Abstract
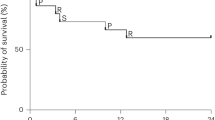
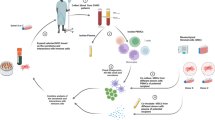
The therapeutic potential of donor-derived mesenchymal stromal cells (MSCs) has been investigated in diverse diseases1, including steroid-resistant acute graft versus host disease (SR-aGvHD)2. However, conventional manufacturing approaches are hampered by challenges with scalability and interdonor variability, and clinical trials have shown inconsistent outcomes3,4. Induced pluripotent stem cells (iPSCs) have the potential to overcome these challenges, due to their capacity for multilineage differentiation and indefinite proliferation5,6. Nonetheless, human clinical trials of iPSC-derived cells have not previously been completed. CYP-001 (iPSC-derived MSCs) is produced using an optimized, good manufacturing practice (GMP)-compliant manufacturing process. We conducted a phase 1, open-label clinical trial (no. NCT02923375) in subjects with SR-aGvHD. Sixteen subjects were screened and sequentially assigned to cohort A or cohort B (n = 8 per group). One subject in cohort B withdrew before receiving CYP-001 and was excluded from analysis. All other subjects received intravenous infusions of CYP-001 on days 0 and 7, at a dose level of either 1 × 106 cells per kg body weight, to a maximum of 1 × 108 cells per infusion (cohort A), or 2 × 106 cells per kg body weight, to a maximum dose of 2 × 108 cells per infusion (cohort B). The primary objective was to assess the safety and tolerability of CYP-001, while the secondary objectives were to evaluate efficacy based on the proportion of participants who showed a complete response (CR), overall response (OR) and overall survival (OS) by days 28/100. CYP-001 was safe and well tolerated. No serious adverse events were assessed as related to CYP-001. OR, CR and OS rates by day 100 were 86.7, 53.3 and 86.7%, respectively. The therapeutic application of iPSC-derived MSCs may now be explored in diverse inflammatory and immune-mediated diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All reasonable requests for raw and analyzed data that are not included in this manuscript or online content will be promptly reviewed by the senior authors to determine whether the request is subject to any intellectual property or confidentiality obligations. Patient-related data may be subject to patient confidentiality restrictions. Any data and materials that can be shared will be released via a material transfer agreement. All raw and analyzed global gene expression data can be found at the NCBI Gene Expression Archive (accession no. GSE150969).
References
Kabat, M., Bobkov, I., Kumar, S. & Grumet, M. Trends in mesenchymal stem cell clinical trials 2004–2018: is efficacy optimal in a narrow dose range? Stem Cells Transl. Med. 9, 17–27 (2020).
Elgaz, S. et al. Clinical use of mesenchymal stromal cells in the treatment of acute graft-versus-host disease. Transfus. Med. Hemother. 46, 27–34 (2019).
Galipeau, J. The mesenchymal stromal cells dilemma—does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy 15, 2–8 (2013).
François, M. et al. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther. 20, 187–195 (2012).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Galipeau, J. & Sensébé, L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell 22, 824–833 (2018).
Harrell, C. R. et al. Mesenchymal stem cell–derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells 8, 1605 (2019).
Galleu, A. et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 9, 7828 (2017).
Sharma, R. R. et al. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion 54, 1418–1437 (2014).
Wegmeyer, H. et al. Mesenchymal stromal cell characteristics vary depending on their origin. Stem Cells Dev. 22, 2606–2618 (2013).
Martin, I. et al. Challenges for mesenchymal stromal cell therapies. Sci. Transl. Med. 20, eaat2189 (2019).
Ketterl, N. et al. A robust potency assay highlights significant donor variation of human mesenchymal stem/progenitor cell immune modulatory capacity and extended radio-resistance. Stem Cell Res. Ther. 6, 236 (2015).
Siegel, G. et al. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 11, 146 (2013).
Wagner, W. et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS ONE 5, e2213 (2008).
von Bahr, L. et al. Long-term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy. Biol. Blood Marrow Transplant. 18, 557–564 (2012).
Garnett, C. et al. Treatment and management of graft-versus-host disease: improving response and survival. Ther. Adv. Hematol. 4, 366–378 (2013).
Le Blanc, K. et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363, 1439–1441 (2004).
Ozay, E. I. et al. Cymerus™ iPSC-MSCs significantly prolong survival in a pre-clinical, humanized mouse model of graft-vs-host disease. Stem Cell Res. 35, 101401 (2019).
Vodyanik, M. A. et al. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell 7, 718–729 (2010).
Slukvin, I. I. & Kumar, A. The mesenchymoangioblast, mesodermal precursor for mesenchymal and endothelial cells. Cell. Mol. Life Sci. 75, 3507–3520 (2018).
Chen, G. et al. Chemically defined conditions for human iPS cell derivation and culture. Nat. Methods 8, 424–429 (2011).
Uenishi, G. et al. Tenascin C promotes hematoendothelial development and T lymphoid commitment from human pluripotent stem cells in chemically defined conditions. Stem Cell Rep. 3, 1073–1084 (2014).
Slukvin, I., Uenishi, G., Hei, D. & Drier, D. Colony forming medium and use thereof. Patent WO2017156580A1 (2017).
Dominici, M. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317 (2006).
Bader, P. et al. Effective treatment of steroid and therapy-refractory acute graft versus-host disease with a novel mesenchymal stromal cell product (MSC-FFM). Bone Marrow Transplant. 53, 852–862 (2018).
Kebriaei, P. et al. A phase 3 randomized study of remestemcel-L versus placebo added to second-line therapy in patients with steroid-refractory acute graft-versus-host disease. Biol. Blood Marrow Transplant. 26, 835–844 (2020).
Zeiser, R. et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N. Engl. J. Med. 382, 1800–1810 (2020).
Jagasia, M. et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label, phase 2 trial. Blood 135, 1739–1749 (2020).
Park, J. H. et al. Etanercept for steroid-refractory acute graft versus host disease following allogeneic hematopoietic stem cell transplantation. Korean J. Intern. Med. 29, 630–636 (2014).
De Jong, C. N. et al. Etanercept for steroid-refractory acute graft-versus-host disease: a single center experience. PLoS ONE 12, e0187184 (2017).
Greinix, H. T. et al. Extracorporeal photochemotherapy in the treatment of severe steroid-refractory acute graft-versus-host disease: a pilot study. Blood 96, 2426–2431 (2000).
Koch, J. M. et al. Mesenchymoangioblast-derived mesenchymal stromal cells inhibit cell damage, tissue damage and improve peripheral blood flow following hindlimb ischemic injury in mice. Cytotherapy 18, 219–228 (2016).
Royce, S. G. et al. Intranasal administration of mesenchymoangioblast-derived mesenchymal stem cells abrogates airway fibrosis and airway hyperresponsiveness associated with chronic allergic airways disease. FASEB J. 31, 4168–4178 (2017).
Royce, S. G. et al. iPSC- and mesenchymoangioblast-derived mesenchymal stem cells provide greater protection against experimental chronic allergic airways disease compared with a clinically used corticosteroid. FASEB J. 33, 6402–6411 (2019).
Khan, M. A. et al. iPSC-derived MSC therapy induces immune tolerance and supports long-term graft survival in mouse orthotopic tracheal transplants. Stem Cell Res. Ther. 10, 290 (2019).
Millar, J. E. et al. Combined mesenchymal stromal cell therapy and ECMO in ARDS: a controlled experimental study in sheep. Am. J. Respir. Crit. Care Med. https://doi.org/10.1164/rccm.201911-2143OC (2020).
Mandai, M., Kurimoto, Y. & Takahashi, M. Autologous induced stem-cell-derived retinal cells for macular degeneration. N. Engl. J. Med. 377, 792–793 (2017).
Ylä-Herttuala, S. iPSC-derived cardiomyocytes taken to rescue infarcted heart muscle in coronary heart disease patients. Mol. Ther. 26, 2077 (2018).
Stoddard-Bennett, T. & Reijo Pera, R. Treatment of Parkinson’s disease through personalized medicine and induced pluripotent stem cells. Cells 8, 26 (2019).
Mack, A. A. et al. Generation of induced pluripotent stem cells from CD34+ cells across blood drawn from multiple donors with non-integrating episomal vectors. PLoS ONE 6, e27956 (2011).
Borowicz, S. et al. The soft agar colony formation assay. J. Vis. Exp. 92, e51998 (2014).
Przepiorka, D. et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 15, 825–828 (1995).
Acknowledgements
We thank the University of Wisconsin Bioinformatics Resource Center for providing mRNA-seq analysis services, and all site investigators, coinvestigators, study coordinators, other study staff and study participants. Medical writer C. Markey, of Markey Medical Consulting Pty Ltd, assisted in the preparation of an earlier version of a draft manuscript describing this work. J.E.J.R. is supported by a National Health and Medical Research Council Investigator Grant (1177305) for ‘driving clinical cell and gene therapy in Australia’, as well as funding from Cure the Future, Therapeutic Innovation Australia and an anonymous foundation.
Author information
Authors and Affiliations
Contributions
I.S., G.I.U., D.D. and D.H. developed and optimized the manufacturing process. I.S., D.D., D.H. and L.S.L. developed and optimized quality control assays and designed the experiment to verify that iPSCs do not survive M-CFM culture. K.K. designed the clinical trial and wrote the protocol. A.J.C.B., A.P., J.E.G., M.H.G., R.R., D.T.Y. and J.E.J.R. were principal investigators in the clinical trial. K.K. and J.E.J.R. prepared the first drafts of the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Patent applications (some of which have been granted) covering aspects of the technology described in this manuscript have been filed by Wisconsin Alumni Research Foundation (which invests in the University of Wisconsin-Madison), Cellular Dynamics International (now FUJIFILM Cellular Dynamics) and Cynata Therapeutics. The patents with inventors including D.D., D.H., G.I.U., K.K. and I.S. cover the processes used to generate iPSCs and differentiate iPSCs into MSCs, as well as the residual undifferentiated iPSC assay. Cynata Therapeutics funded the work described in this manuscript. K.K. is an employee and shareholder of Cynata Therapeutics. I.S. is a cofounder, shareholder and scientific advisor of Cynata Therapeutics. L.S.L. and D.D. are employees of Waisman Biomanufacturing, University of Wisconsin-Madison, which provides contract manufacturing services to Cynata Therapeutics. D.H. is a former employee of both Waisman Biomanufacturing and Cellular Dynamics International. G.I.U. and J.E.J.R. have received travel grants from Cynata Therapeutics. A.J.C.B., A.P., J.E.G., M.H.G., R.R. and D.T.Y. declare no relevant competing interests.
Additional information
Peer review information Jerome Staal was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 mRNAseq global gene expression analysis showing gene correlation and Pearson correlation coefficients.
mRNAseq global gene expression (transcriptome) analysis from three batches (CYN-IPSC-MSC-P5B-FP-001; CYN-IPSC-MSC-P5B-FP-002; CYN-IPSC-MSC-P5B-FP-003) showing gene correlation [log[2](TPM+1)] and Pearson correlation coefficients (R).
Extended Data Fig. 2 mRNAseq global gene expression analysis showing isoform correlation and Pearson correlation coefficients.
mRNAseq global gene expression (transcriptome) analysis from three batches (CYN-IPSC-MSC-P5B-FP-001; CYN-IPSC-MSC-P5B-FP-002; CYN-IPSC-MSC-P5B-FP-003) showing isoform correlation [log[2](TPM+1)] and Pearson correlation coefficients (R).
Extended Data Fig. 3 CONSORT diagram.
Clinical trial summary (CONSORT diagram).
Supplementary information
Supplementary Information
Supplementary Tables 1–11.
Rights and permissions
About this article
Cite this article
Bloor, A.J.C., Patel, A., Griffin, J.E. et al. Production, safety and efficacy of iPSC-derived mesenchymal stromal cells in acute steroid-resistant graft versus host disease: a phase I, multicenter, open-label, dose-escalation study. Nat Med 26, 1720–1725 (2020). https://doi.org/10.1038/s41591-020-1050-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-020-1050-x
This article is cited by
-
Induced pluripotent stem cell-derived mesenchymal stem cells (iMSCs) inhibit M1 macrophage polarization and reduce alveolar bone loss associated with periodontitis
Stem Cell Research & Therapy (2025)
-
Causes and therapeutic limitations of clinical alopecia and the advent of human pluripotent stem cell follicular transplantation
Stem Cell Research & Therapy (2025)
-
The use of MSCs in steroid-refractory acute GvHD in Europe: a survey from the EBMT cellular therapy & immunobiology working party
Bone Marrow Transplantation (2025)
-
Antifragile Treatment for Efficient Chimerism of Induced Pluripotent Stem Cells Derived Hematopoietic Stem Cells
Stem Cell Reviews and Reports (2025)
-
Mesenchymal Stromal Cells and Graft-versus-Host Disease: Preclinical and Clinical Studies
Stem Cell Reviews and Reports (2025)