Abstract
Chronic stress can change how we learn and, thus, how we make decisions1,2,3,4,5. Here we investigated the neuronal circuit mechanisms that enable this. Using a multifaceted systems neuroscience approach in male and female mice, we reveal a dual-pathway, amygdala–striatal neuronal circuit architecture by which a recent history of chronic stress disrupts the action–outcome learning underlying adaptive agency and promotes the formation of inflexible habits. We found that the projection from the basolateral amygdala to the dorsomedial striatum is activated by rewarding events to support the action–outcome learning needed for flexible, goal-directed decision-making. Chronic stress attenuates this to disrupt action–outcome learning and, therefore, agency. Conversely, the projection from the central amygdala to the dorsomedial striatum mediates habit formation. Following stress, this pathway is progressively recruited to learning to promote the premature formation of inflexible habits. Thus, stress exerts opposing effects on two amygdala–striatal pathways to disrupt agency and promote habit. These data provide neuronal circuit insights into how chronic stress shapes learning and decision-making, and help understanding of how stress can lead to the disrupted decision-making and pathological habits that characterize substance use disorders and mental health conditions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data that support the findings of this study are available from the corresponding author upon request. Source data are provided with this paper.
Code availability
Custom-written MATLAB code is available at Dryad (https://doi.org/10.5061/dryad.2jm63xt00)97 and from the corresponding author upon request.
References
Schwabe, L. & Wolf, O. T. Stress prompts habit behavior in humans. J. Neurosci. 29, 7191–7198 (2009).
Pool, E. R. et al. Determining the effects of training duration on the behavioral expression of habitual control in humans: a multilaboratory investigation. Learn. Mem. 29, 16–28 (2022).
Friedel, E. et al. How accumulated real life stress experience and cognitive speed interact on decision-making processes. Front. Hum. Neurosci. 11, 302 (2017).
Schwabe, L., Dalm, S., Schächinger, H. & Oitzl, M. S. Chronic stress modulates the use of spatial and stimulus-response learning strategies in mice and man. Neurobiol. Learn. Mem. 90, 495–503 (2008).
Dias-Ferreira, E. et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science 325, 621–625 (2009).
Balleine, B. W. The meaning of behavior: discriminating reflex and volition in the brain. Neuron 104, 47–62 (2019).
Graybiel, A. M. Habits, rituals, and the evaluative brain. Annu. Rev. Neurosci. 31, 359–387 (2008).
Dickinson, A. Actions and habits: the development of behavioural autonomy. Phil. Trans. R. Soc.Lond. B 308, 67–78 (1985).
Redish, A. D., Jensen, S. & Johnson, A. A unified framework for addiction: vulnerabilities in the decision process. Behav. Brain Sci. 31, 415–437; discussion 437–487 (2008).
Vandaele, Y. & Ahmed, S. H. Habit, choice, and addiction. Neuropsychopharmacology 46, 689–698 (2021).
Voon, V. et al. Disorders of compulsivity: a common bias towards learning habits. Mol. Psychiatry 20, 345–352 (2015).
Belin, D., Belin-Rauscent, A., Murray, J. E. & Everitt, B. J. Addiction: failure of control over maladaptive incentive habits. Curr. Opin. Neurobiol. 23, 564–572 (2013).
Hogarth, L., Balleine, B. W., Corbit, L. H. & Killcross, S. Associative learning mechanisms underpinning the transition from recreational drug use to addiction. Ann. N. Y. Acad. Sci. 1282, 12–24 (2013).
Ray, L. A. et al. Capturing habitualness of drinking and smoking behavior in humans. Drug Alcohol Depend. 207, 107738 (2020).
Gillan, C. M. et al. Disruption in the balance between goal-directed behavior and habit learning in obsessive-compulsive disorder. Am. J. Psychiatry 168, 718–726 (2011).
Horstmann, A. et al. Slave to habit? Obesity is associated with decreased behavioural sensitivity to reward devaluation. Appetite 87, 175–183 (2015).
Morris, R. W., Cyrzon, C., Green, M. J., Le Pelley, M. E. & Balleine, B. W. Impairments in action–outcome learning in schizophrenia. Transl. Psychiatry 8, 54 (2018).
Griffiths, K. R., Morris, R. W. & Balleine, B. W. Translational studies of goal-directed action as a framework for classifying deficits across psychiatric disorders. Front. Syst. Neurosci. 8, 101 (2014).
Byrne, K. A., Six, S. G. & Willis, H. C. Examining the effect of depressive symptoms on habit formation and habit-breaking. J. Behav. Ther. Exp. Psychiatry 73, 101676 (2021).
Alvares, G. A., Balleine, B. W. & Guastella, A. J. Impairments in goal-directed actions predict treatment response to cognitive-behavioral therapy in social anxiety disorder. PLoS ONE 9, e94778 (2014).
Alvares, G. A., Balleine, B. W., Whittle, L. & Guastella, A. J. Reduced goal-directed action control in autism spectrum disorder. Autism Res. 9, 1285–1293 (2016).
Agid, O., Kohn, Y. & Lerer, B. Environmental stress and psychiatric illness. Biomed. Pharmacother. 54, 135–141 (2000).
Baumeister, D., Lightman, S. L. & Pariante, C. M. The interface of stress and the HPA axis in behavioural phenotypes of mental illness. Curr. Top. Behav. Neurosci. 18, 13–24 (2014).
Brady, K. T. & Sinha, R. Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. Am. J. Psychiatry 162, 1483–1493 (2005).
Duffing, T. M., Greiner, S. G., Mathias, C. W. & Dougherty, D. M. Stress, substance abuse, and addiction. Curr. Top. Behav. Neurosci. 18, 237–263 (2014).
Malvaez, M. & Wassum, K. Regulation of habit formation in the dorsal striatum. Curr. Opin. Behav. Sci. 20, 67–74 (2018).
Balleine, B. W. & O’Doherty, J. P. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35, 48–69 (2010).
Yin, H. H., Ostlund, S. B., Knowlton, B. J. & Balleine, B. W. The role of the dorsomedial striatum in instrumental conditioning. Eur. J. Neurosci. 22, 513–523 (2005).
Balleine, B. W., Killcross, A. S. & Dickinson, A. The effect of lesions of the basolateral amygdala on instrumental conditioning. J. Neurosci. 23, 666–675 (2003).
Pan, W. X., Mao, T. & Dudman, J. T. Inputs to the dorsal striatum of the mouse reflect the parallel circuit architecture of the forebrain. Front. Neuroanat. 4, 147 (2010).
Gowrishankar, R. et al. Endogenous opioid dynamics in the dorsal striatum sculpt neural activity to control goal-directed action. Preprint at BioRxiv https://doi.org/10.1101/2024.05.20.595035 (2024).
Lingawi, N. W. & Balleine, B. W. Amygdala central nucleus interacts with dorsolateral striatum to regulate the acquisition of habits. J. Neurosci. 32, 1073–1081 (2012).
Swanson, L. W. & Petrovich, G. D. What is the amygdala? Trends Neurosci. 21, 323–331 (1998).
Wall, N. R., De La Parra, M., Callaway, E. M. & Kreitzer, A. C. Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron 79, 347–360 (2013).
Heaton, E. C., Seo, E. H., Butkovich, L. M., Yount, S. T. & Gourley, S. L. Control of goal-directed and inflexible actions by dorsal striatal melanocortin systems, in coordination with the central nucleus of the amygdala. Prog. Neurobiol. 238, 102629 (2024).
Moscarello, J. M. & Penzo, M. A. The central nucleus of the amygdala and the construction of defensive modes across the threat-imminence continuum. Nat. Neurosci. 25, 999–1008 (2022).
Roozendaal, B., McEwen, B. S. & Chattarji, S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 10, 423–433 (2009).
Dickinson, A. D., Nicholas, J. & Adams, C. D. The effect of the instrumental training contingency on susceptibility to reinforcer devaluation. Q. J. Exp. Psychol. 35, 35–51 (1983).
Adams, C. D. & Dickinson, A. Instrumental responding following reinforcer devaluation. Q. J. Exp. Psychol. 33, 109–121 (1981).
Hammond, L. J. The effect of contingency upon the appetitive conditioning of free-operant behavior. J. Exp. Anal. Behav. 34, 297–304 (1980).
Malvaez, M. et al. Habits are negatively regulated by histone deacetylase 3 in the dorsal striatum. Biol. Psychiatry 84, 383–392 (2018).
Gremel, C. M. & Costa, R. M. Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat. Commun. 4, 2264 (2013).
Alexander, G. M. et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63, 27–39 (2009).
Zhu, H. et al. Cre-dependent DREADD (Designer Receptors Exclusively Activated by Designer Drugs) mice. Genesis 54, 439–446 (2016).
Amaya, K. A. et al. Habit learning shapes activity dynamics in the central nucleus of the amygdala. Preprint at BioRxiv https://doi.org/10.1101/2024.02.20.580730 (2024).
Tipps, M., Marron Fernandez de Velasco, E., Schaeffer, A. & Wickman, K. Inhibition of pyramidal neurons in the basal amygdala promotes fear learning. eNeuro 5, ENEURO.0272-18.2018 (2018).
Tuscher, J. J., Taxier, L. R., Fortress, A. M. & Frick, K. M. Chemogenetic inactivation of the dorsal hippocampus and medial prefrontal cortex, individually and concurrently, impairs object recognition and spatial memory consolidation in female mice. Neurobiol. Learn. Mem. 156, 103–116 (2018).
Corbit, L. H., Leung, B. K. & Balleine, B. W. The role of the amygdala-striatal pathway in the acquisition and performance of goal-directed instrumental actions. J. Neurosci. 33, 17682–17690 (2013).
Ostlund, S. B. & Balleine, B. W. Differential involvement of the basolateral amygdala and mediodorsal thalamus in instrumental action selection. J. Neurosci. 28, 4398–4405 (2008).
Fisher, S. D., Ferguson, L. A., Bertran-Gonzalez, J. & Balleine, B. W. Amygdala–cortical control of striatal plasticity drives the acquisition of goal-directed action. Curr. Biol. 30, 4541–4546.e5 (2020).
Namburi, P. et al. A circuit mechanism for differentiating positive and negative associations. Nature 520, 675–678 (2015).
Tye, K. M. Neural circuit motifs in valence processing. Neuron 100, 436–452 (2018).
Balleine, B. W. & Killcross, S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 29, 272–279 (2006).
Ugolini, A., Sokal, D. M., Arban, R. & Large, C. H. CRF1 receptor activation increases the response of neurons in the basolateral nucleus of the amygdala to afferent stimulation. Front. Behav. Neurosci. 2, 2 (2008).
Liu, Z. P. et al. Chronic stress impairs GABAergic control of amygdala through suppressing the tonic GABAA receptor currents. Mol. Brain 7, 32 (2014).
Rosenkranz, J. A., Venheim, E. R. & Padival, M. Chronic stress causes amygdala hyperexcitability in rodents. Biol. Psychiatry 67, 1128–1136 (2010).
Hetzel, A. & Rosenkranz, J. A. Distinct effects of repeated restraint stress on basolateral amygdala neuronal membrane properties in resilient adolescent and adult rats. Neuropsychopharmacology 39, 2114–2130 (2014).
Rau, A. R., Chappell, A. M., Butler, T. R., Ariwodola, O. J. & Weiner, J. L. Increased basolateral amygdala pyramidal cell excitability may contribute to the anxiogenic phenotype induced by chronic early-life stress. J. Neurosci. 35, 9730–9740 (2015).
Sharp, B. M. Basolateral amygdala and stress-induced hyperexcitability affect motivated behaviors and addiction. Transl. Psychiatry 7, e1194 (2017).
Masneuf, S. et al. Glutamatergic mechanisms associated with stress-induced amygdala excitability and anxiety-related behavior. Neuropharmacology 85, 190–197 (2014).
Lowery-Gionta, E. G. et al. Chronic stress dysregulates amygdalar output to the prefrontal cortex. Neuropharmacology 139, 68–75 (2018).
Blume, S. R., Padival, M., Urban, J. H. & Rosenkranz, J. A. Disruptive effects of repeated stress on basolateral amygdala neurons and fear behavior across the estrous cycle in rats. Sci. Rep. 9, 12292 (2019).
Partridge, J. G. et al. Stress increases GABAergic neurotransmission in CRF neurons of the central amygdala and bed nucleus stria terminalis. Neuropharmacology 107, 239–250 (2016).
He, F., Ai, H., Wang, M., Wang, X. & Geng, X. Altered neuronal activity in the central nucleus of the amygdala induced by restraint water-immersion stress in rats. Neurosci. Bull. 34, 1067–1076 (2018).
Giovanniello, J. et al. A central amygdala–globus pallidus circuit conveys unconditioned stimulus-related information and controls fear learning. J. Neurosci. 40, 9043–9054 (2020).
Murray, J. E. et al. Basolateral and central amygdala differentially recruit and maintain dorsolateral striatum-dependent cocaine-seeking habits. Nat. Commun. 6, 10088 (2015).
Liu, J. et al. Differential efferent projections of GABAergic neurons in the basolateral and central nucleus of amygdala in mice. Neurosci. Lett. 745, 135621 (2021).
Sah, P., Faber, E. S., Lopez De Armentia, M. & Power, J. The amygdaloid complex: anatomy and physiology. Physiol. Rev. 83, 803–834 (2003).
Limoges, A., Yarur, H. E. & Tejeda, H. A. Dynorphin/kappa opioid receptor system regulation on amygdaloid circuitry: implications for neuropsychiatric disorders. Front. Syst. Neurosci. 16, 963691 (2022).
Daviu, N., Bruchas, M. R., Moghaddam, B., Sandi, C. & Beyeler, A. Neurobiological links between stress and anxiety. Neurobiol. Stress 11, 100191 (2019).
McEwen, B. S., Nasca, C. & Gray, J. D. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 41, 3–23 (2016).
Shan, Q., Ge, M., Christie, M. J. & Balleine, B. W. The acquisition of goal-directed actions generates opposing plasticity in direct and indirect pathways in dorsomedial striatum. J. Neurosci. 34, 9196–9201 (2014).
Belin-Rauscent, A., Everitt, B. J. & Belin, D. Intrastriatal shifts mediate the transition from drug-seeking actions to habits. Biol. Psychiatry 72, 343–345 (2012).
Corbit, L. H., Nie, H. & Janak, P. H. Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biol. Psychiatry 72, 389–395 (2012).
Wendler, E. et al. The roles of the nucleus accumbens core, dorsomedial striatum, and dorsolateral striatum in learning: performance and extinction of Pavlovian fear-conditioned responses and instrumental avoidance responses. Neurobiol. Learn. Mem. 109, 27–36 (2014).
Weera, M. M., Schreiber, A. L., Avegno, E. M. & Gilpin, N. W. The role of central amygdala corticotropin-releasing factor in predator odor stress-induced avoidance behavior and escalated alcohol drinking in rats. Neuropharmacology 166, 107979 (2020).
Franklin, K. B. J. & Paxinos, G. The Mouse Brain in Stereotaxic Coordinates 3rd edn (Elsevier, 2008).
Tye, K. M. et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493, 537–541 (2013).
Cerniauskas, I. et al. Chronic stress induces activity, synaptic, and transcriptional remodeling of the lateral habenula associated with deficits in motivated behaviors. Neuron 104, 899–915.e898 (2019).
Bavley, C. C., Fischer, D. K., Rizzo, B. K. & Rajadhyaksha, A. M. Cav1.2 channels mediate persistent chronic stress-induced behavioral deficits that are associated with prefrontal cortex activation of the p25/Cdk5-glucocorticoid receptor pathway. Neurobiol. Stress 7, 27–37 (2017).
Freymann, J., Tsai, P. P., Stelzer, H. D., Mischke, R. & Hackbarth, H. Impact of bedding volume on physiological and behavioural parameters in laboratory mice. Lab. Anim. 51, 601–612 (2017).
Pałucha-Poniewiera, A., Podkowa, K., Rafało-Ulińska, A., Brański, P. & Burnat, G. The influence of the duration of chronic unpredictable mild stress on the behavioural responses of C57BL/6J mice. Behav. Pharmacol. 31, 574–582 (2020).
La-Vu, M. Q. et al. Sparse genetically defined neurons refine the canonical role of periaqueductal gray columnar organization. eLife 11, e77115 (2022).
Reis, F. M. et al. Dorsal periaqueductal gray ensembles represent approach and avoidance states. eLife 10, e64934 (2021).
Hilário, M. R., Clouse, E., Yin, H. H. & Costa, R. M. Endocannabinoid signaling is critical for habit formation. Front. Integr. Neurosci. 1, 6 (2007).
Lichtenberg, N. T. et al. The medial orbitofrontal cortex–basolateral amygdala circuit regulates the influence of reward cues on adaptive behavior and choice. J. Neurosci. 41, 7267–7277 (2021).
Sias, A. et al. A bidirectional corticoamygdala circuit for the encoding and retrieval of detailed reward memories. eLife 10, e68617 (2021).
Sherathiya, V. N., Schaid, M. D., Seiler, J. L., Lopez, G. C. & Lerner, T. N. GuPPy, a Python toolbox for the analysis of fiber photometry data. Sci. Rep. 11, 24212 (2021).
Roth, B. L. DREADDs for neuroscientists. Neuron 89, 683–694 (2016).
Vazey, E. M. & Aston-Jones, G. Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc. Natl Acad. Sci. USA 111, 3859–3864 (2014).
Qiu, M. H., Chen, M. C., Fuller, P. M. & Lu, J. Stimulation of the pontine parabrachial nucleus promotes wakefulness via extra-thalamic forebrain circuit nodes. Curr. Biol. 26, 2301–2312 (2016).
Pomrenze, M. B. et al. A corticotropin releasing factor network in the extended amygdala for anxiety. J. Neurosci. 39, 1030–1043 (2019).
Malvaez, M., Shieh, C., Murphy, M. D., Greenfield, V. Y. & Wassum, K. M. Distinct cortical–amygdala projections drive reward value encoding and retrieval. Nat. Neurosci. 22, 762–769 (2019).
Collins, A. L. et al. Nucleus accumbens cholinergic interneurons oppose cue-motivated behavior. Biol. Psychiatry 86, 388–396 (2019).
Schmider, E., Ziegler, M., Danay, E., Beyer, L. & Bühner, M. Is it really robust? Reinvestigating the robustness of ANOVA against violations of the normal distribution assumption. Methodology (Gott.) 6, 147–151 (2010).
Knief, U. & Forstmeier, W. Violating the normality assumption may be the lesser of two evils. Behav. Res. Methods 53, 2576–2590 (2021).
Giovanniello, J. et al. A dual-pathway architecture for stress to disrupt agency and promote habit. Dryad https://doi.org/10.5061/dryad.2jm63xt00 (2024).
Monteiro, S. et al. An efficient chronic unpredictable stress protocol to induce stress-related responses in C57BL/6 mice. Front. Psychiatry 6, 6 (2015).
Mineur, Y. S., Belzung, C. & Crusio, W. E. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav. Brain Res. 175, 43–50 (2006).
Fang, X. et al. Chronic unpredictable stress induces depression-related behaviors by suppressing AgRP neuron activity. Mol. Psychiatry 26, 2299–2315 (2021).
Gong, S. et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J. Neurosci. 27, 9817–9823 (2007).
Valjent, E., Bertran-Gonzalez, J., Hervé, D., Fisone, G. & Girault, J. A. Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends Neurosci. 32, 538–547 (2009).
Acknowledgements
This research was supported by NIH grant no. R01DA046679 (K.M.W.), NIH grant no. R01DA058374 (K.M.W.), NIH grant no. R01DA035443 (K.M.W.), NIH grant no. T32DA024635 (J.R.G.), NIH grant no. F32DA056201 (J.R.G.), A.P. Giannini Fellowship (J.R.G.), NIH grant no. K99MH135177 (J.R.G.), NIH grant no. TL4GM118977 (N.P.), NIH grant no. R01MH119089 (A.A.) and the Staglin Center for Behavior and Brain Sciences. UCLA Behavioral Testing Core provided space and behavioural testing equipment for the open field, elevated plus maze and light–dark emergence test.
Author information
Authors and Affiliations
Contributions
J.R.G. and K.M.W. conceptualized and designed the experiments, interpreted the data, and wrote the paper. J.R.G. executed all experiments and analysed the data. N.P., K.L., A. Wiener, A. Wang, C.O., H.O.U., A.L.Y., J.S.P., G.N. and G.E.V. assisted with experiments. M.S. assisted with rabies tracing experiments with resources from A.J.S. F.M.C.V.R. assisted with RTPP experiments, with advice and resources from A.A. A.C.S. wrote initial code for photometry analysis. K.R.-A. analysed spontaneous event frequency and amplitude on photometry data. M.M. contributed to the conceptualization and design of the experiments, initially optimized the instrumental conditioning procedures and data analysis, and provided important contributions to the interpretation of the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Patricia Janak, Stephanie Groman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Chronic mild unpredictable stress does not cause classic anxiety- and depression-like phenotypes.
Mice received 14 consecutive d of chronic mild unpredictable stress (stress) including twice daily exposure to 1 of 6 mild stressors at pseudorandom times and orders: damp bedding (16 h), tilted cage (16 h), white noise (80 db; 2 h), continuous illumination (8 h), physical restraint (2 h), footshock (0.7-mA, 1-s, 5 shocks/10 min) prior to subsequent testing in a battery of behavioral assays classically used to assess anxiety- and depression-like behavior. (a-c) Open field test. Distance traveled (a; 2-sided t-test: t(22) = 0.32, P = 0.75, 95% CI −4.43 – 3.24), time spent in center zone (b; 2-sided t-test: t(22) = 1.10, P = 0.28, 95% CI −16.87 − 5.16), and entries into center zone (c; 2-sided t-test: t(22) = 0.63, P = 0.54, 95% CI −10.03 – 5.36). (d-f) Elevated plus maze. Distance traveled (d; 2-sided t-test: t(22) = 0.08, P = 0.94, 95% CI −2.72 – 2.92), time spent in open arms (e; 2-sided t-test: t(22) = 0.01, P = 0.92, 95% CI −26.17 – 23.70), and entries into open arms (f; 2-sided t-test: t(22) = 0.23, P = 0.82, 95% CI −6.56 – 5.23). (g-i) Light-dark emergence test. Distance traveled in light zone (g; 2-sided t-test: t(22) = 0.97, P = 0.34, 95% CI −0.73 - 2.01), time spent in light zone (h; 2-sided t-test: t(22) = 1.57, P = 0.13, 95% CI −11.93 - 86.98), and entries into light zone (I; 2-sided t-test: t(22) = 1.37, P = 0.19, 95% CI −1.708 to 8.041). (j-k) Sucrose preference test. Average amount consumed of water and 10% sucrose over 24 h (j; 2-way ANOVA: Solution: F(1, 22) = 113.20, P < 0.0001; Stress: F(1, 22) = 0.14, P = 0.71, Solution x Stress: F(1, 22) = 0.02, P = 0.89) and ratio of sucrose:water consumed (k; t(22) = 0.03, P = 0.98, 95% CI −0.064 - 0.063). (l-m) Progressive ratio Tests. Total presses (l; 2-sided t-test: t(22) = 2.13, P = 0.04, 95% CI 72.94 - 5346) and breakpoint (k; Final ratio completed; 2-sided t-test: t(22) = 2.12, P = 0.46, 95% CI 1.02 - 94.31). Control N = 12 (6 male), Stress N = 12 (6 male) mice. Males = closed circles, Females = open circles. Data presented as mean +/− SEM. *P < 0.05, ***P < 0.001. Our stress procedure does not affect general locomotor activity or avoidance of anxiogenic spaces or create an anhedonia phenotype. Rather this stress procedure appears to cause elevated motivation to exert effort to obtain reward. This contrasts with more severe, longer-lasting stress procedures, which do produce anxiety- and depression-like phenotypes in these tasks98,99,100. Thus, our stress procedure models chronic, low-level stress.
Extended Data Fig. 2 Food-port entries during training and probe tests following handling control or chronic stress.
(a) Food-port entry rate across training for subjects in the devaluation experiment. 2-way ANOVA: Training: F(2.42, 108.90) = 3.17, P = 0.04; Stress: F(1, 45) = 0.07, P = 0.79; Training x Stress: F(3, 135) = 0.57, P = 0.64. (b) Food-port entries during the devaluation probe tests. 2-way ANOVA: Value: F(1, 45) = 6.77, P = 0.01, Stress: F(1, 45) = 0.29, P = 0.60; Stress x Value: F(1, 45) = 2.42, P = 0.13. Control N = 22 (13 male), Stress N = 25 (12 male) mice. (c) Food-port entry rate across training for subjects in the contingency degradation experiment. 3-way ANOVA: Training: F(2.84, 62.10) = 6.44, P = 0.001; Stress: F(1, 25) = 0.01, P = 0.91; Future Contingency Degradation group: F(1, 25) = 1.27, P = 0.27; Training x Stress: F(3, 75) = 1.62, P = 0.19; Training x Group: F(3, 75) = 0.24, P = 0.87; Stress x Group: F(1, 25) = 0.004, P = 0.95; Training x Stress x Group: F(3, 75) = 1.49, P = 0.23. (d) Food-port entries during the probe test 24 h following contingency degradation or non-degraded control. 2-way ANOVA: Stress x Contingency Degradation Group: F(1, 25) = 18.88, P = 0.0002; Contingency Degradation: F(1, 25) = 4.29, P = 0.05; Stress: F(1, 25) = 1.41, P = 0.25. Control, Non-degraded N = 7 (3 male), Control, Degraded N = 7 (3 male), Stress Non-degraded N = 7 (3 male) Stress Degraded N = 8 (4 male) mice. Males = solid lines, Females = dashed lines. Data presented as mean +/− SEM. *P < 0.05, **P < 0.01, corrected for multiple comparisons.
Extended Data Fig. 3 Lever presses and food-port entries during contingency degradation.
(a) Contingency degradation Procedure. Following stress and training, half the subjects in each group received a 20-min contingency degradation session during which lever pressing continued to earn reward with a probability of 0.1, but reward was also delivered non-contingently with the same probability. This session was identical for non-degraded controls, except they did not receive free rewards. (b) 3-way ANOVA: Press rate in 1-min bins during the contingency degradation session. Time x Contingency Degradation Group: F(19, 475) = 2.03, P = 0.0063; Time x Stress: F(19, 475) = 2.43, P = 0.0007; Stress x Group: F(1, 25) = 0.0001, P = 0.99; Time: F(9.17, 229.20) = 2.13, P = 0.03; Stress: F(1, 25) = 1.36, P = 0.26; Degradation Group: F(1, 25) = 68.23, P < 0.0001; Time x Stress x Degradation Group: F(19, 475) = 1.30, P = 0.19. Contingency degradation cause lower press rates across the session in both control (Time x Contingency Degradation Group: F(12, 228) = 2.47, P = 0.0009; Time: F(6.62, 79.39) = 2.47, P = 0.03; Degradation Group: F(1, 12) = 45.16, P < 0.0001) and stressed (Contingency Degradation Group: F(1, 13) = 28.22, P = 0.0001; Time: F(6.01, 78.16) = 2.19, P = 0.05; Time x Contingency Degradation Group: F(19, 247) = 1.10, P = 0.35) mice. (c) Rate of entry into the food-delivery port in 1-min bins during the contingency degradation session. 3-way ANOVA: Time x Contingency Degradation Group: F(19, 475) = 3.80, P < 0.0001; Time x Stress: F(19, 475) = 1.20, P = 0.26; Stress x Group: F(1, 25) = 0.006, P = 0.94; Time: F(6.26, 156.60) = 7.53, P < 0.0001; Stress: F(1, 25) = 2.51, P = 0.13; Degradation Group: F(1, 25) = 1.37, P = 0.5; Time x Stress x Degradation Group: F(19, 475) = 0.86, P = 0.63. Control, Non-degraded N = 7 (3 male), Control, Degraded N = 7 (3 male), Stress Non-degraded N = 7 (3 male) Stress Degraded N = 8 (4 male) mice. Males = closed circles/solid lines, Females = open circles/dashed lines. Data presented as mean +/− SEM. *P < 0.05, **P < 0.01, corrected for multiple comparisons.
Extended Data Fig. 4 BLA and CeA directly project to DMS.
(a) Top: Anterograde tracing approach. Infusion of an AAV expressing mCherry into the CeA. Bottom: mCherry labeling at infusion site in CeA (left) and mCherry-labeled fibers in the DMS (right). N = 4 (2 male) mice. We observed mCherry-expressing putative fibers in the DMS but not dorsolateral striatum. Expression was also detected in other well-known CeA projection targets such as the bed nucleus of the stria terminalis. (b) Top: Retrograde tracing approach. We infused the fluorescently labeled retrograde tracer Fluorogold into the DMS. Bottom: Fluorogold labeling at infusion site in DMS (left) and fluorogold-labeled, DMS-projecting cell bodies in BLA and CeA (middle), with CeA magnified (right). Labeled cells was detected in both BLA and CeA, indicating that both BLA and CeA directly project to DMS. Labeling was greater in BLA than CeA, indicating the BLA→DMS pathway is denser than the CeA→DMS pathway. N = 4 (2 male) mice. (c) Top: Approach for rabies trans-synaptic retrograde tracing of DMS Drd1+ striatal neurons. We used rabies tracing to confirm monosynaptic amygdala projections onto DMS neurons. We infused a starter virus expressing cre-dependent TVA-oG-GFP into the DMS of mice expressing cre-recombinase under the control of dopamine receptor 1 (D1-Cre) or adenosine 2a receptor (A2A-Cre) genes101,102, followed by ΔG-deleted rabies-mCherry to retrogradely label cells that synapse onto DMS D1 or A2A neurons. Bottom: Starter oG virus (green) and ΔG-deleted rabies-mCherry (red) expression in DMS Drd1+ neurons (left) and rabies-labeled, DMS D1-projecting cell bodies in the BLA and CeA (right), consistent with prior reports30,34. Representative example from N = 4 (3 males) mice. (d) Top: Approach for rabies trans-synaptic retrograde tracing of DMS Adora2a+ neurons. Bottom: Starter ΔG virus (green) and rabies-mCherry (red) expression in DMS Adora2a+ neurons (left) and rabies-labeled, DMS A2A-projecting cell bodies in the BLA and CeA (right). Representative example N = 4 (3 males) mice. Scale bars = 200 µm. Combined, these data confirm that both BLA and CeA directly project to the DMS and are, thus, poised to influence the learning that supports goal-directed decision making and habit formation.
Extended Data Fig. 5 Food-port entries during training with fiber photometry recording of BLA→DMS or CeA→DMS calcium activity following handling control or chronic stress.
(a) Food-port entry rates across training for BLA→DMS GCaMP8s mice. 2-way ANOVA: Training: F(2.47, 46.99) = 0.65, P = 0.56; Stress: F(1, 19) = 0.05, P = 0.82; Training x Stress: F(3, 57) = 0.24, P = 0.87. BLA Control N = 9 (4 male), BLA Stress N = 12 (5 male) mice. (b) Food-port entry rates across training for CeA→DMS GCaMP8s mice. 2-way ANOVA: Training: F(2.36, 47.19) = 0.89, P = 0.43; Stress: F(1, 20) = 2.71, P = 0.12; Training x Stress: F(3, 60) = 0.09, P = 0.96. CeA Control N = 11 (6 male), CeA Stress N = 11 (4 male) mice. Males = solid lines, Females = dashed lines. Data presented as mean +/− SEM.
Extended Data Fig. 6 BLA→DMS and CeA→DMS pathway baseline activity and pathway responses to unpredicted rewarding and aversive events in control and stressed mice.
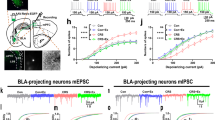
(a-j) Following instrumental training (Fig. 2), we used fiber photometry to record GCaMP8s fluorescent changes in either BLA (top) or CeA (bottom) neurons that project to the DMS in response to unpredicted food-pellet reward deliveries or unpredicted 2-s, 0.7 mA footshocks in control and stressed mice. (a) Trial-averaged Z-scored Δf/F BLA→DMS GCaMP8s fluorescence changes around unpredicted food-pellet reward delivery. (b) Trial-averaged quantification of area under the BLA→DMS GCaMP8s Z-scored ∆f/F curve (AUC) during the 3-s period prior to (baseline) and following reward collection. 2-way ANOVA: Stress x Reward: F(1, 18) = 10.88, P = 0.004; Reward: F(1, 18) = 1.19; P = 0.03; Stress: F(1, 18) = 1.77, P = 0.20. (c) Trial-averaged Z-scored Δf/F CeA→DMS GCaMP8s fluorescence changes around unpredicted food-pellet reward delivery. (d) Trial-averaged quantification CeA→DMS GCaMP8s Z-scored ∆f/F AUC during the 3-s period prior to and following reward collection. 2-way ANOVA: Stress x Reward: F(1, 20) = 11.79, P = 0.02; Reward: F(1, 20) = 8.14, P = 0.01; Stress F(1, 20) = 4.49, P = 0.05. (e) Trial-averaged Z-scored Δf/F BLA→DMS GCaMP8s fluorescence changes around unpredicted footshock. (f) Trial-averaged quantification of BLA→DMS GCaMP8s Z-scored ∆f/F AUC during the 1-s acute shock response compared to a 1-s pre-shock baseline. 2-way ANOVA: Shock: F(1, 18) = 8.53, P = 0.01; Stress: F(1, 18) = 0.14, P = 0.71; Stress x Shock F(1, 18) = 1.73, P = 0.21 (g) Trial-averaged quantification of BLA→DMS GCaMP8s Z-scored ∆f/F AUC during 2-s post-shock period. 2-sided t-test: t(18) = 2.26, P = 0.04, 95% CI −2.68 to −0.10. (h) Trial-averaged Z-scored Δf/F CeA→DMS GCaMP8s fluorescence changes around unpredicted footshock. (i) Trial-averaged quantification of CeA→DMS GCaMP8s Z-scored ∆f/F AUC during the 1-s acute shock response, compared to baseline. 2-way ANOVA: Shock: F(1, 20) = 28.24, P < 0.0001; Stress: F(1, 20) = 0.22, P = 0.64; Stress x Shock: F(1, 20) = 3.20, P = 0.09. (j) Trial-averaged quantification of CeA→DMS GCaMP8s Z-scored ∆f/F AUC during 2-s post-shock period. 2-sided t-test: t(20) = 0.88, P = 0.39, 95% CI −0.99 - 2.43. BLA Control N = 8 (4 male), BLA Stress N = 12 (5 male) mice. CeA Control N = 11 (6 male), CeA Stress N = 11 (4 male) mice. BLA→DMS projections are activated by unpredicted rewards and this is attenuated by prior chronic stress. Conversely, CeA→DMS projections are not normally robustly activated by unpredicted rewards, but are activated by unpredicted rewards following chronic stress. Interestingly, unpredicted rewards robustly activated CeA→DMS projections here, but rewards did not evoke such a response early in instrumental training (Fig. 2m). Rather rewards responses developed with training. This indicates that stress-induced engagement of the CeA→DMS pathway may require repeated reward experience, which may reflect engagement of this pathway with repeated reinforcement and/or opportunity to learn the value or salience of the reward. We speculate this CeA→DMS engagement could be a compensatory mechanism triggered in response to the lack of engagement of the BLA→DMS pathway. Both BLA→DMS and CeA→DMS pathways are acutely activated by unpredicted footshock regardless of prior stress. Chronic stress reduces post-shock activity in the BLA→DMS pathway. (k-l) Frequency (k; 2-way ANOVA: Training: F(2.41, 45.69) = 0.17, P = 0.88; Stress: F(1, 19) = 0.08, P = 0.78; Training x Stress: F(3, 57) = 0.85, P = 0.47) and amplitude (l; 2-way ANOVA: Training: F(2.48, 47.10) = 0.86, P = 0.45; Stress: F(1, 19) = 0.03, P = 0.85; Training x Stress: F(3, 57) = 1.37, P = 0.26) of Z-scored Δf/F spontaneous calcium activity of BLA→DMS projections during the 3-min baseline period prior to each training session in handled control and stressed mice. (m-n) Frequency (m; 2-way ANOVA: Training: F(2.70, 53.97) = 0.21, P = 0.88; Stress F(1, 20) = 3.03, P = 0.10; Training x Stress: F(3, 60) = 0.55, P = 0.65) and amplitude (n; 2-way ANOVA: Training: F(2.59, 51.83) = 0.32, P = 0.78; Stress: F(1, 20) = 3.70, P = 0.07; Training x Stress: F(3, 60) = 0.75, P = 0.52) of Z-scored Δf/F spontaneous calcium activity of CeA→DMS projections during the 3-min baseline period prior to each training session handled control and stressed mice. Chronic stress did not alter baseline spontaneous calcium activity in either pathway. (o) Trial-averaged Z-scored Δf/F CeA→DMS GCaMP8s fluorescence changes aligned to reward collection during training, with 40-s post-collection window. Blue line is the average time of the next lever press (light blue bar = s.e.m.). In stressed mice, CeA→DMS neurons respond to earned reward and this activity takes ~30 s on average to come back to baseline. Control N = 11 (6 male), Stress N = 11 (4 male) mice. Males = solid lines, Females = dashed lines. Data presented as mean +/− SEM. **P < 0.01, corrected for multiple comparisons.
Extended Data Fig. 7 Food-port entries during training with BLA→DMS manipulations and devaluation probe tests.
(a-b) Optogenetic inactivation of BLA→DMS projections at reward during instrumental learning. (a) Food-port entries across training. 2-way ANOVA: Training: F(2.03, 38.55) = 3.30, P = 0.05; Virus: F(1, 19) = 0.14, P = 0.71; Training x Virus: F(3, 57) = 0.43, P = 0.73. (b) Food-port entry rates during devaluation probe tests. 2-way ANOVA: Stress x Value: F(1, 19) = 4.38, P = 0.05; Stress: F(1, 19) = 0.47, P = 0.50; Value: F(1, 19) = 0.39, P = 0.54. eYFP N = 10 (5 males), Arch N = 11 (5 male) mice. (c-d) Optogenetic activation of BLA→DMS projections during post-stress instrumental learning. (c) Food-port entry rate across training. 3-way ANOVA: Training: F(2.5, 82.82) = 6.47, P = 0.001; Stress: F(1, 33) = 3.78, P = 0.06; Virus: F(1, 33) = 0.02, P = 0.89; Training x Stress: F(3, 99) = 0.67, P = 0.57; Training x Virus: F(3, 99) = 0.45, P = 0.72; Stress x Virus: F(1, 33) = 2.18, P = 0.15; Training x Stress x Virus: F(3, 99) = 0.26, P = 0.86. (d) Food-port entry rate during the devaluation probe tests. 3-way ANOVA: Value: F(1, 33) = 15.65, P = 0.0004; Stress: F(1, 33) = 0.23, P = 0.63; Virus: F(1, 33) = 0.20, P = 0.65; Value x Stress: F(1, 33) = 2.75, P = 0.11; Value x Virus: F(1, 33) = 0.09, P = 0.76; Virus x Stress: F(1, 33) = 0.17, P = 0.68; Value x Stress x Virus: F(1, 33) = 1.73, P = 0.20. Control, Value: F(1, 16) = 12.42, P = 0.003; Virus: F(1, 16) = 0.0007, P = 0.98; Value x Virus: F(1, 16) = 0.40, P = 0.53. Stress, Value: F(1, 17) = 3.46, P = 0.08; Virus: F(1, 17) = 0.45, P = 0.51; Value x Virus: F(1, 17) = 1.71, P = 0.21. Control eYFP N = 11 (7 male), Control ChR2 N = 7 (4 males), Stress eYFP N = 9 (2 male), Stress ChR2 N = 10 Stress (3 male) mice. (e-f) Chemogenetic activation of BLA→DMS projections during post-stress instrumental learning. (e) Food-port entry rate across training. 3-way ANOVA: Training: F(2.55, 84.12) = 1.64, P = 0.19; Stress: F(1, 33) = 0.05, P = 0.95; Virus: F(1, 33) = 0.08, P = 0.78; Training x Stress: F(3, 99) = 0.16, P = 0.92; Training x Virus: F(3, 99) = 0.21, P = 0.89; Stress x Virus: F(1, 33) = 0.02, P = 0.89; Training x Stress x Virus: F(3, 99) = 3.07, P = 0.03. (f) Food-port entry rate during the devaluation probe test. Planned comparisons 2-sided t-test valued v. devalued, Control mCherry: t(20) = 1.88, P = 0.07, 95% CI −0.21 − 5.41; Control hM3Dq: t(10) = 1.32, P = 0.20, 95% CI −1.40 − 6.54; Stress mCherry: t(16) = 0.75, P = 0.46, 95% CI −2.04 − 4.44; Stress hM3Dq: t(18) = 3.36, P = 0.002, 95% CI 2.01 − 8.16. Control mCherry N = 12 (7 male), Stress mCherry N = 9 (5 male), Stress hM3Dq N = 10 Stress (5 male) mice. Males = solid lines, Females = dashed lines. Data presented as mean +/− SEM. **P < 0.01, corrected for multiple comparisons.
Extended Data Fig. 8 Manipulation of BLA or CeA terminals in DMS is neither rewarding or aversive.
(a) Following training and testing (Fig. 3h–n) mice receive a real-time place preference test in which 1 side of a 2-chamber apparatus was paired with optogenetic inhibition of BLA axons and terminals in the DMS. Average percent time spent in light-paired chamber across 2, 10-min sessions (one with light paired with each side). 2-sided t-test: t(19) = 0.65, P = 0.52, 95% CI −0.04 − 0.08. eYFP N = 10 (5 male), Arch N = 11 (5 male) mice. Males = closed circles, Females = open circles. Data presented as mean +/− SEM. (b-c) Following training and testing mice receive a real-time place preference test in which 1 side of a 2-chamber apparatus was paired with optogenetic stimulation of DMS-projecting CeA neurons. (b) Average percent time spent in light paired chamber across 2, 10-min sessions (one with light paired with each side) in handled control subjects. 2-sided t-test: t(21) = 1.75, P = 0.10, 95% CI −0.79 − 9.06. eYFP N = 17 (9 male), ChR2 N = 6 (3 male) mice. (c) Average percent time spent in light paired chamber across 2, 10-min sessions (one with light paired with each side) in subjects with a prior once/daily stress for 14 d. 2-sided t-test: t(16) = 0.52, P = 0.61, 95% CI −3.74 − 6.17. eYFP N = 8 (4 male), ChR2 N = 10 (6 male) mice. Males = closed circles, Females = open circles. Data presented as mean +/− SEM.
Extended Data Fig. 9 Food-port entries during training with CeA→DMS manipulations and devaluation probe tests.
(a-b) Optogenetic inhibition of CeA→DMS projections during instrumental overtraining. (a) Food-port entry rates across training. 2-way ANOVA: Training: F(2.29, 45.82) = 1.81, P = 0.17; Virus: F(1, 20) = 0.67, P = 0.42; Training x Virus: F(8, 160) = 0.60, P = 0.77. (b) Food-port entry rates during the devaluation probe tests. 2-way ANOVA: Virus x Value: F(1, 20) = 4.51, P = 0.046; Value: F(1, 20) = 1.47, P = 0.24; Virus: F(1, 20) = 0.41, P = 0.53;. eYFP N = 11 (3 male), Arch N = 11 (7 male) mice. (c-d) Optogenetic inactivation of CeA→DMS projections at reward during post-stress learning. (c) Food-port entry rates across training. 3-way ANOVA: Training: F(2.63, 84.18) = 3.21, P = 0.03; Stress: F(1, 32) = 0.60, P = 0.44; Virus: F(1, 32) = 4.75, P = 0.04; Training x Stress: F(3, 96) = 1.55, P = 0.21; Training x Virus: F(3, 96) = 2.42, P = 0.07; Stress x Virus: F(1, 32) = 0.04, P = 0.84; Training x Stress x Virus: F(3, 96) = 1.14, P = 0.34. (k) Food-port entry rate during the devaluation probe test. 3-way ANOVA: Value x Stress x Virus: F(1, 32) = 0.03, P = 0.86; Value: F(1, 32) = 6.44, P = 0.02; Stress: F(1, 32) = 2.02, P = 0.16; Virus: F(1, 32) = 1.09, P = 0.30; Value x Stress: F(1, 3) = 0.99, P = 0.33; Value x Virus: F(1, 32) = 0.02, P = 0.89; Virus x Stress: F(1, 32) = 0.24, P = 0.63. Control groups, 2-way ANOVA: Value x Virus: F(1, 18) = 0.09, P = 0.77; Value: F(1, 18) = 1.99, P = 0.17; Virus: F(1, 18) = 0.21, P = 0.65. Stress groups, 2-way ANOVA: Value x Virus: F(1, 14) = 0.0005, P = 0.98; Value: F(1, 14) = 3.94, P = 0.06; Virus: F(1, 14) = 0.85, P = 0.87. Control eYFP N = 9 (5 male), Control Arch N = 11 (4 male), Stress eYFP N = 7 (6 male), Stress Arch N = 9 (5 male) mice. (e-f) Chemogenetic inhibition of CeA→DMS projections during post-stress instrumental learning. (e) Food-port entry rates across training. Training: F(1.85, 75.67) = 2.02, P = 0.14; Stress: F(1, 41) = 4.42, P = 0.04; Virus: F(1, 41) = 0.41, P = 0.53; Training x Stress: F(3, 123) = 3.08, P = 0.03; Training x Virus: F(3, 123) = 0.64, P = 0.59; Stress x Virus: F(1, 41) = 0.20, P = 0.66; Training x Stress x Virus: F(3, 123) = 3.23, P = 0.02. (f) Food-port entry rates during the devaluation probe tests. Planned comparisons 2-sided t-test valued v. devalued, Control mCherry: t(11) = 1.94, P = 0.06, 95% CI −0.25 - 12.07; Control hM4Di: t(12) = 0.38, P = 0.71, 95% CI −4.81 − 7.03; Stress mCherry: t(10) = 0.05, P = 0.96, 95% CI −6.33 − 5.99; Stress hM4Di: t(8) = 0.47, P = 0.64, 95% CI −5.47 − 8.76. Control mCherry N = 12 (5 male), Control hM4Di N = 13 (8 male), Stress mCherry N = 11 (5 male), Stress hM4Di N = 9 (4 male) mice. (g-h) Optogenetic stimulation of CeA→DMS projections at reward during learning following subthreshold once daily stress (SubStress). (g) Food-port entry rate across training. 2-way ANOVA: Training: F(1.73, 34.50) = 0.89, P = 0.41; Virus: F(1, 20) = 0.46, P = 0.51; Training x Virus: F(3, 60) = 0.39, P = 0.76. (g) Food-port entry rate during the devaluation probe test. 2-way ANOVA: Virus x Value: F(1, 20) = 1.37, P = 0.26; Virus: F(1, 20) = 0.005, P = 0.94; Value: F(1, 20) = 1.36, P = 0.26. eYFP N = 10 (4 male), ChR2 N = 12 (6 male) mice. Males = solid lines, Females = dashed lines. Data presented as mean +/− SEM.
Extended Data Fig. 10 Optogenetic stimulation of CeA→DMS projections in control mice.
(a) We used an intersectional approach to express the excitatory opsin Channelrhodopsin 2 (ChR2), or a fluorophore control in DMS-projecting CeA neurons and implanted optic fibers above the CeA. (b) Representative images of retro-cre expression in DMS and immunofluorescent staining of cre-dependent ChR2 expression in CeA (scale bars = 200 µm) and map of retro-cre in DMS and cre-dependent ChR2 expression in CeA for all mice. (c) Procedure. Lever presses earned food pellet rewards on a random-ratio (RR) reinforcement schedule. We used blue light (473 nm, 10 mW, 20 Hz, 25-ms pulse width, 2 s) to stimulate CeA→DMS neurons during the collection of each earned reward in mice without a history of stress. Mice were then given a lever-pressing probe test in the Valued state, prefed on untrained food-pellet type to control for general satiety, and Devalued state prefed on trained food-pellet type to induce sensory-specific satiety devaluation (order counterbalanced). (d) Press rates across training. 2-way ANOVA: Training: F(1.85, 38.75) = 62.18, P < 0.0001; Virus: F(1, 21) = 0.23, P = 0.64; Training x Virus: F(3, 63) = 0.05, P = 0.98. (e) Food-port entries across training. 2-way ANOVA: Training: F(2.42, 50.77) = 2.00, P = 0.14; Virus: F(1, 21) = 1.85, P = 0.19; Training x Virus: F(3, 63) = 0.22, P = 0.88. (f) Press rate during the devaluation probe test. 2-way ANOVA: Value: F(1, 21) = 20.32, P = 0.0002; Virus: F(1,21) = 0.92, P = 0.35; Virus x Value: F(1, 21) = 1.17, P = 0.29. (g) Devaluation index. 2-sided t-test: t(21) = 1.37, P = 0.19, 95% CI −0.25 - 0.05. (h) Food-port entries during the devaluation probe tests. 2-way ANOVA: Value: F(1, 21) = 30.07, P < 0.0001; Virus: F(1, 21) = 0.12, P = 0.73; Virus x Value: F(1, 21) = 3.45, P = 0.08. eYFP N = 17 (9 male), ChR2 N = 6 (3 male) mice. Data presented as mean +/− SEM. ** P < 0.01, *** P < 0.001, corrected for multiple comparisons. Optogenetic activation of CeA→DMS projections at reward during learning neither affects affect acquisition of the lever-press behavior, nor the action-outcome learning needed to support flexible goal-directed decision making during the devaluation test.
Supplementary information
Supplementary Tables
Supplementary Tables 1–6 including full statistical reporting for main text data, body weight across training, sensory-specific satiety prefeed consumption, average post-probe-test choice consumption, key reagents and example stress protocol.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Giovanniello, J.R., Paredes, N., Wiener, A. et al. A dual-pathway architecture for stress to disrupt agency and promote habit. Nature 640, 722–731 (2025). https://doi.org/10.1038/s41586-024-08580-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-08580-w
This article is cited by
-
Neurobiology of resilience to early life stress
Neuropsychopharmacology (2025)
-
How chronic stress warps decision-making
Nature (2025)