Abstract
Tirzepatide is a once-weekly GIP/GLP-1 receptor agonist. In this phase 3, randomized, open-label trial, insulin-naive adults (≥18 years of age) with type 2 diabetes (T2D) uncontrolled on metformin (with or without a sulphonylurea) were randomized 1:1:1:1 to weekly tirzepatide 5 mg, 10 mg or 15 mg or daily insulin glargine at 66 hospitals in China, South Korea, Australia and India. The primary endpoint was non-inferiority of mean change in hemoglobin A1c (HbA1c) from baseline to week 40 after treatment with 10 mg and 15 mg of tirzepatide. Key secondary endpoints included non-inferiority and superiority of all tirzepatide doses in HbA1c reduction, proportions of patients achieving HbA1c < 7.0% and weight loss at week 40. A total of 917 patients (763 (83.2%) in China) were randomized to tirzepatide 5 mg (n = 230), 10 mg (n = 228) or 15 mg (n = 229) or insulin glargine (n = 230). All doses of tirzepatide were non-inferior and superior to insulin glargine for least squares mean (s.e.) reduction in HbA1c from baseline to week 40: tirzepatide 5 mg, 10 mg and 15 mg, −2.24% (0.07), −2.44% (0.07) and −2.49% (0.07), respectively, and insulin glargine, −0.95% (0.07), with a treatment difference ranging from −1.29% to −1.54% (all P < 0.001). Proportions of patients achieving HbA1c < 7.0% at week 40 were greater in tirzepatide 5-mg (75.4%), 10-mg (86.0%) and 15-mg (84.4%) groups compared to insulin glargine (23.7%) (all P < 0.001). All tirzepatide doses led to superior body weight reduction at week 40: tirzepatide 5 mg, 10 mg and 15 mg, −5.0 kg (−6.5%), −7.0 kg (−9.3%) and −7.2 kg (−9.4%), respectively, compared to insulin glargine, 1.5 kg (+2.1%) (all P < 0.001). The most common adverse events with tirzepatide were mild to moderate decreased appetite, diarrhea and nausea. No severe hypoglycemia was reported. Tirzepatide demonstrated superior reductions in HbA1c versus insulin glargine in an Asia-Pacific, predominately Chinese, population with T2D and was generally well tolerated. ClinicalTrials.gov registration: NCT04093752.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data from the analyses in this study cannot be publicly available due to the sponsor’s (Eli Lilly and Company) contractual obligations. Eli Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and the European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at https://vivli.org/.
Code availability
No customer code was used for data analysis in this study.
References
Nauck, M. A., Quast, D. R., Wefers, J. & Pfeiffer, A. F. H. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: a pathophysiological update. Diabetes Obes. Metab. 23, 5–29 (2021).
Nauck, M. A., Quast, D. R., Wefers, J. & Meier, J. J. GLP-1 receptor agonists in the treatment of type 2 diabetes—state-of-the-art. Mol. Metab. 46, 101102 (2021).
Gasbjerg, L. S. et al. Evaluation of the incretin effect in humans using GIP and GLP-1 receptor antagonists. Peptides 125, 170183 (2020).
Baggio, L. L. & Drucker, D. J. Glucagon-like peptide-1 receptor co-agonists for treating metabolic disease. Mol. Metab. 46, 101090 (2021).
Min, T. & Bain, S. C. The role of tirzepatide, dual GIP and GLP-1 receptor agonist, in the management of type 2 diabetes: the SURPASS clinical trials. Diabetes Ther. 12, 143–157 (2021).
Samms, R. J., Coghlan, M. P. & Sloop, K. W. How may GIP enhance the therapeutic efficacy of GLP-1? Trends Endocrinol. Metab. 31, 410–421 (2020).
Frias, J. P. et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 392, 2180–2193 (2018).
Papachristou, S., Popovic, D. S. & Papanas, N. The new dual gastric inhibitory peptide/glucagon-like peptide 1 agonist tirzepatide in type 2 diabetes: is the future bright? Diabetes Metab. Res. Rev. 37, e3503 (2021).
Dahl, D. et al. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA 327, 534–545 (2022).
Del Prato, S. et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 398, 1811–1824 (2021).
Frías, J. P. et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N. Engl. J. Med. 385, 503–515 (2021).
Ludvik, B. et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet 398, 583–598 (2021).
Rosenstock, J. et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet 398, 143–155 (2021).
Inagaki, N., Takeuchi, M., Oura, T., Imaoka, T. & Seino, Y. Efficacy and safety of tirzepatide monotherapy compared with dulaglutide in Japanese patients with type 2 diabetes (SURPASS J-mono): a double-blind, multicentre, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 10, 623–633 (2022).
Kadowaki, T., Chin, R., Ozeki, A., Imaoka, T. & Ogawa, Y. Safety and efficacy of tirzepatide as an add-on to single oral antihyperglycaemic medication in patients with type 2 diabetes in Japan (SURPASS J-combo): a multicentre, randomised, open-label, parallel-group, phase 3 trial. Lancet Diabetes Endocrinol. 10, 634–644 (2022).
Davies, M. J. et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 45, 2753–2786 (2022).
IDF Diabetes Atlas, 10th edition. International Diabetes Federation https://www.diabetesatlas.org (2021).
Niswender, K. D. Basal insulin: physiology, pharmacology, and clinical implications. Postgrad. Med. 123, 17–26 (2011).
Chinese Diabetes Society. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chin. J. Diabetes Mellit. 13, 315–409 (2021).
Heise, T. et al. Effects of subcutaneous tirzepatide versus placebo or semaglutide on pancreatic islet function and insulin sensitivity in adults with type 2 diabetes: a multicentre, randomised, double-blind, parallel-arm, phase 1 clinical trial. Lancet Diabetes Endocrinol. 10, 418–429 (2022).
Feng, W., Chen, W., Jiang, S., Du, L. & Zhu, D. Efficacy and safety of LY2963016 insulin glargine versus insulin glargine (Lantus) in Chinese adults with type 2 diabetes: a phase III, randomized, open-label, controlled trial. Diabetes Obes. Metab. 23, 1786–1794 (2021).
Ji, L. et al. Efficacy and safety of insulin glargine 300 U/mL versus insulin glargine 100 U/mL in Asia Pacific insulin-naïve people with type 2 diabetes: the EDITION AP randomized controlled trial. Diabetes Obes. Metab. 22, 612–621 (2020).
Ji, L. et al. Higher versus standard starting dose of insulin glargine 100 U/mL in overweight or obese Chinese patients with type 2 diabetes: results of a multicentre, open-label, randomized controlled trial (BEYOND VII). Diabetes Obes. Metab. 22, 838–846 (2020).
Wang, W. et al. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in mainly Asian patients with type 2 diabetes mellitus on metformin and/or a sulphonylurea: a 52-week open-label, randomized phase III trial. Diabetes Obes. Metab. 21, 234–243 (2019).
Aroda, V. R. et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 5, 355–366 (2017).
Pozzilli, P. et al. Placebo-controlled, randomized trial of the addition of once-weekly glucagon-like peptide-1 receptor agonist dulaglutide to titrated daily insulin glargine in patients with type 2 diabetes (AWARD-9). Diabetes Obes. Metab. 19, 1024–1031 (2017).
Aras, M., Tchang, B. G. & Pape, J. Obesity and diabetes. Nurs. Clin. North Am. 56, 527–541 (2021).
Hills, A. P. et al. Epidemiology and determinants of type 2 diabetes in south Asia. Lancet Diabetes Endocrinol. 6, 966–978 (2018).
Jang, M. & Berry, D. Overweight, obesity, and metabolic syndrome in adults and children in South Korea: a review of the literature. Clin. Nurs. Res 20, 276–291 (2011).
Hou, X. et al. Impact of waist circumference and body mass index on risk of cardiometabolic disorder and cardiovascular disease in Chinese adults: a national diabetes and metabolic disorders survey. PLoS ONE 8, e57319 (2013).
Wang, Y. et al. Prevalence and numbers of diabetes patients with elevated BMI in China: evidence from a nationally representative cross-sectional study. Int J. Environ. Res. Public Health 19, 2989 (2022).
Ji, L. N. & Zou, D. J. Consensus of chinese experts on the remission of type 2 diabetes mellitus. Chin. Gen. Pract. 24, 11 (2021).
Heise, T. et al. Tirzepatide reduces appetite, energy intake, and fat mass in people with type 2 diabetes. Diabetes Care 46, 998–1004 (2023).
Francula-Zaninovic, S. & Nola, I. A. Management of measurable variable cardiovascular disease’ risk factors. Curr. Cardiol. Rev. 14, 153–163 (2018).
Younossi, Z. et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 15, 11–20 (2018).
Aroda, V. R. A review of GLP-1 receptor agonists: evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes. Metab. 20, 22–33 (2018).
Technical Guidance for Clinical Trials of Drugs for the Treatment of Adults with Type 2 Diabetes (China National Medical Products Administration, 2023); https://www.cde.org.cn/main/att/download/e2cb472cc84a13e946378f819cc5c206
Acknowledgements
This study was funded by Eli Lilly and Company. The study sponsor had a role in the study design, data collection, data analysis, interpretation of data, writing of the report and in the decision to submit the paper for publication. Medical writing assistance in the preparation of this article was provided by M. Dyson on behalf of Rude Health Consulting, J. Burrell (Rude Health Consulting) and C. Zeng (Eli Lilly and Company) and was funded by Eli Lilly and Company.
Author information
Authors and Affiliations
Contributions
L.J. led the design, conduct and analysis of the clinical study. L.G., B.L., M.C. and J.K. contributed to the acquisition of data. L.D. led the data analysis, and all authors participated in data interpretation. All authors confirm that they had full access to the complete study data, took part in the development of the manuscript and accept responsibility to submit for publication.
Corresponding author
Ethics declarations
Competing interests
L.H., L.D. and Y.H. are employees of Eli Lilly and Company. L.J. reports receiving consulting and lecture fees from Eli Lilly and Company, Novo Nordisk, Merck, Bayer, Sanofi-Aventis, Roche, Merck Sharp & Dohme, Metronics, AstraZeneca, Boehinger Ingelheim and Abbott. L.J. and L.G. received a research grant for this study from Eli Lilly and Company. M.C. reports having speaker contracts with Novo Nordisk, Sanofi, Biocon, Cipla, USV Ltd. and Abbott. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Victor Volovici, Stefano Del Prato and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Jennifer Sargent, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Change from baseline in HbA1c (a) and proportion of patients achieving HbA1c < 7.0% (b) at week 40 in the full analysis set.
LSMean changes from baseline in HbA1c were estimated using an ANCOVA with return-to-baseline multiple imputation for missing values at week 40 in patients who received at least 1 dose of study drug (5 mg, N = 230; 10 mg, N = 228; 15 mg, N = 229; insulin glargine, N = 220); statistical tests for 10 mg and 15 mg were two sided at a significance level of 0.025, statistical tests for 5 mg were two sided at a significance level of 0.05. Proportion of patients achieving HbA1c < 7.0% was estimated using logistic regression analysis with return-to-baseline multiple imputation for missing values at week 40 in patients who received at least 1 dose of study drug (5 mg, n = 230; 10 mg, n = 228; 15 mg, n = 229; insulin glargine, n = 220); all statistical tests were two sided at a significance level of 0.05. Error bars indicate SE. ANCOVA, analysis of covariance; HbA1c, glycated hemoglobin; LSMean, least-square mean; n, number of patients achieving target with missing value imputed by return-to-baseline multiple imputation; N, number of patients who were randomized and received at least 1 dose of study drug; SE, standard error.
Extended Data Fig. 2 Change from baseline in bodyweight at week 40 in the full analysis set.
Changes from baseline in bodyweight were estimated using an ANCOVA with return-to-baseline multiple imputation for missing values at week 40 in patients who received at least 1 dose of study drug (5 mg, N = 230; 10 mg, N = 228; 15 mg, N = 229; insulin glargine, N = 220); statistical tests for 10 mg and 15 mg were two sided at a significance level of 0.025, statistical tests for 5 mg were two sided at a significance level of 0.05. Data presented are LSMean (SE). Error bars indicate SE. ANCOVA, analysis of covariance; LSMean, least-square mean; N, number of patients who were randomized and received at least 1 dose of study drug; SE, standard error.
Extended Data Fig. 3 Incidence and severity of nausea, vomiting, diarrhea and decreased appetite over time.
Notes: dotted areas indicate the tirzepatide dose escalation period. Severity is indicated by color: green (mild); orange (moderate); red (severe). TZP, tirzepatide.
Extended Data Fig. 4 P-amylase (a) and lipase (b) levels over time.
Data presented are estimate mean (SE). Error bars indicate SE. Baseline values were calculated using an ANOVA, post-baseline measures were calculated from an MMRM, all statistical tests were two sided at a significance level of 0.05. Normal reference values: p-amylase (13–53 IU/L); lipase (13–60 IU/L). ANOVA, analysis of variance; MMRM, mixed model for repeated measures; No., number of patients with baseline and post-baseline value at the specified time point; p-amylase, pancreatic amylase; TZP, tirzepatide; SE, standard error; SFU, safety follow up.
Extended Data Fig. 5 Change in waist circumference over time.
Data presented are LSMean (SE). Error bars indicate SE. LSMean, least-square mean; No., number of patients with baseline and post-baseline value at the specified time point; SE, standard error.
Extended Data Fig. 6 Percent changes in ALT (a) and AST (b) over time.
Data presented are estimate mean (SE). Error bars indicate SE. Baseline values were calculated using an ANOVA, post-baseline measures were calculated from an MMRM, all statistical tests were two sided at a significance level of 0.05. ANOVA, analysis of variance; ALT, alanine aminotransferase; AST, aspartate aminotransferase; MMRM, mixed model for repeated measures; No., number of patients with baseline and post-baseline value at the specified time point; TZP, tirzepatide; SE, standard error; SFU, safety follow up.
Extended Data Fig. 7 Subgroup analysis of change in HbA1c from baseline to week 40 according to baseline oral anti-hyperglycemic medication use and enrolment in China versus out of China.
ETD and CIs in HbA1c at week 40 were estimated using an MMRM without missing-value imputation in the patients who received at least 1 dose of study drug (5 mg, N = 230; 10 mg, N = 228; 15 mg, N = 229; insulin glargine, N = 220); all statistical tests were two sided at a significance level of 0.05 and no adjustments were made for multiplicity. Data presented are LSMean. Error bars indicate 95% CI. P values represent treatment-by-subgroup interaction at week 40. ETD, estimated treatment difference; CI, confidence interval; CHN, China; HbA1c, glycated hemoglobin; LSMean, least-square mean; MMRM, mixed model for repeated measures; N, number of patients who were randomized and received at least 1 dose of study drug; OAM, oral anti-hyperglycemic medication; OCHN, out of China; SU, sulphonylurea; TZP, tirzepatide.
Extended Data Fig. 8 Subgroup analysis of change in bodyweight from baseline to week 40 according to baseline oral anti-hyperglycemic medication use and enrolment in China versus out of China.
ETD and CIs in bodyweight at week 40 were estimated using an MMRM without missing-value imputation in the in patients who received at least 1 dose of study drug (5 mg, N = 230; 10 mg, N = 228; 15 mg, N = 229; insulin glargine, N = 220); all statistical tests were two sided at a significance level of 0.05 and no adjustments were made for multiplicity. Data presented are LSMean. Error bars indicate 95% CI. P values represent treatment-by-subgroup interaction at week 40. ETD, estimated treatment difference; CI, confidence interval; CHN, China; LSMean, least-square mean; MMRM, mixed model for repeated measures; N, number of patients who were randomized and received at least 1 dose of study drug; OAM, oral anti-hyperglycemic medication; OCHN, out of China; SU, sulphonylurea; TZP, tirzepatide.
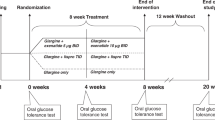
Extended Data Fig. 9 SURPASS-AP-Combo trial design.
FBG, fasting blood glucose; QW, once weekly; QD, once daily; SU, sulfonylurea. a Stable doses of metformin (metformin ≥1000 mg/day and no more than the maximum approved dose per country-specific label) and/or a sulfonylurea for 2 months prior to Visit 1, and during the screening/lead-in Period. b The initial dose of insulin glargine was 6 IU/day for patients who had an average FBG concentration of ≥7.8 mmol/L (140 mg/dL). The initial dose of insulin glargine for patients with an average FBG concentration of <7.8 mmol/L (<140 mg/dL) might be reduced by 1-2 IU/day at the investigator’s discretion. Note: Patients titrated insulin glargine dose in a weekly manner and made the dose decision with the investigator for the first 8 weeks (phone or clinic visit). From week 8 to week 16 patients continued the titration by a phone consultation or clinic visit every other week. It was expected that the insulin dose stayed relatively stable from week 16 onwards.
Extended Data Fig. 10 Graphical multiple-testing procedure for the primary and key secondary efficacy endpoints.
HbA1c, glycated hemoglobin; TZP, tirzepatide.
Supplementary information
Supplementary Information
The file contains Supplementary Tables 1–10; criteria for severe, persistent hyperglycemia; and a complete list of eligibility criteria from the protocol.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, L., Lee, B.W., Chawla, M. et al. Tirzepatide versus insulin glargine as second-line or third-line therapy in type 2 diabetes in the Asia-Pacific region: the SURPASS-AP-Combo trial. Nat Med 29, 1500–1510 (2023). https://doi.org/10.1038/s41591-023-02344-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02344-1
This article is cited by
-
GLP-1-based therapies for type 2 diabetes: from single, dual and triple agonists to endogenous GLP-1 production and L-cell differentiation
Diabetology & Metabolic Syndrome (2025)
-
Type 2 Diabetes Mellitus Remission, Dream or Reality? A Narrative Review of Current Evidence and Integrated Care Strategies
Diabetes Therapy (2025)
-
Perspectives on Stringent Glycemic Control and Weight Loss Among Patients with Type 2 Diabetes in China: A Survey Study
Diabetes Therapy (2025)
-
Robotic versus laparoscopic distal gastrectomy for resectable gastric cancer: a randomized phase 2 trial
Nature Communications (2024)
-
Tirzepatide outcompetes long-acting insulin in managing type 2 diabetes: a meta-analysis of three phase 3 randomized controlled trials
International Journal of Obesity (2024)