Abstract
BCL-2-associated X protein (BAX) is a promising therapeutic target for activating or restraining apoptosis in diseases of pathologic cell survival or cell death, respectively. In response to cellular stress, BAX transforms from a quiescent cytosolic monomer into a toxic oligomer that permeabilizes the mitochondria, releasing key apoptogenic factors. The mitochondrial lipid trans-2-hexadecenal (t-2-hex) sensitizes BAX activation by covalent derivatization of cysteine 126 (C126). In this study, we performed a disulfide tethering screen to discover C126-reactive molecules that modulate BAX activity. We identified covalent BAX inhibitor 1 (CBI1) as a compound that selectively derivatizes BAX at C126 and inhibits BAX activation by triggering ligands or point mutagenesis. Biochemical and structural analyses revealed that CBI1 can inhibit BAX by a dual mechanism of action: conformational constraint and competitive blockade of lipidation. These data inform a pharmacologic strategy for suppressing apoptosis in diseases of unwanted cell death by covalent targeting of BAX C126.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analyzed for this study are included in the manuscript and its Supplementary Information. HDX MS data have been deposited to the ProteomeXchange Consortium via the PRIDE74 partner repository with dataset identifier PXD040917 and are also included in the manuscript as Source Data Fig. 5. The NMR structure of full-length BAX, corresponding to PDB ID: 1F16, was used in this study. Source data are provided with this paper.
Code availability
No code was generated for this study.
References
Walensky, L. D. & Gavathiotis, E. BAX unleashed: the biochemical transformation of an inactive cytosolic monomer into a toxic mitochondrial pore. Trends Biochem. Sci. 36, 642–652 (2011).
Suzuki, M., Youle, R. J. & Tjandra, N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell 103, 645–654 (2000).
Gavathiotis, E. et al. BAX activation is initiated at a novel interaction site. Nature 455, 1076–1081 (2008).
Gavathiotis, E., Reyna, D. E., Davis, M. L., Bird, G. H. & Walensky, L. D. BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol. Cell 40, 481–492 (2010).
Hauseman, Z. J. et al. Homogeneous oligomers of pro-apoptotic BAX reveal structural determinants of mitochondrial membrane permeabilization. Mol. Cell 79, 68–83 (2020).
Bloch, N. B. et al. The conformational stability of pro-apoptotic BAX is dictated by discrete residues of the protein core. Nat. Commun. 12, 4932 (2021).
Chipuk, J. E. et al. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell 148, 988–1000 (2012).
Cohen, D. T., Wales, T. E., McHenry, M. W., Engen, J. R. & Walensky, L. D. Site-dependent cysteine lipidation potentiates the activation of proapoptotic BAX. Cell Rep. 30, 3229–3239 (2020).
Erlanson, D. A., Wells, J. A. & Braisted, A. C. Tethering: fragment-based drug discovery. Annu. Rev. Biophys. Biomol. Struct. 33, 199–223 (2004).
Erlanson, D. A. et al. Site-directed ligand discovery. Proc. Natl Acad. Sci. USA 97, 9367–9372 (2000).
Ostrem, J. M., Peters, U., Sos, M. L., Wells, J. A. & Shokat, K. M. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature 503, 548–551 (2013).
Harvey, E. P. et al. Identification of a covalent molecular inhibitor of anti-apoptotic BFL-1 by disulfide tethering. Cell Chem. Biol. 27, 647–656 (2020).
Burlingame, M. A., Tom, C. T. & Renslo, A. R. Simple one-pot synthesis of disulfide fragments for use in disulfide-exchange screening. ACS Comb. Sci. 13, 205–208 (2011).
Hallenbeck, K. K. et al. A liquid chromatography/mass spectrometry method for screening disulfide tethering fragments. SLAS Discov. 23, 183–192 (2018).
Pritz, J. R. et al. Allosteric sensitization of proapoptotic BAX. Nat. Chem. Biol. 13, 961–967 (2017).
Barclay, L. A. et al. Inhibition of pro-apoptotic BAX by a noncanonical interaction mechanism. Mol. Cell 57, 873–886 (2015).
Garner, T. P. et al. Small-molecule allosteric inhibitors of BAX. Nat. Chem. Biol. 15, 322–330 (2019).
Ma, J. et al. Structural mechanism of Bax inhibition by cytomegalovirus protein vMIA. Proc. Natl Acad. Sci. USA 109, 20901–20906 (2012).
Spitz, A. Z., Zacharioudakis, E., Reyna, D. E., Garner, T. P. & Gavathiotis, E. Eltrombopag directly inhibits BAX and prevents cell death. Nat. Commun. 12, 1134 (2021).
Engen, J. R. Analysis of protein conformation and dynamics by hydrogen/deuterium exchange MS. Anal. Chem. 81, 7870–7875 (2009).
Walensky, L. D. Targeting BAX to drug death directly. Nat. Chem. Biol. 15, 657–665 (2019).
Walensky, L. D. et al. A stapled BID BH3 helix directly binds and activates BAX. Mol. Cell 24, 199–210 (2006).
Gavathiotis, E., Reyna, D. E., Bellairs, J. A., Leshchiner, E. S. & Walensky, L. D. Direct and selective small-molecule activation of proapoptotic BAX. Nat. Chem. Biol. 8, 639–645 (2012).
Reyna, D. E. et al. Direct activation of BAX by BTSA1 overcomes apoptosis resistance in acute myeloid leukemia. Cancer Cell 32, 490–505 (2017).
Lopez, A. et al. Co-targeting of BAX and BCL-XL proteins broadly overcomes resistance to apoptosis in cancer. Nat. Commun. 13, 1199 (2022).
Niu, X. et al. A small-molecule inhibitor of Bax and Bak oligomerization prevents genotoxic cell death and promotes neuroprotection. Cell Chem. Biol. 24, 493–506 (2017).
Sadowsky, J. D. et al. Turning a protein kinase on or off from a single allosteric site via disulfide trapping. Proc. Natl Acad. Sci. USA 108, 6056–6061 (2011).
Pemberton, J. M., Pogmore, J. P. & Andrews, D. W. Neuronal cell life, death, and axonal degeneration as regulated by the BCL-2 family proteins. Cell Death Differ. 28, 108–122 (2021).
Hetz, C. et al. Bax channel inhibitors prevent mitochondrion-mediated apoptosis and protect neurons in a model of global brain ischemia. J. Biol. Chem. 280, 42960–42970 (2005).
Cao, G. et al. Intracellular Bax translocation after transient cerebral ischemia: implications for a role of the mitochondrial apoptotic signaling pathway in ischemic neuronal death. J. Cereb. Blood Flow Metab. 21, 321–333 (2001).
Hochhauser, E. et al. Bax ablation protects against myocardial ischemia-reperfusion injury in transgenic mice. Am. J. Physiol. Heart Circ. Physiol. 284, H2351–H2359 (2003).
Khalaf, K., Tornese, P., Cocco, A. & Albanese, A. Tauroursodeoxycholic acid: a potential therapeutic tool in neurodegenerative diseases. Transl. Neurodegener. 11, 33 (2022).
Paganoni, S. et al. Trial of sodium phenylbutyrate–taurursodiol for amyotrophic lateral sclerosis. N. Engl. J. Med. 383, 919–930 (2020).
Rodrigues, C. M., Solá, S., Sharpe, J. C., Moura, J. J. & Steer, C. J. Tauroursodeoxycholic acid prevents Bax-induced membrane perturbation and cytochrome c release in isolated mitochondria. Biochemistry 42, 3070–3080 (2003).
Amgalan, D. et al. A small-molecule allosteric inhibitor of BAX protects against doxorubicin-induced cardiomyopathy. Nat. Cancer 1, 315–328 (2020).
Studier, F. W. Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 41, 207–234 (2005).
Murray, V., Chen, J., Huang, Y., Li, Q. & Wang, J. Preparation of very-high-yield recombinant proteins using novel high-cell-density bacterial expression methods. Cold Spring Harb. Protoc. 2010, pdb.prot5475 (2010).
Eng, J. K., Jahan, T. A. & Hoopmann, M. R. Comet: an open-source MS/MS sequence database search tool. Proteomics 13, 22–24 (2013).
Elias, J. E. & Gygi, S. P. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods 4, 207–214 (2007).
Huttlin, E. L. et al. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 143, 1174–1189 (2010).
Savitski, M. M., Wilhelm, M., Hahne, H., Kuster, B. & Bantscheff, M. A scalable approach for protein false discovery rate estimation in large proteomic data sets. Mol. Cell Proteom. 14, 2394–2404 (2015).
Beausoleil, S. A., Villen, J., Gerber, S. A., Rush, J. & Gygi, S. P. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat. Biotechnol. 24, 1285–1292 (2006).
Gassaway, B. M. et al. A multi-purpose, regenerable, proteome-scale, human phosphoserine resource for phosphoproteomics. Nat. Methods 19, 1371–1375 (2022).
MacKerell, A. D. et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 102, 3586–3616 (1998).
Mackerell, A. D., Feig, M. & Brooks, C. L. Extending the treatment of backbone energetics in protein force fields: limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J. Comput. Chem. 25, 1400–1415 (2004).
Guvench, O. et al. CHARMM additive all-atom force field for carbohydrate derivatives and its utility in polysaccharide and carbohydrate-protein modeling. J. Chem. Theory Comput. 7, 3162–3180 (2011).
Huang, J. & MacKerell, A. D. CHARMM36 all-atom additive protein force field: validation based on comparison to NMR data. J. Comput. Chem. 34, 2135–2145 (2013).
Vanommeslaeghe, K. et al. CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 31, 671–690 (2010).
Jorgensen, W., Chandrasekhar, J., Madura, J., Impey, R. & Klein, M. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Durell, S., Brooks, B. & Ben-Naim, A. Solvent-induced forces between two hydrophilic groups. J. Phys. Chem. 98, 2198–2202 (1994).
Neria, E., Fischer, S. & Karplus, M. Simulation of activation free energies in molecular systems. J. Chem. Phys. 105, 1902–1921 (1996).
Beglov, D. & Roux, B. Finite representation of an infinite bulk system: solvent boundary potential for computer simulations. J. Chem. Phys. 100, 9050–9063 (1994).
Luo, Y. & Roux, B. Simulation of osmotic pressure in concentrated aqueous salt solutions. J. Phys. Chem. Lett. 1, 183–189 (2010).
Phillips, J. C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
Br ü nger, A., Brooks, C. & Karplus, M. Stochastic boundary conditions for molecular dynamics simulations of ST2 water. Chem. Phys. Lett. 105, 495–500 (1984).
Bussi, G., Donadio, D. & Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 126, 014101 (2007).
Ryckaert, J.-P., Ciccotti, G. & Berendsen, H. Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J. Comput. Phys. 23, 327–341 (1977).
Miyamoto, S. & Kollman, P. Settle: an analytical version of the SHAKE and RATTLE algorithm for rigid water models. J. Comput. Chem. 13, 952–962 (1992).
Ma, Q., Izaguirre, J. & Skeel, R. Verlet-I/R-RESPA/Impulse is limited by nonlinear instabilities. SIAM J. Sci. Comput. 24, 1951–1973 (2003).
Feller, S., Zhang, Y., Pastor, R. & Brooks, B. Constant pressure molecular dynamics simulation: the Langevin piston method. J. Chem. Phys. 103, 4613–4621 (1995).
Tuckerman, M., Berne, B. & Martyna, G. Reversible multiple time scale molecular dynamics. J. Chem. Phys. 97, 1990–2001 (1992).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: an N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Essmann, U., Perera, L. & Berkowitz, M. A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995).
Skeel, R. An alternative construction of the Ewald sum. Mol. Phys. 114, 3166–3170 (2016).
Harris, C. R. et al. Array programming with NumPy. Nature 585, 357–362 (2020).
Hunter, J. D. Matplotlib: a 2D graphics environment. Comput. Sci. Eng. 9, 90–95 (2007).
Hyberts, S. G., Milbradt, A. G., Wagner, A. B., Arthanari, H. & Wagner, G. Application of iterative soft thresholding for fast reconstruction of NMR data non-uniformly sampled with multidimensional Poisson Gap scheduling. J. Biomol. NMR 52, 315–327 (2012).
Schanda, P. & Brutscher, B. Very fast two-dimensional NMR spectroscopy for real-time investigation of dynamic events in proteins on the time scale of seconds. J. Am. Chem. Soc. 127, 8014–8015 (2005).
Vranken, W. F. et al. The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins 59, 687–696 (2005).
Williamson, M. P. Using chemical shift perturbation to characterise ligand binding. Prog. Nucl. Magn. Reson. Spectrosc. 73, 1–16 (2013).
Marintchev, A., Frueh, D. & Wagner, G. NMR methods for studying protein–protein interactions involved in translation initiation. Methods Enzymol. 430, 283–331 (2007).
Masson, G. R. et al. Recommendations for performing, interpreting and reporting hydrogen deuterium exchange mass spectrometry (HDX-MS) experiments. Nat. Methods 16, 595–602 (2019).
Wales, T. E. & Engen, J. R. Hydrogen exchange mass spectrometry for the analysis of protein dynamics. Mass Spectrom. Rev. 25, 158–170 (2006).
Perez-Riverol, Y. et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 50, D543–D552 (2022).
Acknowledgements
We thank E. Smith for assistance with figure preparation; J. Lee for performing intact mass spectrometry analysis of tBID at the Dana-Farber Molecular Biology Core; J. Sun for NMR technical support at the Dana-Farber NMR Core and the Harvard Medical School BioNMR Core; and G. Bird and B. Moyer for synthesizing BIM SAHB. This study was funded by National Institutes of Health (NIH) grant R35CA197583 to L.D.W., NIH grant R01AI070292 and the Harry and Dianna Professorship in Pharmaceutical Sciences to J.A.W., NIH grant R01GM67945 to S.P.G., National Science Foundation and Landry Cancer Biology Research pre-doctoral fellowships to M.W.M. and NIH grant 5T32HL007574 to C.M.C.
Author information
Authors and Affiliations
Contributions
M.W.M. and L.D.W. designed the study. D.T.C. and T.J.R. conducted the disulfide tethering screen, under the supervision of J.A.W. M.W.M. synthesized the small molecules, produced BAX proteins and performed all biochemical, mitochondrial and structural experiments. P.S. assisted M.W.M. with biochemical and mitochondrial experiments. C.M.C. performed the molecular dynamics simulations and assisted M.W.M. with the HMQC NMR experiments. U.A., M.A.G. and K.Y. conducted the chemoproteomics experiment, under the supervision of S.P.G. M.W.M. and T.E.W. performed the HDX MS analyses, under the supervision of J.R.E. L.D.W. wrote the manuscript, which was reviewed by all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Haiming Dai, Glenn Masson, Tudor Moldoveanu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Comparative inhibitory effects of small molecule hits from the disulfide tethering screen on tBID-triggered BAX-mediated liposomal release.
a-d, Liposomal release in response to BAX WT, tBID, the indicated molecule, or the combination of tBID and BAX WT pre-incubated with or without increasing doses of 1C18 (a), 1A18 (b), 1E4 (c), or 3A20 (d). Data are mean ± s.e.m. for experiments performed in technical quadruplicate and repeated using independent preparations of liposomes, proteins, and molecules with similar results.
Extended Data Fig. 2 Effect of CBI1 on BIM SAHB-triggered BAX-mediated liposomal release.
Dose-responsive inhibition of BIM SAHB-triggered, BAX-mediated liposomal poration (dark gray) upon addition of CBI1 (blue). Data are mean ± s.e.m. for experiments performed in technical quadruplicate and repeated using independent preparations of liposomes, protein, and compounds with similar results.
Extended Data Fig. 3 No inhibitory effect of CBI1 on Fos-12-induced oligomerization of BAX.
SEC profiles of monomeric BAX (gray) and Fos-12-induced BAX oligomer in the presence (blue) or absence (black) of CBI1. The experiment was performed twice with independent preparations of protein, detergent, and small molecule with similar results.
Extended Data Fig. 4 NMR analysis of the BAX/CBI1 interaction.
a, Measured chemical shift changes of 15N-BAX (20 μM) upon addition of CBI1 (5:1 of CBI1:BAX), plotted as a function of BAX residue number. Chemical shift changes above the 2 s.d. cutoff (significance threshold of 0.0536 p.p.m.) are colored maroon and those above the 1 s.d. cutoff (significance threshold of 0.0347 p.p.m.) are colored red. Residues whose cross peaks experienced prominent signal attenuation or chemical shift perturbation upon CBI1 incubation are colored beige. b-c, Chemical shift perturbations of BAX cross peaks corresponding to residues F116 (b, dashed box) and S118 (c) upon addition of CBI1. Whereas BAX exhibited one cross peak each for F116 and S118 (gray), the addition of CBI1 at a CBI1:BAX ratio of 5:1 resulted in the appearance of a second cross peak for each residue (red). Upon incubation with CBI1 at a CBI1:BAX ratio of 10:1, the original cross peaks shifted completely to the second locations (blue). In contrast, D142 (b) experienced little to no change in its corresponding cross peak upon addition of CBI1. d, Prominent signal attenuation or chemical shift perturbation of A81 in wild-type BAX upon CBI1 titration, as reflected by disappearance of the cross peak. e, In contrast, in the context of BAX C126A, the A81 cross peak demonstrates progressive migration upon CBI1 titration, consistent with fast exchange between the unbound and bound forms of BAX C126A, as expected for non-covalent interaction.
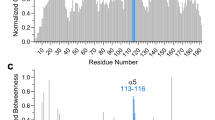
Extended Data Fig. 5 Influence of CBI1 on the conformational dynamics of the BAX α1-α2 loop.
a, A difference distance matrix plot derived from molecular dynamics simulations of wild-type BAX in the presence or absence of CBI1 demonstrated the greatest effect of small molecule C126-derivatization on the protein dynamics of the α1-α2 loop. b-c, Representative images from the molecular dynamics (MD) simulations of BAX in the absence (b) and presence (c) of CBI1, demonstrating distinct positioning of the α1-α2 loop (purple).
Extended Data Fig. 6 Comparative NMR analyses of the BAX WT/CBI1, BAX WT/N-CBI1, and BAX C126A/CBI1 interactions.
a–c, Measured chemical shift changes of 15N-BAX WT (40 μM) upon addition of CBI1 (5:1 small molecule:protein) (a), 15N-BAX WT (40 μM) upon addition of N-CBI1 (5:1 small molecule:protein) (b), and 15N-BAX C126A (40 μM) upon addition of CBI1 (5:1 small molecule:protein) (c), plotted as a function of BAX residue number. Chemical shift changes above the 2 s.d. cutoff (significance thresholds of 0.0499, 0.0187, 0.01579 p.p.m. for a, b, c, respectively) are colored maroon and those above the 1 s.d. cutoff (significance thresholds of 0.0329, 0.0125, 0.0107 p.p.m. for a, b, c, respectively) are colored red. Residues whose cross peaks experienced prominent signal attenuation or chemical shift perturbation upon small molecule incubation are colored beige.
Extended Data Fig. 7 Relative impact of covalent vs. non-covalent small molecule interaction on BAX F116A-mediated liposomal permeabilization.
a, b, Comparative inhibitory effects of CBI1 and N-CBI1 on liposomal release by BAX F116A (a) or BAX F116A/C126A (b). Data are mean ± s.e.m. for experiments performed in technical quadruplicate and repeated using independent preparations of liposomes, proteins, and small molecules with similar results.
Extended Data Fig. 8 CBI1 reverses the conformational activation of BAX F116A.
a, b, Difference distance matrix plots derived from molecular dynamics simulations of BAX F116A compared to BAX WT (a) and BAX F116A in the presence or absence of CBI1 (b) demonstrated striking reversal of the auto-activating conformational changes induced by BAX F116A mutagenesis upon CBI1 covalent derivatization of C126.
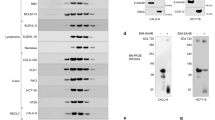
Extended Data Fig. 9 CBI1 blocks the mitochondrial translocation of BAX F116A.
a, b, Distribution of BAX F116A (a) or BAX F116A/C126A (b) (2 μM) between supernatant and BAX/BAK-deficient mitochondrial fractions, as detected by BAX western analysis after pre-treating BAX proteins with escalating doses of CBI1 (200 nM–4 μM, lanes 2–7), incubation with mitochondria, isolation of the supernatant and pellet fractions by centrifugation, and SDS PAGE. Isolation of the mitochondrial pellet fraction was verified by VDAC1 western analysis. The experiment was performed three times using independent preparations of mitochondria, proteins, and small molecule.
Extended Data Fig. 10 CBI1 blocks t-2-hex lipidation and induced homo-oligomerization of BAX F116A.
Incubation of BAX F116A (5 μM) with t-2-hex (2.5 mM) in the presence or absence of increasing amounts (5 μM-100 μM) of CBI1 (lanes 3–7) or N-CBI1 (lanes 9–13) for 2 hours at 37 °C followed by detection of lipidated BAX by addition of Cy5-hydrazide, gel electrophoresis, and fluorescence scan. BAX protein lipidation, t-2-hex-induced homo-oligomerization (as reflected by laddering), and comparative dose-responsive suppression by CBI1 and N-CBI1, was detected by fluorescence scan of the indicated BAX mixtures (top and middle panels). The influence of t-2-hex and co-treatment with CBI1 or N-CBI1 on the level of monomeric BAX F116A was monitored by protein stain of the electrophoresed BAX mixtures (bottom panel). The experiment was performed twice using independent preparations of protein, lipid, and small molecules.
Supplementary information
Supplementary Information
Supplementary Tables 1–4, Supplementary Figs. 1–6 and Supplementary Note 1.
Supplementary Video
Molecular dynamics simulation of the CBI1–BAX interaction.
Supplementary Data 1
Source data for Supplementary Figs. 1–3, 5 and 6.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data and unprocessed western blots and gels.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 9
Unprocessed western blots.
Source Data Extended Data Fig. 10
Unprocessed gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McHenry, M.W., Shi, P., Camara, C.M. et al. Covalent inhibition of pro-apoptotic BAX. Nat Chem Biol 20, 1022–1032 (2024). https://doi.org/10.1038/s41589-023-01537-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-023-01537-6