Abstract
Successful pregnancy relies directly on the placenta’s complex, dynamic, gene-regulatory networks. Disruption of this vast collection of intercellular and intracellular programs leads to pregnancy complications and developmental defects. In the present study, we generated a comprehensive, spatially resolved, multimodal cell census elucidating the molecular architecture of the first trimester human placenta. We utilized paired single-nucleus (sn)ATAC (assay for transposase accessible chromatin) sequencing and RNA sequencing (RNA-seq), spatial snATAC-seq and RNA-seq, and in situ sequencing and hybridization mapping of transcriptomes at molecular resolution to spatially reconstruct the joint epigenomic and transcriptomic regulatory landscape. Paired analyses unraveled intricate tumor-like gene expression and transcription factor motif programs potentially sustaining the placenta in a hostile uterine environment; further investigation of gene-linked cis-regulatory elements revealed heightened regulatory complexity that may govern trophoblast differentiation and placental disease risk. Complementary spatial mapping techniques decoded these programs within the placental villous core and extravillous trophoblast cell column architecture while simultaneously revealing niche-establishing transcriptional elements and cell–cell communication. Finally, we computationally imputed genome-wide, multiomic single-cell profiles and spatially characterized the placental chromatin accessibility landscape. This spatially resolved, single-cell multiomic framework of the first trimester human placenta serves as a blueprint for future studies on early placental development and pregnancy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data related to the present study are available at the Broad Institute Single Cell Portal (https://singlecell.broadinstitute.org/single_cell/study/SCP2601) (snRNA-seq, snATAC-seq, Slide-tags) and via Zenodo at https://zenodo.org (ref. 97) under accession no. 10981713 (STARmap). Public datasets used in the present study include: Human GRCh38 sequences (https://www.gencodegenes.org/human/release_32.html), 1000 Genome Project (https://www.internationalgenome.org/data), cis-BP database (https://cisbp.ccbr.utoronto.ca), UKBB (http://www.ukbiobank.ac.uk/register-apply; other utilized placental GWASs can be found in refs. 78,79,80), Roadmap Epigenomics (http://www.roadmapepigenomics.org), ABC (https://www.engreitzlab.org/resources) and ENCODE (https://www.encodeproject.org/help/project-overview). Source data are provided with this paper.
Code availability
Code for comprehensive analysis as described in the present study, and figure generation as shown, can be found at https://github.com/jian-shu-lab/hPlacenta-architecture.
References
Maltepe, E. & Fisher, S. J. Placenta: the forgotten organ. Annu. Rev. Cell Dev. Biol. 31, 523–552 (2015).
Lee, E. D. & Mistry, H. D. Placental related disorders of pregnancy 2.0. Int. J. Mol. Sci. 24, 14286 (2023).
Burton, G. J. & Fowden, A. L. The placenta: a multifaceted, transient organ. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370, 20140066 (2015).
Costanzo, V., Bardelli, A., Siena, S. & Abrignani, S. Exploring the links between cancer and placenta development. Open Biol. 8, 180081 (2018).
Vento-Tormo, R. et al. Single-cell reconstruction of the early maternal–fetal interface in humans. Nature 563, 347–353 (2018).
Greenbaum, S. et al. A spatially resolved timeline of the human maternal–fetal interface. Nature 619, 595–605 (2023).
Arutyunyan, A. et al. Spatial multiomics map of trophoblast development in early pregnancy. Nature 616, 143–151 (2023).
Russell, A. J. C. et al. Slide-tags enables single-nucleus barcoding for multimodal spatial genomics. Nature 625, 101–109 (2024).
Wang, X. et al. Three-dimensional intact-tissue sequencing of single-cell transcriptional states. Science 361, eaat5691 (2018).
Haider, S. et al. Transforming growth factor-β signaling governs the differentiation program of extravillous trophoblasts in the developing human placenta. Proc. Natl Acad. Sci. USA 119, e2120667119 (2022).
Upadhyay, G. Emerging role of lymphocyte antigen-6 family of genes in cancer and immune cells. Front. Immunol. 10, 819 (2019).
Sebastian, A. et al. Single-cell transcriptomic analysis of tumor-derived fibroblasts and normal tissue-resident fibroblasts reveals fibroblast heterogeneity in breast cancer. Cancers 12, 1307 (2020).
Lake, D. F. & Faigel, D. O. The emerging role of QSOX1 in cancer. Antioxid. Redox Signal. 21, 485–496 (2014).
Lu, M. et al. FGFR1 promotes tumor immune evasion via YAP-mediated PD-L1 expression upregulation in lung squamous cell carcinoma. Cell. Immunol. 379, 104577 (2022).
Zhang, P. et al. Targeting myeloid derived suppressor cells reverts immune suppression and sensitizes BRAF-mutant papillary thyroid cancer to MAPK inhibitors. Nat. Commun. 13, 1588 (2022).
Gabut, M. et al. An alternative splicing switch regulates embryonic stem cell pluripotency and reprogramming. Cell 147, 132–146 (2011).
Vasilopoulou, E. et al. Triiodothyronine regulates angiogenic growth factor and cytokine secretion by isolated human decidual cells in a cell-type specific and gestational age-dependent manner. Hum. Reprod. 29, 1161–1172 (2014).
Purvis, G. S. D., Solito, E. & Thiemermann, C. Annexin-A1: therapeutic potential in microvascular disease. Front. Immunol. 10, 938 (2019).
Neuper, L. et al. Candesartan does not activate PPARγ and its target genes in early gestation trophoblasts. Int. J. Mol. Sci. 23, 12326 (2022).
Radford, B. N. et al. Defects in placental syncytiotrophoblast cells are a common cause of developmental heart disease. Nat. Commun. 14, 1174 (2023).
Rooda, I. et al. Target prediction and validation of microRNAs expressed from FSHR and aromatase genes in human ovarian granulosa cells. Sci. Rep. 10, 2300 (2020).
Hu, L.-T., Wang, B.-Y., Fan, Y.-H., He, Z.-Y. & Zheng, W.-X. Exosomal miR-23b from bone marrow mesenchymal stem cells alleviates oxidative stress and pyroptosis after intracerebral hemorrhage. Neural Regen. Res. 18, 560–567 (2023).
Renaud, S. J., Kubota, K., Rumi, M. A. K. & Soares, M. J. The FOS transcription factor family differentially controls trophoblast migration and invasion. J. Biol. Chem. 289, 5025–5039 (2014).
Kohli, S. et al. p45 NF-E2 regulates syncytiotrophoblast differentiation by post-translational GCM1 modifications in human intrauterine growth restriction. Cell Death Dis. 8, e2730 (2017).
Guo, H., Wang, Y., Jia, W. & Liu, L. MiR-133a-3p relieves the oxidative stress induced trophoblast cell apoptosis through the BACH1/Nrf2/HO-1 signaling pathway. Physiol. Res. 70, 67–78 (2021).
Hornbachner, R. et al. MSX2 safeguards syncytiotrophoblast fate of human trophoblast stem cells. Proc. Natl Acad. Sci. USA 118, e2105130118 (2021).
Song, H., Wang, X., Li, J.-C. & Lv, Y.-H. MiR-655-3p inhibits growth and invasiveness of trophoblasts via targeting PBX3 and thus deteriorates preeclampsia. Eur. Rev. Med. Pharmacol. Sci. 24, 10346–10351 (2020).
Chen, Q. et al. Association of lncRNA SH3PXD2A-AS1 with preeclampsia and its function in invasion and migration of placental trophoblast cells. Cell Death Dis. 11, 583 (2020).
Song, J. et al. Transcription factor AP-4 promotes tumorigenic capability and activates the Wnt/β-catenin pathway in hepatocellular carcinoma. Theranostics 8, 3571–3583 (2018).
Dietrich, B., Haider, S., Meinhardt, G., Pollheimer, J. & Knöfler, M. WNT and NOTCH signaling in human trophoblast development and differentiation. Cell. Mol. Life Sci. 79, 292 (2022).
Varberg, K. M. et al. ASCL2 reciprocally controls key trophoblast lineage decisions during hemochorial placenta development. Proc. Natl Acad. Sci. USA 118, e2016517118 (2021).
Zhou, G. et al. EBF1 Gene mRNA levels in maternal blood and spontaneous preterm birth. Reprod. Sci. 27, 316–324 (2020).
Pagani, F., Tratta, E., Dell’Era, P., Cominelli, M. & Poliani, P. L. EBF1 is expressed in pericytes and contributes to pericyte cell commitment. Histochem. Cell Biol. 156, 333–347 (2021).
Kim, M. et al. A MYC-ZNF148-ID1/3 regulatory axis modulating cancer stem cell traits in aggressive breast cancer. Oncogenesis 11, 60 (2022).
Miftah, H. et al. NR2F6, a new immune checkpoint that acts as a potential biomarker of immunosuppression and contributes to poor clinical outcome in human glioma. Front. Immunol. 14, 1139268 (2023).
Schep, A. N., Wu, B., Buenrostro, J. D. & Greenleaf, W. J. chromVAR: inferring transcription-factor-associated accessibility from single-cell epigenomic data. Nat. Methods 14, 975–978 (2017).
Gong, H., Lu, F., Zeng, X. & Bai, Q. E2F transcription factor 1 (E2F1) enhances the proliferation, invasion and EMT of trophoblast cells by binding to Zinc Finger E-Box Binding Homeobox 1 (ZEB1). Bioengineered 13, 2360–2370 (2022).
Fang, L. et al. EGF stimulates human trophoblast cell invasion by downregulating ID3-mediated KISS1 expression. Cell Commun. Signal. 19, 101 (2021).
Morimoto, M., Takahashi, Y., Endo, M. & Saga, Y. The Mesp2 transcription factor establishes segmental borders by suppressing Notch activity. Nature 435, 354–359 (2005).
David, R. et al. MesP1 drives vertebrate cardiovascular differentiation through Dkk-1-mediated blockade of Wnt-signalling. Nat. Cell Biol. 10, 338–345 (2008).
Jena, S. K., Das, S., Chakraborty, S. & Ain, R. Molecular determinants of epithelial mesenchymal transition in mouse placenta and trophoblast stem cell. Sci. Rep. 13, 10978 (2023).
Butler, T. M., Pater, J. A. & MacPhee, D. J. Integrin linked kinase regulates syncytialization of BeWo trophoblast cells. Biol. Reprod. 96, 673–685 (2017).
Baczyk, D. et al. Complex patterns of GCM1 mRNA and protein in villous and extravillous trophoblast cells of the human placenta. Placenta 25, 553–559 (2004).
Shaut, C. A. E., Keene, D. R., Sorensen, L. K., Li, D. Y. & Stadler, H. S. HOXA13 Is essential for placental vascular patterning and labyrinth endothelial specification. PLoS Genet. 4, e1000073 (2008).
Matsubara, K. et al. HMGA1 Is a potential driver of preeclampsia pathogenesis by interference with extravillous trophoblasts invasion. Biomolecules 11, 822 (2021).
Ma, S. et al. Chromatin potential identified by shared single-cell profiling of RNA and chromatin. Cell 183, 1103–1116.e20 (2020).
Sekita, Y. et al. Role of retrotransposon-derived imprinted gene, Rtl1, in the feto-maternal interface of mouse placenta. Nat. Genet. 40, 243–248 (2008).
Sturtzel, C. et al. FOXF1 mediates endothelial progenitor functions and regulates vascular sprouting. Front. Bioeng. Biotechnol. 6, 76 (2018).
Sheng, F. et al. Aberrant expression of imprinted lncRNA MEG8 causes trophoblast dysfunction and abortion. J. Cell. Biochem. 120, 17378–17390 (2019).
Liang, Y., Wang, P., Shi, Y., Cui, B. & Meng, J. Long noncoding RNA maternally expressed gene 3 improves trophoblast dysfunction and inflammation in preeclampsia through the Wnt/β-Catenin/nod-like receptor pyrin ___domain-containing 3 axis. Front Mol. Biosci. 9, 1022450 (2022).
Fan, H. et al. Aberrant Kank1 expression regulates YAP to promote apoptosis and inhibit proliferation in OSCC. J. Cell. Physiol. 235, 1850–1865 (2020).
Nolan, M. E. et al. The polarity protein Par6 induces cell proliferation and is overexpressed in breast cancer. Cancer Res. 68, 8201–8209 (2008).
Mukherjee, A. et al. A review of FOXI3 regulation of development and possible roles in cancer progression and metastasis. Front. Cell Dev. Biol. 6, 69 (2018).
Suenaga, Y. et al. TAp63 represses transcription of MYCN/NCYM gene and its high levels of expression are associated with favorable outcome in neuroblastoma. Biochem. Biophys. Res. Commun. 518, 311–318 (2019).
Figeac, N. et al. VGLL3 operates via TEAD1, TEAD3 and TEAD4 to influence myogenesis in skeletal muscle. J. Cell Sci. 132, jcs225946 (2019).
Zhao, H.-J. et al. Bone morphogenetic protein 2 promotes human trophoblast cell invasion and endothelial-like tube formation through ID1-mediated upregulation of IGF binding protein-3. FASEB J. 34, 3151–3164 (2020).
Fleury, M., Eliades, A., Carlsson, P., Lacaud, G. & Kouskoff, V. FOXF1 inhibits hematopoietic lineage commitment during early mesoderm specification. Development 142, 3307–3320 (2015).
Steimle, J. D. & Moskowitz, I. P. TBX5: a key regulator of heart development. Curr. Top. Dev. Biol. 122, 195–221 (2017).
Lange, M. et al. CellRank for directed single-cell fate mapping. Nat. Methods 19, 159–170 (2022).
Wuebben, E. L., Mallanna, S. K., Cox, J. L. & Rizzino, A. Musashi2 is required for the self-renewal and pluripotency of embryonic stem cells. PLoS ONE 7, e34827 (2012).
Sugimoto, J., Sugimoto, M., Bernstein, H., Jinno, Y. & Schust, D. A novel human endogenous retroviral protein inhibits cell-cell fusion. Sci. Rep. 3, 1462 (2013).
You, J. et al. The BMP2 signaling axis promotes invasive differentiation of human trophoblasts. Front. Cell Dev. Biol. 9, 607332 (2021).
Lee, S.-W. et al. Peroxidasin is essential for endothelial cell survival and growth signaling by sulfilimine crosslink-dependent matrix assembly. FASEB J. 34, 10228–10241 (2020).
Jin, S. et al. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 12, 1088 (2021).
Clemente, L. & Bird, I. M. The epidermal growth factor receptor in healthy pregnancy and preeclampsia. J. Mol. Endocrinol. 70, e220105 (2023).
Ma, Y., Yu, X., Li, Y.-X. & Wang, Y.-L. HGF/c-Met signaling regulates early differentiation of placental trophoblast cells. J. Reprod. Dev. 67, 89–97 (2021).
Ball, M. et al. Expression of pleiotrophin and its receptors in human placenta suggests roles in trophoblast life cycle and angiogenesis. Placenta 30, 649–653 (2009).
Becsky, D. et al. Syndecan-4 modulates cell polarity and migration by influencing centrosome positioning and intracellular calcium distribution. Front. Cell Dev. Biol. 8, 575227 (2020).
Haider, S. et al. Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Rep. 11, 537–551 (2018).
Haider, S. et al. Notch1 controls development of the extravillous trophoblast lineage in the human placenta. Proc. Natl Acad. Sci. USA 113, E7710–E7719 (2016).
Li, B. et al. Cumulus provides cloud-based data analysis for large-scale single-cell and single-nucleus RNA-seq. Nat. Methods 17, 793–798 (2020).
Granja, J. M. et al. ArchR is a scalable software package for integrative single-cell chromatin accessibility analysis. Nat. Genet. 53, 403–411 (2021).
Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296 (2019).
Huber, W. et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat. Methods 12, 115–121 (2015).
Neavin, D. et al. Demuxafy: improvement in droplet assignment by integrating multiple single-cell demultiplexing and doublet detection methods. Genome Biol. 25, 94 (2024).
1000 Genomes Project Consortium et al. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018).
Zhao, L., Bracken, M. B. & DeWan, A. T. Genome-wide association study of pre-eclampsia detects novel maternal single nucleotide polymorphisms and copy-number variants in subsets of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study cohort. Ann. Hum. Genet. 77, 277–287 (2013).
Jilling, T. et al. Surgical necrotizing enterocolitis in extremely premature neonates is associated with genetic variations in an intergenic region of chromosome 8. Pediatr. Res. 83, 943–953 (2018).
Workalemahu, T. et al. Genetic variations and risk of placental abruption: a genome-wide association study and meta-analysis of genome-wide association studies. Placenta 66, 8–16 (2018).
Finucane, H. K. et al. Heritability enrichment of specifically expressed genes identifies disease-relevant tissues and cell types. Nat. Genet. 50, 621–629 (2018).
Jagadeesh, K. A. et al. Identifying disease-critical cell types and cellular processes by integrating single-cell RNA-sequencing and human genetics. Nat. Genet. 54, 1479–1492 (2022).
Roadmap Epigenomics Consortium et al. Integrative analysis of 111 reference human epigenomes. Nature 518, 317–330 (2015).
Fulco, C. P. et al. Activity-by-contact model of enhancer-promoter regulation from thousands of CRISPR perturbations. Nat. Genet. 51, 1664–1669 (2019).
Bulik-Sullivan, B. K. et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291–295 (2015).
ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012).
de Leeuw, C. A., Mooij, J. M., Heskes, T. & Posthuma, D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput. Biol. 11, e1004219 (2015).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021).
McInnes, L., Healy, J., Saul, N. & Großberger, L. UMAP: Uniform Manifold Approximation and Projection. J. Open Source Softw. 3, 861 (2018).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Bivand, R. S. & Wong, D. W. S. Comparing implementations of global and local indicators of spatial association. Test 27, 716–748 (2018).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272 (2020).
Axelrod, S. et al. starfish: scalable pipelines for image-based transcriptomics. J. Open Source Softw. 6, 2440 (2021).
He, Y. et al. ClusterMap for multi-scale clustering analysis of spatial gene expression. Nat. Commun. 12, 5909 (2021).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Lohoff, T. et al. Integration of spatial and single-cell transcriptomic data elucidates mouse organogenesis. Nat. Biotechnol. 40, 74–85 (2022).
Ounadjela, J., et al. Spatial multiomic landscape of the human placenta at molecular resolution. Zenodo https://zenodo.org/records/10981713 (2024).
Acknowledgements
This paper is part of the Human Cell Atlas: www.humancellatlas.org/publications. We are profoundly grateful to the patients for donating their tissues for research. We thank R. Jaenisch and E. Lander for early discussions and support. We thank lab members in the Chen lab, the Haider lab and the Shu lab for insightful discussions. This work was supported by funds from Massachusetts Life Science Center, Broad Institute of MIT and Harvard and Massachusetts General Hospital to J.S. and by the Austrian Science Funds (nos. P34588 and P-36159), assigned to S.H.
Author information
Authors and Affiliations
Contributions
J.S. conceived, designed and directed the study. J.S., S.H. and F.C. co-supervised the collaborative project. S.H. established and collected human placenta tissues and performed TB-ORG/TSC experiments with contributions from T.M. and A.M.P. J.R.O. and K.Z. generated single-cell multiome data with contributions from C.C. and F.V. K.Z. generated STARmap-ISS and STARmap-ISH data with contributions from C.C. and F.V. A.J.C.R. generated Slide-tags data with contributions from N.N., R.R. and K.Z. K.J.K.K., K.J. and A.L. analyzed data with contributions from Q.G., M.L.Z., M.H. and X.L. W.M., M.K. and F.C. provided conceptual, methodological suggestions and feedback. S.H. and K.Z. assembled figures with input from all authors. J.R.O., S.H. and J.S. wrote the manuscript with contributions from all authors. All authors read and accepted the manuscript.
Corresponding authors
Ethics declarations
Competing interests
A patent application related to this work about discovering novel immune modulators has been filed by the Massachusetts General Hospital. J.S. is a scientific advisor for Johnson & Johnson. F.C. is an academic co-founder of Curio Bioscience and Doppler Bio, and an advisor to Amber Bio. F.C., A.J.C.R. and N.M.N. are listed as inventors on a patent application related to Slide-tags. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Fabian Theis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Sonia Muliyil, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Further characterization of multiomic data.
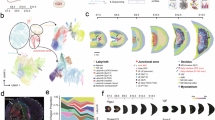
a, snRNA-seq quality control metrics, separated by samples. Left: number of genes detected per cell (Gex_nGenes) for each sample. Right: unique molecular identifiers per cell (Gex_nUMI) for each sample. Further QC metrics can be found in Supplementary Table 1. b, snATAC-seq quality control metrics showing TSS Enrichment scores and fragment counts per cell across all samples. c, UMAP plot showing cell origins (maternal origin or fetal origin) (Methods). d–e, UMAP plots (d) and dot plot (e) showing selected canonical marker genes expressed across main placental cell types including vCTB (PAGE4, PEG10), vCTBp (MKI67, TOP2A), EVT (HLA-G, CCNE1), STB (CYP19A1, ERVFRD-1), Endo (PECAM1, KDR), MAC (CD14, SPP1), and FIB (COL3A1, COL6A2). Further resolved clusters included vCTB1 (TBL1X), vCTB2 (SMAGP, IFI6), vCTB3 (LRP5), FIB1 (PDGFR1B, AGTR1), FIB2 (PDGFRA, CXCL14), Unknown 1 (Unk. 1) (HGF, DCN), mat. FIB (ALDH1A2, FAM155A), and Unknown 2 (Unk. 2) (PDN4, RSPO3). Manual sub-clustering revealed EVT1 (UTRN), EVT2, (HAPLN3, LY6E), EVT3 (AOC1, PAPPA2), mat. MAC (CD74, LYZ), HBC (LYVE1, ADAMTS17), and myeloid_unknown (myel. Unk) (GNA12, FGF13). f, Bar plot showing time point/donor composition per cluster. g, Myeloid cells further subclustered by Louvain clustering colored by sample. Maternal macrophages: mat. MAC, Hofbauer cells: HBC, and myeloid_unknown: myel. Unk. h, EVTs further subclustered by Louvain clustering colored by sample. i, Myeloid cells further subclustered by Louvain clustering colored by subcluster identity. j, EVTs further subclustered by louvain clustering colored by subcluster identity. k, STARmap-ISH (n = 3) characterization of trophoblast markers EGFR (pan-trophoblast), SMAGP (vCTB2), and LY6E (EVT) in sample W7-2. Stippled line demarcates the villous core from the vCTB layers. l, Gene expression patterns for novel motility and immunotolerance-promoting genes expressed in EVTs (PLXNB2, RUNX1, C12orf75, QSOX1, RASGRF2, PDCD1LG2, JAK1, MYCN, CD276, EBI3, FGFR1) and STBs (BRAF, TBX3) via UMAP visualization, with accompanying spatial visualization of FGFR1 using STARmap-ISH (n = 3). Stippled lines demarcate the villous core from vCTB layers. m, Heatmap showing novel differentially expressed genes across clusters. All differentially expressed genes from snRNA-seq can be found in Supplementary Table 2.
Extended Data Fig. 2 Chromatin accessibility dynamics across the human placenta.
a, Left: FOXP1 expression in 3D trophoblast organoids (TB-ORG, n = 4). WB analyses of TB-ORG protein lysates under stemness and EVT differentiation conditions (DIFF1= EVT differentiation under TGF-beta (TGFβ) inhibition; DIFF2=EVT differentiation under TGFβ activation) detect FOXP1 in TB-ORG under stemness conditions and its almost absence in EVTs. Right: siRNA-based downregulation of FOXP1 in trophoblast stem cells (TSCs) under stemness conditions, showing that siRNA treatment of FOXP1 reduces proliferation (CCNA2) and provokes markers for STB differentiation of TSCs (CGB, ENDOU). n = 3 independent TSC lines, two replicates each. Data are represented as mean values +/− SD. Normal distribution was tested using Kolmogorov-Smirnov test and subsequent unpaired t-tests were applied. P-values are depicted in the blots. b, Visual comparison of gene expression (RNA) and gene activity score (ATAC) UMAP plots for LY6E, with accompanying spatial visualization using STARmap-ISH (n = 3). Stippled line demarcates villous core from vCTBs. c, UMAP representation of novel trophoblast-associated microRNAs miR23B (vCTB) and miR7973-1 (STB), identified by snATAC-seq analyses. Verification of expression was performed through qPCR in isolated vCTB (TP63), STB (CGB), and EVT cells (Methods) (n = 3 donors). Per donor, qPCR was repeated twice (TP63, CGB), six times (miRNA23B), and four times (miRNA7973), respectively. Data are represented as mean values +/− SD. Kolmogorov-Smirnov test (normal distribution) was followed by either Friedman test and Dunn’s multiple comparison (non-parametric miRNA23b and CGB), or one-way ANOVA and Geisser-Greenhouse correction (miRNA7973 and TP63). P-values are depicted in the blots. d, Heatmap showing differentially accessible genes across clusters. All differentially accessible genes from snATAC-seq are listed in Supplementary Table 3. e, Corresponding gene expression (Gene Expression Matrix) and gene accessibility (Gene Score Matrix) of TF motifs from intercluster motif enrichment comparisons, ChromVAR analyses, and identified positive TF regulators. Intercluster motif enrichments can be found in Supplementary Table 4, while ChromVAR enrichments and positive TF regulators can be found in Supplementary Table 5. f, Positive TF regulators identified based on gene activity scores. Positive TF regulator motifs such as BACH2, GCM1, TFAP2C, TEAD1, and MESP2 are significantly enriched. Specific motifs and enrichment values can be found in Supplementary Table 5.
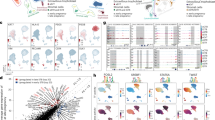
Extended Data Fig. 3 Peak-gene analysis to uncover disease risk.
a, Heatmap of representative DORCs for each cluster, revealing lineage-specific enrichment of DORCs in each cell type including FIB/MAC/Unk. 1-2 (CXCL14, COL6A3, TBX5), Endo (PECAM1), TB (TFAP2C, IRX2, VGLL3), vCTB (EGFR, KANK1, PARD6B, FOXI3), STB (CYP19A1), and EVT (HLA-G, DIO2, VGLL3). DORC scores, defined by the normalized sum of counts in all significantly correlated peaks per gene for all cells, were normalized. DORCs and DORC scores can be found in Supplementary Table 7. b, UMAPs confirming overlapping DORC scores (DORC) and gene expression (RNA) of EGFR (CTB), VGLL3 (EVT), CYP19A1 (STB), COL6A3 (FIB), CXCL14 (FIB2), and PECAM1 (Endo). DORC scores are defined by the normalized sum of counts in all significantly correlated peaks per gene for all cells. DORCs and DORC scores can be found in Supplementary Table 7. c, Transition probabilities across all trophoblasts for terminal differentiation states (vCTB2, STB, and EVT) calculated by chromatin potential and CellRank analysis. Transition probability (colorbar) is defined as the probability of a cell reaching a terminal state (Methods). d, Average heritability enrichment across 17 cell types and 9 traits (n = 8 donor placentas) explained by (i) SNPs in a 100kb window around genes specifically enriched in expression across cell types (LDSC-SEG), (ii) SNPs linked to cell type-specific genes through the union of Roadmap and Activity-By-Contact enhancer-gene maps in placenta biosamples as proposed in sc-linker (sc-linker (ABC + Roadmap)), (iii) SNPs in peaks linked to any gene in a cell type (Multiome), and (iv) SNPs linked to cell type-specific genes by the peak-gene links (sc-linker (Multiome)) (Methods). Data are represented as mean values +/− SD. Numerical results are reported in Supplementary Table 9. e, Illustration of a GWAS hit (rs117659937) associated with excessive vomiting during pregnancy linked to the TP53INP2 gene by ArchR peak-gene linkage in EVT3 cells. TP53 is a Phase 1 clinical trial drug target.
Extended Data Fig. 4 Additional spatial cell-type mapping.
a, Multiomics-derived cell types identified in space with STARmap-ISS (n = 4) via Seurat integration on samples W9, W7-1, and W11. b, Canonical marker gene spatial expression for vCTB (PAGE4), STB (CGA), EVT (NOTUM), and the stromal core (VIM) identified by STARmap-ISS (n = 4) on samples W7-1, W9, and W11. c, Canonical marker gene spatial expression for FIB (COL3A1), Endo (KDR), HBC (CD163), STB (CYP19A1), and EVT (HAPLN3, AOC1), identified by STARmap-ISS (n = 4) on samples W9, W7-1, W11. Insets depict magnified areas of villous (COL3A1, KDR, CD163, CYP19A1) and extravillous (HAPLN3, AOC1) areas.
Extended Data Fig. 5 Global overview of spatial cluster localization across all four samples.
a - d, Spatial localization of STARmap-ISS-identified placental cell clusters across the entire sections of samples (n = 4). W7-1 (a), W9 (b), W11 (c), and W8-2 (d).
Extended Data Fig. 6 Expression of tumor and immunomodulation-associated genes.
STARmap-ISH (n = 3) co-detecting multiple tumor and immunomodulation-associated genes expressed by vCTBs (KLF5, PARP1, IDH1, ABL1, SMARCA4, CEBPA, BTG2, ATM) and EVTs (KLF5, RAD50, MSH3, FGFR1, DIAPH2, CEBPA, BTG2, ATM, HRAS). Stippled lines demarcate the villous core from vCTB layers. Genes are listed and described in Supplementary Table 11.
Extended Data Fig. 7 Cell column visualization.
Spatial visualization of cell column-associated genes (overview and magnified insets) via STARmap-ISS (n = 4) on samples W8-2, W9, and W7-1. By using canonical and newly identified markers associated with various EVT maturation states (EVT1-3), we classified cell columns into three categories: cell column type 1 (associated with EVT1) contained proliferative EVT progenitors (CPS1, SPINT1, MKI67, CDK1, CDK7) and low expression of differentiated EVT markers. Cell column type 2 (associated with EVT2) demonstrated reduced areas of proliferative EVT progenitors and increasingly expressed markers for differentiated EVTs (HSPG2 and ADAM19). In cell column type 3 (associated with EVT3), proliferative markers were absent and cells highly expressed gene patterns associated with a mature EVT phenotype predominantly detected in maternal decidua (ITGB4 and PAPPA2). Images at the bottom illustrate the direction of estimated EVT differentiation (arrows) within each cell column.
Extended Data Fig. 8 Investigating shared gene expression patterns.
a–c, Spatial visualization of gene expression using STARmap-ISS (n = 4) across samples W8-2, W9, and W7-1. Insets depict magnified areas, stippled lines demarcate the villous core from vCTB layers. a, Canonical, epithelial trophoblast markers KRT7, KRT19, and KRT23 are shown. KRT7 and KRT19 are substantially enhanced in EVTs while KRT23 is restricted to vCTBs. b, Spatial exploration of gene expression shared between STBs and EVTs (ADAM12, EBI3), as well as vCTBs and EVTs (ADAMTS20, ANK2) are depicted. c, Spatial visualization of EVT-expressed COL27A1 and COL4A1 suggests an EVT-provided local source for fibrin-matrix-type fibrinoid constituting the materno-fetal border as part of the decidua basalis.
Extended Data Fig. 9 Imputation performance evaluation.
a, Cumulative curves of the imputation performance scores using different numbers of snRNA-seq nearest neighbors in samples W7-1, W8-2, W9, and W11. b, Comparison of STARmap-ISS (n = 4), STARmap-ISH (n = 3), and imputation-based (Imputed-RNA) gene expression for selected canonical markers across cell types to validate imputation results, including SMAGP (vCTB), AGTR1 (FIB1), CD163 (HBC), and KDR (Endo). Insets show magnified areas of Imputed-RNA visualization. Stippled lines in STARmap-ISH demarcate the villous core from vCTB layers. Scale bar = 50 µm c, Comparison between STARmap-ISH-detected (n = 3) and imputed (Imputed-RNA) gene expression of ATP1B3 reveals expression in selected stromal cells and vCTBs. Inset shows magnified villous area of Imputed-RNA visualization. Stippled line in STARmap-ISH demarcates the villous core from vCTB layers. Scale bar = 50 µm. d, Comparison of STARmap-ISS (n = 4) and imputed gene activity scores (Imputed-ATAC) for select genes AOC1 (EVT3), PAPPA2 (EVT3, STB), and KDR (Endo) to validate Imputed-ATAC.
Extended Data Fig. 10 Evaluation of cell-cell interactions.
a, Dotplot of identified ligand-receptor (L-R) pairs across all clusters and shared between samples. We were able to detect L-R pairs within the same cell clusters including BMP5 - BMPR1B (FIB1 - FIB1), PGF - VEGFR1 (EVT3 - EVT3), VEGFC - VEGFR2 (Endo - Endo), MDK-LRP1 (FIB2 - FIB2), and ANXA1 - FPR1 (HBC - HBC) indicating autocrine regulation. However, we also detected numerous paracrine L-R pairs such as AREG - EGFR (FIB1 - vCTB), GDF15 - TGFBR2 (EVT2 - EVT3), NAMPT - ITGA5/ITGB1 (EVT2 - EVT3), PGF - VEGFR1 (EVT2 - EVT3), VEGFA - VEGFR1/VEGFR1R2/VEGFR2 (FIB2 - Endo), MIF - CD74/CD44 (FIB2 - HBC), PDGFC - PDGFRA (HBC - FIB2), PGF - VEGFR1 (STB - vCTB2), and NAMPT - INSR/ITGA5/ITGB1 (vCTB2 - EVT2). Interaction (communication) probabilities and significance were computed by permutation test (p < 0.05). b, Spatial visualization of canonical L-R pair HGF- MET via STARmap-ISS (n = 4) across samples W7-1, W9, and W8-2 to support CellChat findings. HGF - MET has not yet been characterized in the placenta. MET expression in vCTBs and corresponding HGF expression in COL3A1-positive FIB suggests paracrine interactions between FIB1 and vCTBs. Insets depict magnified areas, stippled lines demarcate the villous core from vCTB layers.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11, Tables 1–13 and References.
Supplementary Table 1
Information about the human primary samples isolated from first trimester placentas and used in the present study along with accompanying assays: Multiome (combined snRNA-seq and snATAC-seq), Slide-tags, STARmap-ISS and STARmap-ISH. Each assay is accompanied by relevant QC metrics.
Supplementary Table 2
DEGs discovered by snRNA-seq.
Supplementary Table 3
Differentially accessible genes discovered by snATAC-seq.
Supplementary Table 4
Intercluster motif enrichment comparisons. Differentially accessible peaks were identified using a two-sided Wilcoxon’s test (FDR ≤ 0.1, log2(FC) ≥ 0.5). Intercluster motif enrichments were calculated via a hypergeometric test to generate P values. For instance, ‘vCTBvsEVT’ lists motifs enriched across peaks that are more accessible in vCTB clusters compared with EVT clusters.
Supplementary Table 5
ChromVAR motif enrichment analysis and accompanying positive TF regulators. Correlation between variables (motif enrichment, gene expression, gene accessibility) was calculated using Pearson’s correlation coefficient and statistical significance was assessed using P values adjusted by the Benjamini–Hochberg method (Padj < 0.01).
Supplementary Table 6
Cluster-specific differentially expressed marker genes across all clusters identified by Slide-tags, along with spatially autocorrelated genes and TF motifs with ___location-dependent expression and enrichment as identified by Slide-tags analysis using Moran’s I (includes correlations across all cells as well as within individual cell types). P values were adjusted by the Benjamini–Hochberg method (Padj < 0.05). Cluster numbering can be found in Supplementary Fig. 5.
Supplementary Table 7
Inferred peak–gene links, DORCs and per-cluster DORC scores. SnRNA-seq-identified tumor invasion and immunomodulation-related genes overlapped with DORCs, namely RUNX1, C12orf75, QSOX1, RASGRF2, PLXNB2, JAK1 and MYCN. Overlap with snATAC-seq-identified genes included ATP11A, DIO2, ANXA1, KLF6, ASCL2, NR2F6, SNAI2, TCF21, FLI1, PITX1, GRHL1 and NFIX.
Supplementary Table 8, 12
Supplementary Table 8 Lineage drivers for chromatin potential/CellRank-derived terminal states (EVT3, STB, vCTB2) calculated by CellRank analyses. P values of the two-sided Fisher transformation tests were calculated and adjusted using the Storey–Tibshirani procedure for multiple hypothesis comparisons. Suppplementary Table 12 DEGs identified by STARmap-ISS. Statistics were derived using Wilcoxon’s test (two sided). P values were adjusted using the Benjamini–Hochberg method.
Supplementary Table 9
Description of UKBB pregnancy-related traits and accompanying heritability enrichments across cell types.
Supplementary Table 10
MAGMA GSEA of cell type-specific programs for each cell type as well as enrichment analysis of top GWAS hits for each pregnancy-related trait across cell types.
Supplementary Table 11
Genes, 1,001, for STARmap-ISS and 48 genes for STARmap-ISH and accompanying probe sequences used for ISS and ISH.
Supplementary Table 13
Ligand–receptor interactions across clusters and samples. Interaction (communication) probabilities and significance were computed by permutation test (P < 0.05).
Source data
Source Data Fig. 2a
Uncropped and unprocessed blots corresponding to Fig. 2a. Please note that. for the lower plot detecting p63, HLA-G and CGβ, an additional internal loading control has been added (TOPOIIbeta). The red stippled lines demarcate the protein bands shown in Fig. 2a.
Source Data Extended Data Fig. 2a
Uncropped and unprocessed blots corresponding to Extended Data Fig. 2a. The red stippled lines demarcate the protein bands shown in Extended Data Fig. 2a.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ounadjela, J.R., Zhang, K., Kobayashi-Kirschvink, K.J. et al. Spatial multiomic landscape of the human placenta at molecular resolution. Nat Med 30, 3495–3508 (2024). https://doi.org/10.1038/s41591-024-03073-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-024-03073-9