Abstract
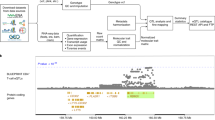
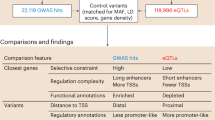
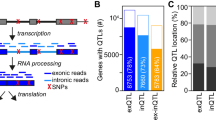
Complete characterization of the genetic effects on gene expression is needed to elucidate tissue biology and the etiology of complex traits. In the present study, we analyzed 2,344 subcutaneous adipose tissue samples and identified 34,774 conditionally distinct expression quantitative trait locus (eQTL) signals at 18,476 genes. Over half of eQTL genes exhibited at least two eQTL signals. Compared with primary eQTL signals, nonprimary eQTL signals had lower effect sizes, lower minor allele frequencies and less promoter enrichment; they corresponded to genes with higher heritability and higher tolerance for loss of function. Colocalization of eQTLs with genome-wide association study (GWAS) signals for 28 cardiometabolic traits identified 1,835 genes. Inclusion of nonprimary eQTL signals increased discovery of colocalized GWAS–eQTL signals by 46%. Furthermore, 21 genes with ≥2 colocalized GWAS–eQTL signals showed a mediating gene dosage effect on the GWAS trait. Thus, expanded eQTL identification reveals more mechanisms underlying complex traits and improves understanding of the complexity of gene expression regulation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The AdipoExpress meta-analysis results are available via Zenodo at https://zenodo.org/records/13845120 (ref. 87). Results include full marginal eQTL summary statistics for all individuals and only European-ancestry individuals, men and women, along with the conditional AB1 eQTL summary statistics for each signal. Colocalized GWAS–eQTL signals can be downloaded from Zenodo at https://zenodo.org/records/13845120 (ref. 87) and visualized at https://adipose.colocus.app. METSIM genotypes and gene expression data are available at dbGaP phs000743. FUSION genotypes and gene expression data are available at dbGaP phs001048. GTEx whole-genome sequencing and gene expression data are available at dbGaP phs000424.v8.p2. TwinsUK RNA-seq data are available in the European Genome–phenome Archive under accession EGAS00001000805. TwinsUK genotypes are available upon application to the TwinsUK Resource Executive Committee. For information on how to apply, see https://twinsuk.ac.uk/resources-for-researchers/access-our-data. GWAS datasets were accessed according to the links and publications listed in Supplementary Table 18. The following databases and datasets were used to perform analyses for this manuscript: GENCODE v.19 (https://www.gencodegenes.org/human/release_19.html); Cis-BP v.2.00 (http://cisbp2.ccbr.utoronto.ca/index.php); Human Protein Atlas (proteinatlas.org); UKBB (application 25953; http://www.ukbiobank.ac.uk); STARNET (PMID: 27540175); eQTLGen (http://www.eqtlgen.org); gnomAD (https://gnomad.broadinstitute.org/downloads#v2); NIH Roadmap Epigenomics chromatin states (https://egg2.wustl.edu/roadmap/web_portal/chr_state_learning.html); and ATAC peaks (PMID: 34699533). Source data are provided with this paper.
Code availability
Analyses described in this manuscript were conducted using the following freely available software: Fastx-toolkit v.0.0.14; Cutadapt v.1.18; STAR v.2.4.0.1, v.2.4.2a and v.2.7.3a; vcftools v.0.1.15; QTLtools v.1.1; QoRTs v.1.3.6; R v.4.0.3, v.4.1.0 and v.4.1.3; edgeR v.3.36.0; ggplot2 v.3.4.0; PEER v.1.3; APEX v.0.2; apex2R; PLINK v.1.90b3; METAL v.2011-03-25; LocusZoom v.1.4; SusieR v.0.12.35; GCTA v.1.94.1; coloc v.5.1.0.1; swiss v.1.1.1; bedtools v.2.3.0; SMR v.1.3.1; MRLocus v.0.0.26; and GARFIELD v.2.
References
GTEx Consortium. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 369, 1318–1330 (2020).
Umans, B. D., Battle, A. & Gilad, Y. Where are the disease-associated eQTLs? Trends Genet. 37, 109–124 (2021).
Nicolae, D. L. et al. Trait-associated SNPs are more likely to be eQTLs: annotation to enhance discovery from GWAS. PLoS Genet. 6, e1000888 (2010).
Gallagher, M. D. & Chen-Plotkin, A. S. The post-GWAS era: from association to function. Am. J. Hum. Genet 102, 717–730 (2018).
Nica, A. C. & Dermitzakis, E. T. Expression quantitative trait loci: present and future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120362 (2013).
Raulerson, C. K. et al. Adipose tissue gene expression associations reveal hundreds of candidate genes for cardiometabolic traits. Am. J. Hum. Genet. 105, 773–787 (2019).
Wu, Y. et al. Colocalization of GWAS and eQTL signals at loci with multiple signals identifies additional candidate genes for body fat distribution. Hum. Mol. Genet. 28, 4161–4172 (2019).
Dobbyn, A. et al. Landscape of conditional eQTL in dorsolateral prefrontal cortex and co-localization with schizophrenia GWAS. Am. J. Hum. Genet. 102, 1169–1184 (2018).
Zeng, B. et al. Comprehensive multiple eQTL detection and its application to GWASinterpretation. Genetics 212, 905–918 (2019).
Zhu, Z. et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat. Genet. 48, 481–487 (2016).
Hormozdiari, F. et al. Widespread allelic heterogeneity in complex traits. Am. J. Hum. Genet 100, 789–802 (2017).
Jansen, R. et al. Conditional eQTL analysis reveals allelic heterogeneity of gene expression. Hum. Mol. Genet. 26, 1444–1451 (2017).
Lappalainen, T. et al. Transcriptome and genome sequencing uncovers functional variation in humans. Nature 501, 506–511 (2013).
Spracklen, C. N. et al. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature 582, 240–245 (2020).
Zeng, B. et al. Multi-ancestry eQTL meta-analysis of human brain identifies candidate causal variants for brain-related traits. Nat. Genet. 54, 161–169 (2022).
Brown, M., Greenwood, E., Zeng, B., Powell, J. E. & Gibson, G. Effect of all-but-one conditional analysis for eQTL isolation in peripheral blood. Genetics 223, iyac162 (2023).
Mostafavi, H., Spence, J. P., Naqvi, S. & Pritchard, J. K. Systematic differences in discovery of genetic effects on gene expression and complex traits. Nat. Genet. 55, 1866–1875 (2023).
Connally, N. J. et al. The missing link between genetic association and regulatory function. eLife 11, e74970 (2022).
Delaneau, O. et al. A complete tool set for molecular QTL discovery and analysis. Nat. Commun. 8, 15452 (2017).
Yang, J., Lee, S. H., Goddard, M. E. & Visscher, P. M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 88, 76–82 (2011).
Quick, C. et al. A versatile toolkit for molecular QTL mapping and meta-analysis at scale. Preprint at bioRxiv https://doi.org/10.1101/2020.12.18.423490 (2020).
GTEx Consortium. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 348, 648–660 (2015).
GTEx Consortium et al. Genetic effects on gene expression across human tissues. Nature 550, 204–213 (2017).
Arvanitis, M., Tayeb, K., Strober, B. J. & Battle, A. Redefining tissue specificity of genetic regulation of gene expression in the presence of allelic heterogeneity. Am. J. Hum. Genet. 109, 223–239 (2022).
Ha, E. E. & Bauer, R. C. Emerging roles for adipose tissue in cardiovascular disease. Arter. Thromb. Vasc. Biol. 38, e137–e144 (2018).
Considine, R. V. et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 334, 292–295 (1996).
Cornier, M.-A. et al. The metabolic syndrome. Endocr. Rev. 29, 777–822 (2008).
Civelek, M. et al. Genetic regulation of adipose gene expression and cardio-metabolic traits. Am. J. Hum. Genet. 100, 428–443 (2017).
Grundberg, E. et al. Mapping cis- and trans-regulatory effects across multiple tissues in twins. Nat. Genet. 44, 1084–1089 (2012).
El-Sayed Moustafa, J. S. et al. ACE2 expression in adipose tissue is associated with cardio-metabolic risk factors and cell type composition—implications for COVID-19. Int. J. Obes. 46, 1478–1486 (2022).
Franzén, O. et al. Cardiometabolic risk loci share downstream cis- and trans-gene regulation across tissues and diseases. Science 353, 827–830 (2016).
Gudbjartsson, D. F. et al. Large-scale whole-genome sequencing of the Icelandic population. Nat. Genet. 47, 435–444 (2015).
Valencak, T. G., Osterrieder, A. & Schulz, T. J. Sex matters: the effects of biological sex on adipose tissue biology and energy metabolism. Redox Biol. 12, 806–813 (2017).
Võsa, U. et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat. Genet. 53, 1300–1310 (2021).
Karczewski, K. J. et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443 (2020).
Aragam, K. G. et al. Discovery and systematic characterization of risk variants and genes for coronary artery disease in over a million participants. Nat. Genet. 54, 1803–1815 (2022).
Mahajan, A. et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 50, 1505–1513 (2018).
Yengo, L. et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum. Mol. Genet. 27, 3641–3649 (2018).
Pulit, S. L. et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum. Mol. Genet. 28, 166–174 (2019).
Graham, S. E. et al. The power of genetic diversity in genome-wide association studies of lipids. Nature 600, 675–679 (2021).
Evangelou, E. et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat. Genet. 50, 1412–1425 (2018).
Chen, J. et al. The trans-ancestral genomic architecture of glycemic traits. Nat. Genet. 53, 840–860 (2021).
Agrawal, S. et al. Inherited basis of visceral, abdominal subcutaneous and gluteofemoral fat depots. Nat. Commun. 13, 3771 (2022).
Wallace, C. Eliciting priors and relaxing the single causal variant assumption in colocalisation analyses. PLoS Genet. 16, e1008720 (2020).
Wallace, C. A more accurate method for colocalisation analysis allowing for multiple causal variants. PLoS Genet. 17, e1009440 (2021).
Granade, M. E. et al. Feeding desensitizes A1 adenosine receptors in adipose through FOXO1-mediated transcriptional regulation. Mol. Metab. 63, 101543 (2022).
Zhu, A. et al. MRLocus: identifying causal genes mediating a trait through Bayesian estimation of allelic heterogeneity. PLoS Genet. 17, e1009455 (2021).
Jin, Y.-R. & Yoon, J. K. The R-spondin family of proteins: emerging regulators of WNT signaling. Int. J. Biochem. Cell Biol. 44, 2278–2287 (2012).
Tocci, J. M., Felcher, C. M., García Solá, M. E. & Kordon, E. C. R-spondin-mediated WNT signaling potentiation in mammary and breast cancer development. IUBMB Life 72, 1546–1559 (2020).
Nagaoka, T., Shirakawa, T., Balon, T. W., Russell, J. C. & Fujita-Yamaguchi, Y. Cyclic nucleotide phosphodiesterase 3 expression in vivo: evidence for tissue-specific expression of phosphodiesterase 3A or 3B mRNA and activity in the aorta and adipose tissue of atherosclerosis-prone insulin-resistant rats. Diabetes 47, 1135–1144 (1998).
Hanna, R. et al. Cardiac phosphodiesterases are differentially increased in diabetic cardiomyopathy. Life Sci. 283, 119857 (2021).
Kundaje, A. et al. Integrative analysis of 111 reference human epigenomes. Nature 518, 317–330 (2015).
Fischer-Posovszky, P., Newell, F. S., Wabitsch, M. & Tornqvist, H. E. Human SGBS cells—a unique tool for studies of human fat cell biology. Obes. Facts 1, 184–189 (2008).
Perrin, H. J. et al. Chromatin accessibility and gene expression during adipocyte differentiation identify context-dependent effects at cardiometabolic GWAS loci. PLoS Genet. 17, e1009865 (2021).
Nam, J. S., Ahn, C. W., Park, H. J. & Kim, Y. S. Semaphorin 3C is a novel adipokine representing exercise-induced improvements of metabolism in metabolically healthy obese young males. Sci. Rep. 10, 10005 (2020).
Yang, W.-J. et al. Semaphorin-3C signals through Neuropilin-1 and PlexinD1 receptors to inhibit pathological angiogenesis. EMBO Mol. Med. 7, 1267–1284 (2015).
Shungin, D. et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 518, 187–196 (2015).
Mejhert, N. et al. Semaphorin 3C is a novel adipokine linked to extracellular matrix composition. Diabetologia 56, 1792–1801 (2013).
Scott, L. J. et al. The genetic regulatory signature of type 2 diabetes in human skeletal muscle. Nat. Commun. 7, 11764 (2016).
Brotman, S. M., Oravilahti, A., Rosen, J. D., Alvarez, M. & Heinonen, S. Cell-type composition affects adipose gene expression associations with cardiometabolic traits. Diabetes 72, 1707–1718 (2023).
Palowitch, J., Shabalin, A., Zhou, Y.-H., Nobel, A. B. & Wright, F. A. Estimation of cis-eQTL effect sizes using a log of linear model. Biometrics 74, 616–625 (2018).
Laakso, M. et al. METabolic Syndrome In Men (METSIM) Study: a resource for studies of metabolic and cardiovascular diseases. J. Lipid Res. 58, 481–493 (2017).
Hysi, P. G. et al. A genome-wide association study for myopia and refractive error identifies a susceptibility locus at 15q25. Nat. Genet. 42, 902–905 (2010).
Buil, A. et al. Gene–gene and gene–environment interactions detected by transcriptome sequence analysis in twins. Nat. Genet. 47, 88–91 (2015).
Glastonbury, C. A. A. et al. Adiposity-dependent regulatory effects on multi-tissue transcriptomes. Am. J. Hum. Genet. 99, 567–579 (2016).
McCarthy, S. et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 48, 1279–1283 (2016).
Danecek, P. et al. The variant call format and VCFtools. Bioinformatics 27, 2156–2158 (2011).
Frankish, A. et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 47, D766–D773 (2019).
Hartley, S. W. & Mullikin, J. C. QoRTs: a comprehensive toolset for quality control and data processing of RNA-Seq experiments. BMC Bioinf. 16, 224 (2015).
Robinson, M. D. & Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11, R25 (2010).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Stegle, O., Parts, L., Piipari, M., Winn, J. & Durbin, R. Using probabilistic estimation of expression residuals (PEER) to obtain increased power and interpretability of gene expression analyses. Nat. Protoc. 7, 500–507 (2012).
Ongen, H., Buil, A., Brown, A. A., Dermitzakis, E. T. & Delaneau, O. Fast and efficient QTL mapper for thousands of molecular phenotypes. Bioinformatics 32, 1479–1485 (2016).
Domínguez Islas, C. & Rice, K. M. Addressing the estimation of standard errors in fixed effects meta‐analysis. Stat. Med 37, 1788–1809 (2018).
Uhlén, M. et al. Proteomics: tissue-based map of the human proteome. Science 347, 1260419 (2015).
Weirauch, M. T. et al. Determination and inference of eukaryotic transcription factor sequence specificity. Cell 158, 1431–1443 (2014).
Chang, C. C. et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience 4, s13742-015-0047–8 (2015).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018).
Wickham, H. Ggplot2: Elegant Graphics for Data Analysis (Springer-Verlag, 2016).
Willer, C. J., Li, Y. & Abecasis, G. R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010).
Brown, G. W. & Mood, A. M. On median tests for linear hypotheses. In Proc. Second Berkeley Symposium on Mathematical Statistics and Probability Vol. 2, 159–167 (Univ. California Press,1951).
Pruim, R. J. et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 26, 2336–2337 (2010).
Wright, S. Statistical methods in biology. J. Am. Stat. Assoc. 26, 155–163 (1931).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Iotchkova, V. et al. GARFIELD classifies disease-relevant genomic features through integration of functional annotations with association signals. Nat. Genet. 51, 343–353 (2019).
Fogarty, M. P., Cannon, M. E., Vadlamudi, S., Gaulton, K. J. & Mohlke, K. L. Identification of a regulatory variant that binds FOXA1 and FOXA2 at the CDC123/CAMK1D type 2 diabetes GWAS Locus. PLoS Genet. 10, e1004633 (2014).
Brotman, S. et al. AdipoExpress: Adipose eQTL meta-analysis summary statistics [Data set]. Zenodo https://doi.org/10.1101/2023.10.26.563798 (2023).
King’s College London. King’s Computational Research, Engineering and Technology Environment (CREATE); https://doi.org/10.18742/rnvf-m076 (2024).
Acknowledgements
We thank the METSIM, FUSION and TwinsUK study investigators and participants for providing the subcutaneous adipose tissue samples and genotypes that made the present study possible. We thank E. Hill-Burns for analyses performed in revision. The present study was supported by the following NIH grants: R01DK093757, R01DK072193 and U01DK105561 (to K.L.M.), R01DK132775 (to P.P., K.L.M. and L.J.S.), R01DK062370 (to M.B. and L.J.S.), R01HL170604 and R01HG010505 (to P.P.), R01HG009976 (to M.B.), UM1DK126185 (to K.L.M. and S.C.J.P.), ZIAHG000024 (to F.S.C.), F31HL154730 and T32GM007092 (to S.M.B.), F31HL146121 (to K.W.C.) and T32HL129982 (to K.A.B.). The present study was also supported by opportunity pool funds from the Accelerating Medical Partnerships Type 2 Diabetes consortium (grant U01DK105554/UM1DK105554 to K.L.M. and K.S.S.), the Academy of Finland (grant 321428 to M.L.), Sigrid Juselius Foundation, Finnish Foundation for Cardiovascular Research and Centre of Excellence of Cardiovascular and Metabolic Diseases (grant 0245896-3 to M.L.), Medical Research Council (grants MR/M004422/1 and MR/R023131/1 to K.S.S.), National Institute for Health Research (NIHR) Biomedical Research Centre (to M.T. and Y.R.), King’s China Scholarship Council PhD scholarship (to X.Y. and D.W.) and the University of North Carolina Global Partnership Initiative (to S.M.B.). TwinsUK is funded by the Wellcome Trust, Medical Research Council, Versus Arthritis, European Union Horizon 2020, Chronic Disease Research Foundation, Zoe and the NIHR Clinical Research Network and Biomedical Research Centre based at the Guy’s and St Thomas’s NHS Foundation Trust in partnership with King’s College London. This project utilized the King’s Computational Research, Engineering and Technology Environment88. This research has been conducted using the UKBB resource under application 25953. The GTEx Project was supported by the Common Fund of the Office of the Director of the NIH (commonfund.nih.gov/GTEx). Additional funds were provided by the National Cancer Institute, National Human Genome Research Institute, National Heart, Lung, and Blood Institute, National Institute on Drug Abuse, National Institute of Mental Health and National Institute of Neurological Disorders and Stroke. Donors were enrolled at biospecimen source sites funded by NCI/Leidos Biomedical Research subcontracts to the National Disease Research Interchange (10XS170), Roswell Park Cancer Institute (10XS171) and Science Care (X10S172). The Laboratory, Data Analysis, and Coordinating Center was funded through a contract (HHSN268201000029C) to the Broad Institute. Biorepository operations were funded through a Leidos Biomedical Research subcontract to Van Andel Research Institute (10ST1035). Additional data repository and project management were provided by Leidos Biomedical Research (HHSN261200800001E). The Brain Bank supported supplements to University of Miami grant DA006227. Statistical methods development grants were made to the University of Geneva (grants MH090941 and MH101814), the University of Chicago (grants MH090951, MH090937, MH101825 and MH101820), the University of North Carolina—Chapel Hill (grant MH090936), North Carolina State University (grant MH101819), Harvard University (grant MH090948), Stanford University (grant MH101782), Washington University (grant MH101810) and the University of Pennsylvania (grant MH101822). The datasets used for the analyses described in the present study were obtained from dbGaP at http://www.ncbi.nlm.nih.gov/gap through dbGaP accession phs000424.v8.p2.
Author information
Authors and Affiliations
Contributions
S.M.B., J.S.E.-S.M., L.G., K.L.M., K.S.S. and L.J.S. conceived and designed the study. S.M.B., J.S.E.-S.M. and L.G. generated data. S.M.B., J.S.E.-S.M., L.G., K.A.B., D.W., A.U.J., R.W., K.W.C., M.T., S.V. and M.I.L. performed analyses. H.M.S., A.L.R., T.A.L., A.O., L.F.S., N.N., M.R.E., T.Y., L.L.B., C.K.R., Y.R., X.Y., S.C.J.P., J.K., P.P., J.T., F.S.C., M.B., H.A.K. and M.L. provided resources. S.M.B., J.S.E.-S.M., L.G., K.L.M., K.S.S. and L.J.S. interpreted results and wrote the manuscript. All co-authors provided critical feedback and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks Johan Bjorkegren, Swapan Das and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Comparison of effect sizes in sex-stratified meta-analyses.
(A) Effect sizes of lead variants per gene identified in the female marginal eQTL meta-analysis looked up in the male marginal eQTL analysis. (B) Effect sizes of lead variants per gene identified in the male marginal eQTL meta-analysis looked up in the female marginal eQTL analysis. Each point is a variant-gene pair. The linear regression lines are shown in blue and the black diagonal line indicates slope = 1. R2 values are the Pearson correlations.
Extended Data Fig. 2 Overlap of adipose and STARNET eQTL signals.
Stacked bar charts show the proportion of significant adipose eQTL signals that overlapped with significant STARNET eQTL signals (FDR ≤ 0.05). Each bar shows eQTL signals from a different tissue in STARNET or at least one tissue (“Any tissue”) versus primary adipose eQTL signals. Gray indicates the proportion of primary adipose eQTL signals with an eQTL signal detected in STARNET (LD r2 ≥ 0.2), purple indicates the proportion of primary adipose eQTL signals with the same eQTL gene detected in STARNET but the signal differed (LD r2 < 0.2), and white indicates the primary adipose eQTL signals with a gene not reported in STARNET.
Extended Data Fig. 3 Overlap of adipose and blood eQTL signals.
Stacked bar charts show the proportion of significant adipose eQTL signals that overlapped with significant blood eQTL signals (FDR ≤ 0.05). The top bar shows all adipose eQTL signals, the middle bar shows only primary adipose eQTL signals, and the bottom bar shows non-primary adipose eQTL signals. Gray indicates the proportion of primary adipose eQTL signals with an eQTL signal detected in eQTLGen (LD r2 ≥ 0.2), purple indicates the proportion of primary adipose eQTL signals with the same eQTL gene detected in eQTLGen but the signal differed (LD r2 < 0.2), and white indicates the primary adipose eQTL signals with a gene not reported in eQTLGen.
Extended Data Fig. 4 Characteristics of eQTL signal variants.
Violin plots with inset boxplots of the (A) absolute value of the effect sizes of lead variants, (B) MAF, and (C) distance of the lead variants to the gene TSS for the indicated signals in order of discovery. The 5 sets of violin plots represent genes with exactly 1,2,3,4 or 5+ signals and include 9,148, 5,310, 2,309, 917, and 661 genes (or 1,209 signals for 5 + ), per set respectively. Boxplot center lines represent median values, limits represent upper and lower quartiles, whiskers represent 1.5x interquartile ranges, and black circles represent outliers. The black lines connect the median values of each signal group. In C, 163 points with a distance to TSS greater than 600 were excluded.
Extended Data Fig. 5 Heritability of genes with an eQTL grouped by gene expression.
(A) Boxplots of heritability in TwinsUK for genes with zero to five or more eQTL signals. Boxplots are split into subgroups based on the quartile of gene expression in TwinsUK. The number of genes with 0, 1, 2, 3, 4 and 5+ eQTL signals per expression quantile are Q1 (2,589, 1,819, 839, 288, 117, 87), Q2 (1,696, 1,830, 1,221, 590, 242, 159), Q3 (1,295, 1,995, 1,378, 635, 236, 199), and Q4 (1,160, 1,923, 1,465, 687, 295, 209), respectively. Lowly expressed genes are in quartile 1 shown in white, while highly expressed genes are in quartile 4 shown in darkest blue. Boxplot center lines represent the median value, box limits represent the upper and lower quartiles, whiskers represent the 1.5x interquartile range, and the black circles represent outliers. The black line connects the median of the boxplots. (B) Boxplots of heritability for genes separated by gene expression quartile. Numbers are the median heritability values. Boxplots as in A, with addition of the kernel density plots. Quartiles Q1-Q4 contain 5,738 or 5,739 genes.
Extended Data Fig. 6 pLI scores of eQTL genes with 1 through 5 or more signals.
(A) Proportion of genes with a pLI score ≥0.9 among all genes with a pLI score, by TwinUK gene expression quartile. Quartile 1 indicates the genes with the lowest expression and quartile 4 indicates genes with the highest expression. Expression quartiles were formed using all genes in TwinsUK, including those without pLI scores. The number of genes in each quartile (Q1-Q4) with a pLI score are 1,951, 3,230, 4,979, and 5,476 respectively. (B) Proportion of genes in TwinsUK with a given number of signals, by pLI score <0.9 or ≥0.9 and gene expression quartile. N genes with pLI <0.9 and pLI≥0.9 for Q1 (1,761 and 190), Q2 (2,906 and 324), Q3 (4,188 and 791), and Q4 (3,868 and 1,608). The darkest blue are the genes without an eQTL signal and the lightest blue are genes with five or more eQTL signals. For each expression level quartile, the proportion of genes with multiple signals was substantially lower for genes with pLI ≥0.9 than for genes with pLI <0.9: this trend was particularly pronounced in the highest expression category. (C) Proportion of genes in TwinsUK for each eQTL signal number with a pLI score ≥0.9. Each set of bar graphs is for a different gene expression quartile.
Extended Data Fig. 7 Proportion of eQTL signals colocalized with GWAS signals.
Per signal number proportion of eQTL signals colocalized with ≥ 1 GWAS signal. (A) all eQTL signals; (B) eQTL signals by quartiles of meta-analysis eQTL significance. Significance threshold for inclusion of eQTL in plots is P ≤ 1e-6. (A, B) Points are the proportion of eQTL signals colocalized with ≥1 GWAS signal; error bars are the standard errors of the proportion of signals colocalized. The total number of signals included in each plot and p-values for the one sample test of proportions (one-sided) are listed in Supplementary Table 23.
Extended Data Fig. 8 Mendelian randomization using MRLocus for select allelic series.
Plots of the effect size of the GWAS signals (y-axis) versus the effect size of the eQTL signals (x-axis) from MRLocus. These six eQTL gene-trait pairs have 2 or 3 pairs of GWAS and eQTL signals colocalized with each other. Each point represents the effect sizes of the colocalized GWAS-eQTL signals; x and y bars represent the standard errors of the eQTL and GWAS effect sizes, respectively. The solid blue lines represent the slope of the effect of the gene on the trait, and dotted blue lines represent an 80% credible interval on the slope.
Extended Data Fig. 9 WHRadjBMI GWAS and ZNRF3 eQTL conditional signal plots.
LocusZoom plots for WHRadjBMI signal 1 conditioned on signal 2 (top left) and WHRadjBMI signal 2 conditioned on signal 1 (bottom left). Plots for ZNRF3 signal 1 conditioned on signal 2 (top right) and ZNRF3 signal 2 conditioned on signal 1 (bottom right). The dots are colored by LD with the lead variant. Both plots are colored by the GWAS lead variant represented by a purple diamond. ‘AB1’ indicates all-but-one. Significance threshold for eQTLs is P ≤ 1e-6 and for GWAS is P ≤ 5e-8.
Extended Data Fig. 10 HDL-C GWAS and PDE3A eQTL conditional signal plots.
Left column, LocusZoom plot for HDL-C signal 1 conditioned on all the other signals, followed by plots for each signal conditioned on all other signals. Right column, plot for PDE3A signal 1 conditioned on all the other signals, followed by plots for signal 4 conditioned on all other signals, then signal 2 and signal 3. y-axes show the -log10 P-value after conditioning. The dots are colored by LD with the lead variant. Both plots are colored by the GWAS lead variant represented by a purple diamond. ‘AB1’ indicates all-but-one. Significance threshold for eQTLs is P ≤ 1e-6 and for GWAS is P ≤ 5e-8.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Results, Supplementary Figs. 1–8 and Supplementary References.
Supplementary Tables
Supplementary Tables 1–34.
Supplementary Data 1
Table of read depth, number of genes tested and samples sizes of each eQTL study.
Supplementary Data 2
ADIPOQ counts per million for each individual in each study.
Supplementary Data 3
Table of number of eQTL genes, eQTL signals and sample size per study.
Supplementary Data 4
The eQTL variants and P values for each of the five studies and the meta-analysis (EUR) near the ADIPOQ locus.
Supplementary Data 5
Median gene expression levels in METSIM by meta-analysis signal number and including pLI score.
Supplementary Data 6
GARFIELD enrichment results by eQTL signal number.
Supplementary Data 7
Luciferase assay raw data for hWAT and SGBS cells.
Supplementary Data 8
Luciferase assay raw data for LHCN-M2 cells.
Source data
Source Data Fig. 1
Conditionally distinct eQTL signals for each of the five studies and the two meta-analyses.
Source Data Fig. 2
The eQTL variants and P values for each of the five studies and the meta-analysis (EUR) near the GLYCTK locus.
Source Data Fig. 3
The eQTL signals with TSS and MAF information. Heritability estimates and pLI scores for eQTL genes.
Source Data Fig. 4
The eQTL variants and P values for the male and female eQTL meta-analysis near the ADORA1 locus.
Source Data Fig. 5
ZNRF3 and PDE3A MRLocus results and eQTL variants and P values for the eQTL meta-analysis near the PDE3A and ZNRF3 loci.
Source Data Fig. 6
All-but-one eQTL variants and P values near the SEMA3C locus, promoter and enhancer enrichment, and luciferase assay raw data.
Source Data Extended Data Fig. 1
Male and female eQTL results.
Source Data Extended Data Fig. 2
Counts and percentages of adipose eQTL signals overlapping with STARNET eQTL signals.
Source Data Extended Data Fig. 3
Counts and percentages of adipose eQTL signals overlapping with eQTLGen eQTL signals.
Source Data Extended Data Fig. 4
The eQTL signals with TSS and MAF information.
Source Data Extended Data Fig. 5
Heritability estimates from TwinsUK by meta-analysis eQTL signal number.
Source Data Extended Data Fig. 6
Heritability estimates from TwinsUK by meta-analysis eQTL signal number for genes with pLI scores.
Source Data Extended Data Fig. 7
Proportion of eQTL signals colocalized with GWAS signals separated by signal number and strength of eQTL.
Source Data Extended Data Fig. 8
MRLocus results for six eQTL-GWAS allelic series.
Source Data Extended Data Fig. 9
All-but-one eQTL variants and P values near the ZNRF3 locus
Source Data Extended Data Fig. 10
All-but-one eQTL variants and P values near the PDE3A locus
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brotman, S.M., El-Sayed Moustafa, J.S., Guan, L. et al. Adipose tissue eQTL meta-analysis highlights the contribution of allelic heterogeneity to gene expression regulation and cardiometabolic traits. Nat Genet 57, 180–192 (2025). https://doi.org/10.1038/s41588-024-01982-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-024-01982-6
This article is cited by
-
Diabetes mellitus polygenic risk scores: heterogeneity and clinical translation
Nature Reviews Endocrinology (2025)