Abstract
Clonal haematopoiesis arises when a haematopoietic stem cell (HSC) acquires a mutation that confers a competitive advantage over wild-type HSCs, resulting in its clonal expansion. Individuals with clonal haematopoiesis are at increased risk of developing haematologic neoplasms and other age-related inflammatory illnesses1,2,3,4. Suppressing the expansion of mutant HSCs may prevent these outcomes; however, such interventions have not yet been identified. The most common clonal haematopoiesis driver mutations are in the DNMT3A gene, with arginine 882 (R882) being a mutation hotspot1,2,3,5,6,7. Here we show that mouse haematopoietic stem and progenitor cells (HSPCs) carrying the Dnmt3aR878H/+ mutation, equivalent to human DNMT3AR882H/+, have increased mitochondrial respiration compared with wild-type cells and are dependent on this metabolic reprogramming for their competitive advantage. Treatment with metformin, an anti-diabetic drug that inhibits mitochondrial respiration8, reduced the competitive advantage of Dnmt3aR878H/+ HSCs. Through a multi-omics approach, we found that metformin acts by enhancing methylation potential in Dnmt3aR878H/+ HSPCs and reversing the aberrant DNA CpG methylation and histone H3 K27 trimethylation profiles in these cells. Metformin also reduced the competitive advantage of human DNMT3AR882H HSPCs generated by prime editing. Our findings provide preclinical rationale for investigating metformin as a preventive intervention against DNMT3A R882 mutation-driven clonal haematopoiesis in humans.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Raw and processed data from the bulk RNA-seq, scRNA-seq, RRBS and ChIP–seq experiments are deposited in a publicly accessible Gene Expression Omnibus (GEO) repository under the accession number GSE255101. Source data are provided with this paper.
Code availability
All code used for bioinformatics analysis is publicly available via GitHub at https://github.com/veroniquevoisin/DNMT3A.
References
Jaiswal, S. et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 377, 111–121 (2017).
Jaiswal, S. et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 371, 2488–2498 (2014).
Genovese, G. et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 371, 2477–2487 (2014).
Weeks, L. D. & Ebert, B. L. Causes and consequences of clonal hematopoiesis. Blood 142, 2235–2246 (2023).
Bick, A. G. et al. Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature 586, 763–768 (2020).
Buscarlet, M. et al. DNMT3A and TET2 dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood 130, 753–762 (2017).
Ley, T. J. et al. DNMT3A mutations in acute myeloid leukemia. N. Engl. J. Med. 363, 2424–2433 (2010).
Bridges, H. R. et al. Structural basis of mammalian respiratory complex I inhibition by medicinal biguanides. Science 379, 351–357 (2023).
Venugopal, K., Feng, Y., Shabashvili, D. & Guryanova, O. A. Alterations to DNMT3A in hematologic malignancies. Cancer Res. 81, 254–263 (2021).
Young, A. L., Tong, R. S., Birmann, B. M. & Druley, T. E. Clonal hematopoiesis and risk of acute myeloid leukemia. Haematologica 104, 2410–2417 (2019).
Jawad, M. et al. DNMT3A R882 mutations confer unique clinicopathologic features in MDS including a high risk of AML transformation. Front. Oncol. 12, 849376 (2022).
Larsson, C. A., Cote, G. & Quintas-Cardama, A. The changing mutational landscape of acute myeloid leukemia and myelodysplastic syndrome. Mol. Cancer Res. 11, 815–827 (2013).
Russler-Germain, D. A. et al. The R882H DNMT3A mutation associated with AML dominantly inhibits wild-type DNMT3A by blocking its ability to form active tetramers. Cancer Cell 25, 442–454 (2014).
Kim, S. J. et al. A DNMT3A mutation common in AML exhibits dominant-negative effects in murine ES cells. Blood 122, 4086–4089 (2013).
Smith, A. M. et al. Functional and epigenetic phenotypes of humans and mice with DNMT3A overgrowth syndrome. Nat. Commun. 12, 4549 (2021).
Loberg, M. A. et al. Sequentially inducible mouse models reveal that Npm1 mutation causes malignant transformation of Dnmt3a-mutant clonal hematopoiesis. Leukemia 33, 1635–1649 (2019).
Nam, A. S. et al. Single-cell multi-omics of human clonal hematopoiesis reveals that DNMT3A R882 mutations perturb early progenitor states through selective hypomethylation. Nat. Genet. 54, 1514–1526 (2022).
LaMoia, T. E. & Shulman, G. I. Cellular and molecular mechanisms of metformin action. Endocr. Rev. 42, 77–96 (2021).
Seo, B. B. et al. Molecular remedy of complex I defects: rotenone-insensitive internal NADH-quinone oxidoreductase of Saccharomyces cerevisiae mitochondria restores the NADH oxidase activity of complex I-deficient mammalian cells. Proc. Natl Acad. Sci. USA 95, 9167–9171 (1998).
Wheaton, W. W. et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife 3, e02242 (2014).
Dowling, R. J. et al. Metformin pharmacokinetics in mouse tumors: implications for human therapy. Cell Metab. 23, 567–568 (2016).
Izzo, F. et al. DNA methylation disruption reshapes the hematopoietic differentiation landscape. Nat. Genet. 52, 378–387 (2020).
Ducker, G. S. et al. Human SHMT inhibitors reveal defective glycine import as a targetable metabolic vulnerability of diffuse large B-cell lymphoma. Proc. Natl Acad. Sci. USA 114, 11404–11409 (2017).
Konteatis, Z. et al. Discovery of AG-270, a first-in-class oral MAT2A inhibitor for the treatment of tumors with homozygous MTAP deletion. J. Med. Chem. 64, 4430–4449 (2021).
Dai, Y. J. et al. Conditional knockin of Dnmt3a R878H initiates acute myeloid leukemia with mTOR pathway involvement. Proc. Natl Acad. Sci. USA 114, 5237–5242 (2017).
Fiumara, M. et al. Genotoxic effects of base and prime editing in human hematopoietic stem cells. Nat. Biotechnol. 42, 877–891 (2024).
Cuyas, E. et al. Metformin regulates global DNA methylation via mitochondrial one-carbon metabolism. Oncogene 37, 963–970 (2018).
Cuyas, E. et al. Metformin directly targets the H3K27me3 demethylase KDM6A/UTX. Aging Cell 17, e12772 (2018).
Garcia-Calzon, S. et al. DNA methylation partially mediates antidiabetic effects of metformin on HbA1c levels in individuals with type 2 diabetes. Diabetes Res. Clin. Pract. 202, 110807 (2023).
Kristensen, D. T. et al. Metformin use and risk of myeloproliferative neoplasms: a Danish population-based case–control study. Blood Adv. 8, 4478–4485 (2024).
Nelson, J. W. et al. Engineered pegRNAs improve prime editing efficiency. Nat. Biotechnol. 40, 402–410 (2022).
Akalin, A. et al. methylKit: a comprehensive R package for the analysis of genome-wide DNA methylation profiles. Genome Biol. 13, R87 (2012).
Akalin, A. et al. Base-pair resolution DNA methylation sequencing reveals profoundly divergent epigenetic landscapes in acute myeloid leukemia. PLoS Genet. 8, e1002781 (2012).
Wang, H. Q., Tuominen, L. K. & Tsai, C. J. SLIM: a sliding linear model for estimating the proportion of true null hypotheses in datasets with dependence structures. Bioinformatics 27, 225–231 (2011).
Lee, S., Cook, D. & Lawrence, M. plyranges: a grammar of genomic data transformation. Genome Biol. 20, 4 (2019).
Wang, Q. et al. Exploring epigenomic datasets by ChIPseeker. Curr. Protoc. 2, e585 (2022).
Yu, G., Wang, L. G. & He, Q. Y. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 31, 2382–2383 (2015).
Gehrke, S. et al. Red blood cell metabolic responses to torpor and arousal in the hibernator Arctic ground squirrel. J. Proteome Res. 18, 1827–1841 (2019).
Nemkov, T., Reisz, J. A., Gehrke, S., Hansen, K. C. & D’Alessandro, A. High-throughput metabolomics: isocratic and gradient mass spectrometry-based methods. Methods Mol. Biol. 1978, 13–26 (2019).
Nemkov, T., Hansen, K. C. & D’Alessandro, A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun. Mass Spectrom. 31, 663–673 (2017).
Acknowledgements
This project was supported by funds from an Invest in Research Award from the Princess Margaret Cancer Foundation, a Medicine by Design (MbD) Award from the University of Toronto, a New Investigator Award from the Leukemia Research Foundation, and a Project Grant (PJT-175186) from the Canadian Institutes of Health Research. J.E.D. is supported by funds from the Princess Margaret Cancer Centre Foundation, Canadian Institutes for Health Research (RN380110–409786), Canadian Cancer Society (grants 703212 (end date 2019) and 706662 (end date 2025)), Terry Fox New Frontiers Program Project Grant (project 1106), a Canada Research Chair, Princess Margaret Cancer Centre, The Princess Margaret Cancer Foundation and Ontario Ministry of Health. The authors thank R. Culp-Hill for metabolomics data acquisition. J.A.R. and A.D. acknowledge support from the University of Colorado Cancer Center Support Grant (P30CA046934).
Author information
Authors and Affiliations
Contributions
M.H. and S.M.C. conceived the study and designed the experiments. M.H. performed and analysed most experiments. V.V., A.C. and S.P. performed the bioinformatics analyses with assistance from Y.W. and A.C.H.L. F.G., A.D.S., G.D.B. and G.E. provided input and supervised the bioinformatics analyses. S.C., D.M.A., A.C.H.L., Y.Y., V.W., A.M. and E.G. performed experiments. J.A.R. and A.D. performed and analysed the metabolomics experiments. A.V. performed the prime editing experiments under the supervision of S.Z.X. and J.E.D. M.F. designed the prime editing strategy under the supervision of L.N. and S.F. M.H. and S.M.C. interpreted the data and wrote the manuscript. K.Y. and J.J.T. reviewed and edited the manuscript. All authors provided input in the preparation of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
S.M.C. has received research funding from the Centre for Oncology and Immunology in Hong Kong, Celgene/BMS, AbbVie Pharmaceuticals, Agios Pharmaceuticals and Servier Laboratories. F.G. serves as a consultant for S2 Genomics Inc. A.D.S. has received research funding from Takeda Pharmaceuticals, BMS and Medivir AB, and consulting fees/honorarium from Takeda, Novartis, Jazz and Otsuka Pharmaceuticals. A.D.S. is named on a patent application for the use of DNT cells to treat AML. A.D.S. is a member of the Medical and Scientific Advisory Board of the Leukemia and Lymphoma Society of Canada. A.D.S. holds the Ronald N. Buick Chair in Oncology Research. J.E.D. has received research funding from Celgene/BMS, and has patents licensed to Trillium Therapeutics/Pfizer. J.J.T. has received research funding from H3 Biomedicine, Inc. and patent royalties from Fate Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Navdeep Chandel, Erik Eldering and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
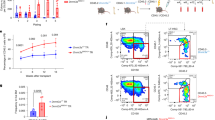
Extended Data Fig. 1 Dnmt3aR878H/+ HSPCs have increased mitochondrial respiration compared with Dnmt3a+/+ cells and are dependent on this metabolic reprogramming for their competitive advantage.
a, Gene set enrichment plots comparing DNMT3AR882 or DNMT3Anon-R882 mutated AML samples versus DNMT3AWT AML samples using two publicly available gene expression datasets (GSE27187 and GSE68833). Number of patients in each cohort is shown at the bottom of the plot. b, Gene set enrichment plots comparing DNMT3AR882 versus DNMT3AWT CD34+ HSPCs from individuals with CH using the publicly available GSE158067 gene expression dataset and analyzed as pseudobulk populations. Number of cells in each pseudobulk population is shown at the bottom of the plot. c, OCRs of whole bone marrow cells of the indicated genotype at baseline and at different time points following treatment with oligomycin A (Oligo A), FCCP, and rotenone plus antimycin A (R&A). n = 3 biologically independent samples for each condition. d, Schematic diagram showing the design of the in vitro competition assay. Created in BioRender. Chan, S. (2025) https://BioRender.com/ks3i0wn. e, Proportion of CD45.2+ and CD45.1+ cells in a competition assay between CD45.2+ LK cells of the indicated genotype and CD45.1+ Dnmt3a+/+ LK cells after the 1st passage (n = 3 biologically independent samples each) and 2nd passage (n = 3 biologically independent samples each). In c, e, data are presented as mean values ± SEM. Statistical significance was calculated using two-sided Student’s t-test. * P < 0.05, ** P < 0.01.
Extended Data Fig. 2 Dnmt3aR878H/+ HSPCs have increased mitochondrial respiration compared with Dnmt3a+/+ cells and are dependent on this metabolic reprogramming for their competitive advantage.
a, Expression of the indicated genes in Dnmt3a+/+ or Dnmt3aR878H/+ LK cells transduced with a lentiviral vector expressing a non-targeting shRNA or a shRNA targeting Ndufv1 (shNdufv1) or Cox15 (shCox15). n = 3 technical replicates for 1 biological sample in each condition. b, Number of colony forming units in the second plating from Dnmt3a+/+ or Dnmt3aR878H/+ LK HSPCs in the absence or presence of metformin. n = 3 biologically independent samples per condition. c, Schematic diagram showing the design of the in vivo competitive repopulation experiment. Created in BioRender. Chan, S. (2025) https://BioRender.com/wkp2h9m. d, Proportion of CD45.2+ vs. CD45.1+ cells in the myeloid (CD11b+), T (CD3+), and B (B220+) cell compartments in peripheral blood cells collected from mice after 4 months of treatment with metformin or vehicle in the experiment shown in Fig. 1h. n = 7 biologically independent samples per condition. In a,b, the box represents the interquartile range with the median indicated by the line inside the box. Whiskers extend to the minimum and maximum values. In d, data are presented as mean values ± SEM. Statistical significance was calculated using two-sided Student’s t-test for all comparisons. * P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001.
Extended Data Fig. 3 Metformin suppresses the competitive advantage of Dnmt3aR878H/+ HSCs.
a, Gating strategy for the identification of murine HSPCs. b, Proportion of CD45.1+ Dnmt3a+/+ cells vs. CD45.2+ Dnmt3aR878H/+ or Dnmt3a+/+cells in each immunophenotypically-defined HSPC subset. The bone marrow samples were collected from untreated and metformin-treated mice in the experiment shown in Fig. 1h after 4 months of treatment. The subsets were defined as: HSC (CD150+CD48-LS+K), MPP (CD150-CD48-LS+K), HPC1 (CD150-CD48+LS+K), HPC2 (CD150+CD48+LS+K), CMP (CD34+CD16/32LoLS-K), GMP (CD34+CD16/32HiLS-K), and MEP (CD34-CD16/32-LS-K). For HSC, MPP, HPC1 and HPC2, the number of biologically independent samples in the Dnmt3a+/+ VEH, Dnmt3a+/+ MET, Dnmt3aR878H/+ VEH, and Dnmt3aR878H/+ MET groups was 10, 12, 12, and 8, respectively. For CMP, GMP, and MEP, the number of biologically independent samples in the Dnmt3a+/+ VEH, Dnmt3a+/+ MET, Dnmt3aR878H/+ VEH, and Dnmt3aR878H/+ MET groups was 9, 17, 11, and 11, respectively. Data are presented as mean values ± SEM. Statistical significance was calculated using two-sided Student’s t-test for all comparisons. * P < 0.05, ** P < 0.01. c, Schematic diagram showing the design of the non-competitive repopulation experiment in which lethally-irradiated recipient mice were transplanted with CD45.2+ Dnmt3a+/+ or Dnmt3aR878H/+ whole bone marrow (WBM) cells. Five weeks after transplantation, the mice were either left untreated or treated with metformin in the drinking water. After 4 weeks of treatment, BM cells were collected and used for analysis. Created in BioRender. Chan, S. (2025) https://BioRender.com/2k9dcho.
Extended Data Fig. 4 Metformin suppresses OXPHOS in Dnmt3aR878H/+ HSPCs.
a, Normalized enrichment scores (NES) for the indicated gene set in HSPC subsets using scRNA-seq gene expression data and analyzed as pseudobulk populations. b, Gene set enrichment plots for the indicated gene set comparing Dnmt3aR878H/+ cells versus Dnmt3a+/+ cells and metformin-treated Dnmt3aR878H/+ versus vehicle-treated Dnmt3aR878H/+ cells in the HSC fraction using scRNA-seq gene expression data and analyzed as pseudobulk populations. c, Basal and maximal OCRs in LK HSPCs collected from mice transplanted with WBM cells of the indicated genotype and treated with or without metformin for 1 month. n = 9 biologically independent samples for each condition. d, Mean fluorescence intensity of TMRE staining in LK HSPCs collected from mice transplanted with WBM cells of the indicated genotype and treated with or without metformin for 1 month. n = 3 biologically independent samples for each condition. e, Gene set enrichment plots of bulk RNA-seq data comparing metformin-treated Dnmt3aR878H/+ LK cells (n = 3 biologically independent samples) versus vehicle-treated Dnmt3aR878H/+ LK cells (n = 3 biologically independent samples) using the indicated self-renewal and stemness-related gene sets. f, Gene set enrichment plots of bulk RNA-seq data comparing metformin-treated Dnmt3aR878H/+ LK cells (n = 3 biologically independent samples) versus vehicle-treated Dnmt3aR878H/+ LK cells (n = 3 biologically independent samples) using the indicated mitochondrial OXPHOS-related gene sets. In c, d, the box represents the interquartile range with the median indicated by the line inside the box. Whiskers extend to the minimum and maximum values. Statistical significance was calculated using two-sided Student’s t-test for all comparisons. * P < 0.05, ** P < 0.01.
Extended Data Fig. 5 Metformin suppresses the competitive advantage of Dnmt3aR878H/+ HSPCs by enhancing their methylation potential.
a, Proportion of CD45.2+ and CD45.1+ cells in a competition assay between CD45.2+ Dnmt3aR878H/+ LK cells and CD45.1+ Dnmt3a+/+ LK cells in the presence or absence of the indicated compounds. n = 2 biologically independent samples for each condition. b, Expression of Dnmt3b in Dnmt3a+/+ LK cells transduced with a shRNA vector expressing a non-targeting shRNA (shNT) or shDnmt3b. n = 3 technical replicates for 1 biological sample in each condition. c, Proportion of CD45.2+ and CD45.1+ cells in a competition assay between CD45.2+ Dnmt3aR878H/+ LK cells and CD45.1+ Dnmt3a+/+ LK cells in the presence or absence of exogenous SAM. Both populations were transduced with the indicated shRNA vectors. n = 1 biological sample per condition. The mean value of 5 technical replicates is shown for each condition. d, Proportion of CD45.2+ and CD45.1+ cells in a competition assay between CD45.2+ Dnmt3aR878H/+ or Dnmt3a+/+ LK cells and CD45.1+ Dnmt3a+/+ LK cells in the presence or absence of folic acid supplementation at 100 μM. n = 2 biologically independent samples per condition. e, Proportion of CD45.2+ and CD45.1+ cells in a competition assay between CD45.2+ Dnmt3aR878H/+ LK cells and CD45.1+ Dnmt3a+/+ LK cells in the presence or absence of the indicated compounds. n = 3 biologically independent samples for each condition. f, Proportion of CD45.2+ and CD45.1+ cells in a competition assay between CD45.2+ Dnmt3aR878H/+ LK cells and CD45.1+ Dnmt3a+/+ LK cells in the presence or absence of the indicated compounds. n = 3 biologically independent samples for each condition. g, Proportion of CD45.2+ and CD45.1+ cells in a competition assay between CD45.2+ Dnmt3aR878H/+ LK cells and CD45.1+ Dnmt3a+/+ LK cells in the presence or absence of the indicated compounds. n = 3 biologically independent samples per condition. h, Expression of Shmt2 in Dnmt3a+/+ LK cells transduced with an empty shRNA vector control (shEV) or a shRNA vector expressing shShmt2. n = 5 technical replicates for 1 biological sample in each condition. i, Proportion of CD45.2+ and CD45.1+ cells in a competition assay between CD45.2+ Dnmt3aR878H/+ LK cells and CD45.1+ Dnmt3a+/+ LK cells in the presence or absence of metformin. Both populations were transduced with the indicated shRNA vectors. n = 3 biologically independent samples per condition. In a, c, d, the bar blot represents the mean value. In b, h, the box represents the interquartile range with the median indicated by the line inside the box. Whiskers extend to the minimum and maximum values. In e, f, g, i, data are presented as mean values ± SEM. Statistical significance was calculated using two-sided Student’s t-test for all comparisons. * P < 0.05, ** P < 0.01, and **** P < 0.0001.
Extended Data Fig. 6 Metformin reverses the aberrant DNA CpG methylation and H3K27me3 profiles in Dnmt3aR878H/+ HSPCs.
a, Knockout score for Dnmt3a in Dnmt3aR878H/+ or Dnmt3a+/+ LK cells as determined by Inference of CRISPR Edits (ICE) analysis 4 days after nucleofection with CRISPR/Cas9 ribonucleoprotein (RNP) complexes targeting the Dnmt3a gene. b, Proportion of CD45.2+ and CD45.1+ cells in a competition assay between CD45.2+ LK cells of the indicated genotype and CD45.1+ Dnmt3a+/+ LK cells in the presence or absence of metformin. The CD45.2+ LK cells were nucleofected with Cas9 alone (-) or with RNPs targeting Dnmt3a (+) prior to mixing and plating. The competing CD45.1+ LK cells were nucleofected with Cas9 alone prior to plating. n = 3 biologically independent samples for each condition. c, Venn diagram showing the overlap of DMRs between untreated Dnmt3aR878H/+ samples versus untreated Dnmt3a+/+ samples and between metformin-treated Dnmt3aR878H/+ samples versus untreated Dnmt3aR878H/+ samples. d, Average methylation levels of DMRs in LK cells of the indicated genotype and treated with or without metformin for 1 month. DMRs were restricted to the ones that were hypomethylated in vehicle-treated Dnmt3aR878H/+ samples relative to Dnmt3aR878H/+ samples. Each dot represents the average value of the DMRs in one sample. n = 3 biologically independent samples for untreated Dnmt3a+/+ samples and metformin-treated Dnmt3aR878H/+ samples. n = 4 biologically independent samples for metformin-treated Dnmt3a+/+ samples and untreated Dnmt3aR878H/+ samples. In d, the box represents the interquartile range with the median indicated by the line inside the box. Whiskers extend to the minimum and maximum values. In b, data are presented as mean values ± SEM. Statistical significance was calculated using two-sided Student’s t-test for all comparisons. * P < 0.05, ** P < 0.01, and **** P < 0.0001.
Extended Data Fig. 7 Dnmt3aR878H mutation decreases promoter methylation and increases expression of genes involved in mitochondrial respiration.
a, Normalized enrichment scores (NES) for the indicated gene sets from GSEA using genes ranked by differential promoter methylation or gene expression in the comparison between vehicle-treated Dnmt3aR878H/+ cells versus vehicle-treated Dnmt3a+/+ cells and between metformin treated Dnmt3aR878H/+ cells versus vehicle-treated Dnmt3aR878H/+ cells. b, Normalized enrichment scores (NES) for the indicated gene sets from GSEA using genes ranked by differential promoter methylation or gene expression in the comparison between DNMT3AR882-mutated versus DNMT3AWT HSPCs as pseudobulk populations in the GSE158067 dataset. GSEA was performed using all Gene Ontology (GO) gene sets. The top 10 enriched GO gene sets ranked based on NES from gene expression are shown. The ones shown in green color are related to mitochondrial respiration.
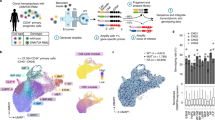
Extended Data Fig. 8 Metformin decreases the competitive advantage of human DNMT3AR882H HSPCs.
a, Expression of DNMT3A mRNA in human CD34+ enriched HSPCs transduced with a lentiviral vector expressing a non-targeting shRNA (shNT) or DNMT3A shRNA. n = 3 technical replicates for 1 biological sample in each condition. b, Intracellular flow cytometry staining for DNMT3A protein in human CD34+ enriched HSPCs transduced with a lentiviral vector expressing a non-targeting shRNA (shNT) or DNMT3A shRNA. The histogram depicting isotype control antibody staining represents cells expressing the non-targeting shRNA (shNT). c, Proportion of BFP+ and GFP+ cells in a competition assay between BFP+ HSPCs expressing shNT or shDNMT3A and GFP+ HSPCs expressing shNT in the absence or presence of metformin at 50 μM. Analysis was gated on the CD34+ cells in the left panel and CD34- cells in the right panel. n = 2 biologically independent samples for each condition. d, Concentration SAM and SAH in human HSPCs expressing the indicated shRNA and treated with or without metformin. n = 3 biologically independent samples for each condition. e, Representative Sanger sequencing chromatogram of the sequences surrounding the DNMT3A p.R882H (c.2645 G > A) missense mutation in a prime-edited HSPC cell pool. In a, d, the box represents the interquartile range with the median indicated by the line inside the box. Whiskers extend to the minimum and maximum values. In c, bar plots represent mean values. Statistical significance was calculated using two-sided Student’s t-test for all comparisons. *** P < 0.001.
Supplementary information
Supplementary Information
This file contains Supplementary Fig. 1 and legends for Supplementary Tables 1–6.
Supplementary Tables
Supplementary Tables 1–6.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hosseini, M., Voisin, V., Chegini, A. et al. Metformin reduces the competitive advantage of Dnmt3aR878H HSPCs. Nature 642, 421–430 (2025). https://doi.org/10.1038/s41586-025-08871-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-025-08871-w