Methods). Plot shows the individual downsampled effects (thin green lines), the average across 1000 downsampling trials (yellow line), the 95% confidence interval from downsampling (green shading), and the mean marginal effects from the full dataset with 95% confidence intervals (black line and shading). c) Uncertainty in the marginal effects of average temperature on the sensitivity of turnover rate to temperature change, calculated by downsampling each time series of dissimilarities (see Methods). Plot shows the individual downsampled effects (thin lines), the average across 1000 downsampling trials (thick lines), and the 95% confidence interval from downsampling (vertical error bars) for warming (orange) and cooling (blue). The mean marginal effects from the full dataset with 95% confidence intervals are also shown (black lines and error bars).
Extended Data Fig. 5 Turnover rate model interactions.
a) Interaction of Tchange (x-axis) with Tave (y-axis) from the Tchange × Tave × Year × Realm model (Table 1). Two average temperature levels (0 °C and 25 °C) from this interaction are plotted in Fig. 2c. b) Marginal effects of temperature change on the turnover rate (lines) as predicted from the best environmental interaction model identified by AIC (Extended Data Table 5). The model included effects of microclimate availability (colors).
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pinsky, M.L., Hillebrand, H., Chase, J.M. et al. Warming and cooling catalyse widespread temporal turnover in biodiversity. Nature 638, 995–999 (2025). https://doi.org/10.1038/s41586-024-08456-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-08456-z