Abstract
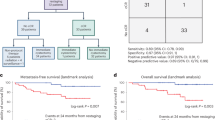
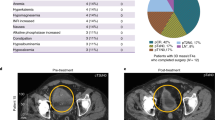
There is a critical unmet need for safe and efficacious neoadjuvant treatment for cisplatin-ineligible patients with muscle-invasive bladder cancer. Here we launched a phase 1b study using the combination of intravesical cretostimogene grenadenorepvec (oncolytic serotype 5 adenovirus encoding granulocyte–macrophage colony-stimulating factor) with systemic nivolumab in cisplatin-ineligible patients with cT2-4aN0-1M0 muscle-invasive bladder cancer. The primary objective was to measure safety, and the secondary objective was to assess the anti-tumor efficacy as measured by pathologic complete response along with 1-year recurrence-free survival. No dose-limiting toxicity was encountered in 21 patients enrolled and treated. Combination treatment achieved a pathologic complete response rate of 42.1% and a 1-year recurrence-free survival rate of 70.4%. Pathologic response was associated with baseline free E2F activity and tumor mutational burden but not PD-L1 status. Although T cell infiltration was broadly induced after intravesical oncolytic immunotherapy, the formation, enlargement and maturation of tertiary lymphoid structures was specifically associated with complete response, supporting the importance of coordinated humoral and cellular immune responses. Together, these results highlight the potential of this combination regimen to enhance therapeutic efficacy in cisplatin-ineligible patients with muscle-invasive bladder cancer, warranting additional study as a neoadjuvant therapeutic option. ClinicalTrials.gov identifier: NCT04610671.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information. Additional data required, including individual de-identified participant clinical data or raw data from correlative studies, can be accessed by request. In accordance with the National Institutes of Health’s Genomic Data Sharing Policy, de-identified patient DNA/RNA sequencing data will be managed with institutional review board guidelines within an appropriate access-controlled repository. These data and other anonymized individual and/or study-level data will be available to be shared upon reasonable request after publication of this study and if regulatory activities and other criteria are met. Qualified scientific and medical researchers are eligible to request access to the data and can expect a response within 14 d, with a turnover of up to 90 d. Upon approval, and governed by a legal agreement, data can be accessed for a limited period of 1 year, which may be extended upon request. Data must not be used for commercial purposes but only for research purposes. Requests for data should be directed to the corresponding author, R.L. ([email protected]), and will be reviewed and approved by the first (R.L.) and senior (J.J.M. and J.R.C.-G.) authors, respectively.
Code availability
Any computer code or algorithm used to generate results and central to the main claims within this paper will be made available upon reasonable request per the methods detailed above.
References
Stein, J. P. et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J. Clin. Oncol. 19, 666–675 (2001).
Grossman, H. B. et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 349, 859–866 (2003).
International Collaboration of Trialists et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J. Clin. Oncol. 29, 2171–2177 (2011).
Flaig, T. W. et al. NCCN Guidelines® Insights: Bladder Cancer, Version 2.2022. J. Natl Compr. Canc. Netw. 20, 866–878 (2022).
Galsky, M. D. et al. Treatment of patients with metastatic urothelial cancer ‘unfit’ for cisplatin-based chemotherapy. J. Clin. Oncol. 29, 2432–2438 (2011).
Gao, J. et al. Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with cisplatin-ineligible operable high-risk urothelial carcinoma. Nat. Med. 26, 1845–1851 (2020).
Necchi, A. et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J. Clin. Oncol. 36, 3353–3360 (2018).
Powles, T. et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat. Med. 25, 1706–1714 (2019).
van Dijk, N. et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat. Med. 26, 1839–1844 (2020).
Ramesh, N. et al. CG0070, a conditionally replicating granulocyte macrophage colony-stimulating factor–armed oncolytic adenovirus for the treatment of bladder cancer. Clin. Cancer Res.12, 305–313 (2006).
Burke, J. M. et al. A first in human phase 1 study of CG0070, a GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J. Urol. 188, 2391–2397 (2012).
Tyson M. D. et al. P2-02: Pivotal results from BOND-003: a phase 3, single-arm study of intravesical cretostimogene grenadenorepvec for the treatment of high risk, bcg-unresponsive non-muscle invasive bladder cancer with carcinoma in situ. J. Urol. https://doi.org/10.1097/01.JU.0001015816.87470.c9.02 (2024).
Li, R. et al. Final results of CORE-001: a phase-2, single arm study of cretostimogene grenadenorepvec in combination with pembrolizumab in patients with BCG-unresponsive, non-muscle invasive bladder cancer with carcinoma in situ. J. Clin. Oncol. 42, 4601 (2024).
Li, R. et al. Oncolytic adenoviral therapy plus pembrolizumab in BCG-unresponsive non-muscle-invasive bladder cancer: the phase 2 CORE-001 trial. Nat. Med. 30, 2216–2223 (2024).
Dai, P. et al. Intratumoral delivery of inactivated modified vaccinia virus Ankara (iMVA) induces systemic antitumor immunity via STING and Batf3-dependent dendritic cells. Sci. Immunol. 2, eaal1713 (2017).
Packiriswamy, N. et al. Oncolytic measles virus therapy enhances tumor antigen-specific T-cell responses in patients with multiple myeloma. Leukemia 34, 3310–3322 (2020).
Svensson-Arvelund, J. et al. Expanding cross-presenting dendritic cells enhances oncolytic virotherapy and is critical for long-term anti-tumor immunity. Nat. Commun. 13, 7149 (2022).
Shabsigh, A. et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur. Urol. 55, 164–174 (2009).
Chow, A. et al. The ectonucleotidase CD39 identifies tumor-reactive CD8+ T cells predictive of immune checkpoint blockade efficacy in human lung cancer. Immunity 56, 93–106 (2023).
Creelan, B. C. et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial. Nat. Med. 27, 1410–1418 (2021).
Conejo-Garcia, J. R., Biswas, S., Chaurio, R. & Rodriguez, P. C. Neglected no more: B cell-mediated anti-tumor immunity. Semin. Immunol. 65, 101707 (2023).
Crichton, E. S., Zeng, S., La Muraglia, G. M. 2nd & Badell, I. R. CXCL13 is an indicator of germinal center activity and alloantibody formation following transplantation. Transplant Direct. 7, e785 (2021).
Meylan, M. et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity 55, 527–541 (2022).
Mazor, R. D. et al. Tumor-reactive antibodies evolve from non-binding and autoreactive precursors. Cell 185, 1208–1222 (2022).
Pfister, C. et al. Randomized phase III trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin, or gemcitabine and cisplatin as perioperative chemotherapy for patients with muscle-invasive bladder cancer. Analysis of the GETUG/AFU V05 VESPER trial secondary endpoints: chemotherapy toxicity and pathological responses. Eur. Urol. 79, 214–221 (2021.
Grivas, P. et al. PrECOG PrE0807: a phase 1b feasibility trial of neoadjuvant nivolumab without and with lirilumab in patients with muscle-invasive bladder cancer ineligible for or refusing cisplatin-based neoadjuvant chemotherapy. Eur. Urol. Oncol. 7, 914–922 (2023).
Guercio, B. J. et al. Neoadjuvant nivolumab (N) +/- ipilimumab (I) in cisplatin-ineligible patients (pts) with muscle-invasive bladder cancer (MIBC). J. Clin. Oncol. 40, 498 (2022).
Koshkin, V. S. et al. Phase II trial of escalating doses of neoadjuvant atezolizumab for patients with non-metastatic urothelial carcinoma ineligible for cisplatin-based neoadjuvant chemotherapy. J. Clin. Oncol. 39, 442 (2021).
Martinez Chanza, N. et al. Avelumab as the basis of neoadjuvant regimen in platinum-eligible and -ineligible patients with nonmetastatic muscle-invasive bladder cancer: AURA (Oncodistinct-004) trial. J. Clin. Oncol. 40, 4517 (2022).
van Dorp, J. et al. High- or low-dose preoperative ipilimumab plus nivolumab in stage III urothelial cancer: the phase 1B NABUCCO trial. Nat. Med. 29, 588–592 (2023).
Wei, X. X. et al. Durvalumab as neoadjuvant therapy for muscle-invasive bladder cancer: preliminary results from the Bladder Cancer Signal Seeking Trial (BLASST)-2. J. Clin. Oncol. 38, 507 (2020).
Blanc, J. et al. Avelumab (A) as neoadjuvant therapy in patients (pts) with muscle-invasive urothelial carcinoma (MIUC): survival data of AURA trial, Oncodistinct 004. J. Clin. Oncol. 42, 4516 (2024).
Szabados, B. et al. Final results of neoadjuvant atezolizumab in cisplatin-ineligible patients with muscle-invasive urothelial cancer of the bladder. Eur. Urol. 82, 212–222 (2022).
Galsky, M. D. et al. Gemcitabine and cisplatin plus nivolumab as organ-sparing treatment for muscle-invasive bladder cancer: a phase 2 trial. Nat. Med. 29, 2825–2834 (2023).
Li, R., Zhang, J., Gilbert, S. M., Conejo-Garcia, J. & Mulé, J. J. Using oncolytic viruses to ignite the tumour immune microenvironment in bladder cancer. Nat. Rev. Urol. 18, 543–555 (2021).
Oh, D. Y. et al. Intratumoral CD4+ T cells mediate anti-tumor cytotoxicity in human bladder cancer. Cell 181, 1612–1625 (2020).
Chaurio, R. A. et al. TGF-β-mediated silencing of genomic organizer SATB1 promotes Tfh cell differentiation and formation of intra-tumoral tertiary lymphoid structures. Immunity 55, 115–128 (2022).
Qi, H. T follicular helper cells in space-time. Nat. Rev. Immunol. 16, 612–625 (2016).
Sautès-Fridman, C., Petitprez, F., Calderaro, J. & Fridman, W. H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 19, 307–325 (2019).
Coppola, D. & Mulé, J. J. Ectopic lymph nodes within human solid tumors. J. Clin. Oncol. 26, 4369–4370 (2008).
Coppola, D. et al. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am. J. Pathol. 179, 37–45 (2011).
Messina, J. L. et al. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci. Rep. 2, 765 (2012).
Cabrita, R. et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565 (2020).
Helmink, B. A. et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020).
Petitprez, F. et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560 (2020).
van Dijk, N. et al. The tumor immune landscape and architecture of tertiary lymphoid structures in urothelial cancer. Front. Immunol. 12, 793964 (2021).
Ng, K. W. et al. Antibodies against endogenous retroviruses promote lung cancer immunotherapy. Nature 616, 563–573 (2023).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. https://doi.org/10.14806/ej.17.1.200 (2011).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011).
Johnson, W. E., Li, C. & Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8, 118–127 (2006).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Ashburner, M. et al. Gene Ontology: tool for the unification of biology. Nat. Genet. 25, 25–29 (2000).
Liberzon, A. et al. Molecular signatures database (MSigDB) 3.0. Bioinformatics 27, 1739–1740 (2011).
Kim, J. et al. The cancer genome atlas expression subtypes stratify response to checkpoint inhibition in advanced urothelial cancer and identify a subset of patients with high survival probability. Eur. Urol. 75, 961–964 (2019).
Danziger, S. A. et al. ADAPTS: automated deconvolution augmentation of profiles for tissue specific cells. PLoS ONE 14, e0224693 (2019).
Ellrott, K. et al. Scalable open science approach for mutation calling of tumor exomes using multiple genomic pipelines. Cell Syst. 6, 271–281 (2018).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Cibulskis, K. et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat. Biotechnol. 31, 213–219 (2013).
Larson, D. E. et al. SomaticSniper: identification of somatic point mutations in whole genome sequencing data. Bioinformatics 28, 311–317 (2012).
Fan, Y. et al. MuSE: accounting for tumor heterogeneity using a sample-specific error model improves sensitivity and specificity in mutation calling from sequencing data. Genome Biol. 17, 178 (2016).
Garrison, E. P. & Marth, G. Haplotype-based variant detection from short-read sequencing. Preprint at https://arxiv.org/abs/1207.3907 (2012).
Ye, K., Schulz, M. H., Long, Q., Apweiler, R. & Ning, Z. Pindel: a pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics 25, 2865–2871 (2009).
Saunders, C. T. et al. Strelka: accurate somatic small-variant calling from sequenced tumor-normal sample pairs. Bioinformatics 28, 1811–1817 (2012).
Tate, J. G. et al. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res. 47, D941–D947 (2019).
Lek, M. et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291 (2016).
Forbes, S. A. et al. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 43, D805–D811 (2015).
Sherry, S. T. et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 29, 308–311 (2001).
Aken, B. L. et al. The Ensembl gene annotation system. Database 2016, baw093 (2016).
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, e164 (2010).
Mayakonda, A., Lin, D.-C., Assenov, Y., Plass, C. & Koeffler, H. P. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 28, 1747–1756 (2018).
Martincorena, I. et al. Universal patterns of selection in cancer and somatic tissues. Cell 171, 1029–1041 (2017).
Tate, J. G. et al. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res. 47, D941–D947 (2018).
Orenbuch, R. et al. arcasHLA: high-resolution HLA typing from RNAseq. Bioinformatics 36, 33–40 (2019).
Kawaguchi, S., Higasa, K., Shimizu, M., Yamada, R. & Matsuda, F. HLA-HD: an accurate HLA typing algorithm for next-generation sequencing data. Hum. Mutat. 38, 788–797 (2017).
Song, L., Bai, G., Liu, X. S., Li, B. & Li, H. Efficient and accurate KIR and HLA genotyping with massively parallel sequencing data. Genome Res. 33, 923–931 (2023).
Jurtz, V. et al. NetMHCpan-4.0: improved peptide–MHC class I interaction predictions integrating eluted ligand and peptide binding affinity data. J. Immunol. 199, 3360–3368 (2017).
Reynisson, B. et al. Improved prediction of MHC II antigen presentation through integration and motif deconvolution of mass spectrometry MHC eluted ligand data. J. Proteome Res. 19, 2304–2315 (2020).
Hall, M. S. et al. Neoantigen-specific CD4+ tumor-infiltrating lymphocytes are potent effectors identified within adoptive cell therapy products for metastatic melanoma patients. J. Immunother. Cancer 11, e007288 (2023).
Gouin, K. H. 3rd et al. An N-Cadherin 2 expressing epithelial cell subpopulation predicts response to surgery, chemotherapy and immunotherapy in bladder cancer. Nat. Commun. 12, 4906 (2021).
Chu, T., Wang, Z., Pe’er, D. & Danko, C. G. Cell type and gene expression deconvolution with BayesPrism enables Bayesian integrative analysis across bulk and single-cell RNA sequencing in oncology. Nat. Cancer 3, 505–517 (2022).
Ivanova, A., Qaqish, B. F. & Schell, M. J. Continuous toxicity monitoring in phase II trials in oncology. Biometrics 61, 540–545 (2005).
Acknowledgements
This study was funded by CG Oncology. The funders contributed to the study design but did not participate in data collection, analysis and interpretation. The primary investigator (R.L.) had full access to the data. Additional support for investigators include W81XWH-22-1-0395 (CA210846) (R.L.) and the Moffitt Cancer Center Schulze Award (R.L.) It is also supported, in part, by a generous donation from the Campbell Family Foundation. In addition, we thank the Analytical Microscopy lab and the Advanced Analytical and Digital lab as well as the Quantitative Imaging, Molecular Genomics Tissue and Pathology, Biostatistics and Bioinformatics cores at H. Lee Moffitt Cancer Center for providing support. We also thank the patients and their families for participating in this clinical trial.
Author information
Authors and Affiliations
Contributions
R.L.: conceptualization, formal analysis, supervision, investigation, original writing and writing—review and editing. N.Y.V.: formal analysis, investigations and writing—review and editing. X.Y.: formal analysis, investigations and writing—review and editing. J.O.J.: formal analysis and writing—review and editing. G.B.: investigations and writing—review and editing. J.D.: investigations and writing—review and editing. C.M.M.-S.: investigations and writing—review and editing. Y.K.: formal analysis and writing—review and editing. N.F.: investigations. D.D.: investigations. J.J.P.: investigations. W.J.S.: investigations and writing—review and editing. P.E.S.: investigations and writing—review and editing. M.A.P.: investigations and writing—review and editing. L.Z.: investigations and writing—review and editing. S.M.G.: investigations and writing—review and editing. J.Z.: conceptualization and writing—review and editing. J.M.P.-S.: conceptualization and writing—review and editing. A.R.A.A.: formal analysis and writing—review and editing. T.L.: formal analysis and writing—review and editing. X.W.: formal analysis and writing—review and editing. G.D.G.: writing—review and editing. J.M.B.: conceptualization and writing—review and editing. C.P.N.D.: conceptualization, formal analysis and writing—review and editing. P.C.R.: formal analysis and writing—review and editing. R.K.J.: conceptualization, investigations and writing—review and editing. J.J.M.: conceptualization, formal analysis, supervision, formal analysis and writing—review and editing. J.R.C.-G.: conceptualization, formal analysis, supervision, investigations, formal analysis and writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
R.L. reports research support from Predicine, Veracyte, CG Oncology, Valar Labs, Merck and Janssen; clinical trial protocol committee participation with CG Oncology, Merck and Janssen; and is scientific advisor/consultant for Bristol Myers Quibb, Merck, Fergene, Arquer Diagnostics, Urogen Pharma, Lucence, CG Oncology, Janssen, Thericon, Iconovir, ImmunityBio and Pfizer. W.J.S. reports consultant work for Pacific Edge and Urogen Pharma. P.E.S. reports being the Vice Chair of the NCCN guidelines committee for bladder and penile cancer. J.Z. reports consultant work for Sanofi, AstraZeneca, Dendreon, Seagen, Bayer and Pfizer. G.D.G. reports consultant work for MyCareGorithm and stock options in Lantheus. J.M.B. reports consultant work for CG Oncology and Kalivir Immunotherapeutics and being a shareholder of CG Oncology and Kalivir Immunotherapeutics. C.P.N.D. reports consultant work for AstraZeneca and CG Oncology and intellectual property ownership related to the use of genetic alterations as a predictive biomarker for response to nadofaragene firadenovec. R.K.J. reports consultant work for AVEO, Bristol Myers Squibb, Sanofi, EMD Serono, Gilead Sciences, IDEOlogy Pfizer and Seattle Genetics/Astellas; speaker’s bureau for Gilead Sciences, Seagen and Seattle Genetics/Astellas; research funding from Bristol Myers Squibb, Gilead Sciences and the National Cancer Institute; and honoraria from FLASCO, Curio Science, DAVA Oncology, NCCN/Pfizer and OncLive/MJH Life Sciences. J.J.M. reports membership on the CG Oncology Board of Directors; being the Associate Center Director at Moffitt Cancer Center; ownership interest in Aleta Biotherapeutics, CG Oncology, Turnstone Biologics, Ankyra Therapeutics and AffyImmune Therapeutics; and consultant work for ONCoPEP, CG Oncology, Turnstone Biologics, Vault Pharma, Ankyra Therapeutics, AffyImmune Therapeutics, UbiVac, Vycellix and Aleta Biotherapeutics. J.R.C.-G. reports consultant work for Anixa Biosciences and Alloy Therapeutics; stock options in Compass Therapeutics, Anixa Biosciences and Alloy Therapeutics; patent licensed by Anixa Biosciences; intellectual property filed with Compass Therapeutics; and being the co-founder of Cellepus Therapeutics, a CAR T cell company. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Masahiro Kojima and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Saheli Sadanand and Ulrike Harjes, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Increases in viral replication is not associated with tumor intrinsic molecular features.
Fold change in GM-CSF, used as a surrogate marker for viral replication, was associated with neither baseline E2F target expression levels (n = 18; Pearson correlation R2 = 0.019; p = 0.58) (a), nor with molecular subtypes (n = 17; p = 0.228) (b).
Extended Data Fig. 2 Increase in T lymphocyte infiltration is not associated with baseline tumor/immune features nor treatment response.
Fold changes in infiltrating T lymphocyte levels were not associated with baseline tumor mutational burden (n = 19; CD3+ p = 0.33; CD4+ p = 0.23; CD8+ p = 0.81) (a); pre-treatment CD3+ lymphocytic infiltration (n = 19; CD3+ p = 0.27; CD4+ p = 0.44; CD8+ p = 0.43) (b); baseline tumor molecular subtypes (n = 17; CD3+ p = 0.11; CD4+ p = 0.305; CD8+ p = 0.16) (c); nor with treatment response (n = 19; CD3+ p = 0.97; CD4+ p = 0.9; CD8+ p = 0.5) (d). For dot-plots in a-c, correlation coefficients and P values were calculated using Pearson Correlation. For boxplots in d, boxes represent median (center) and first/third quartile (bottom and top, respectively) values; Tukey whiskers represent the ± 1.5 interquartile range (IQR); individual data points are shown in dots; Two-sided P values were calculated by Wilcoxon’s signed-rank test.
Extended Data Fig. 3 Phenotypic changes in T cell markers from pre- to post-treatment.
a, Gene expression levels of PDCD1 (PD-1), TNFRSF4 (b); LAG3 (c); and HAVCR2 (TIM3) (d) on bulk RNA sequencing were not different from pre- to post-treatment in pathologic complete responders vs. non-responders. Two-sided P values were calculated by Wilcoxon signed-rank test. Boxes represent median (center) and first/third quartile (bottom and top, respectively) values; Tukey whiskers represent the ± 1.5 interquartile range (IQR); individual data points are shown in dots colored by CR (blue) or NR (gray).
Extended Data Fig. 4 Enzyme-linked immunosorbent spot (ELISpot) analysis indicates heightened cell-mediated anti-tumor systemic reactivity in patients with pathologic complete response.
a, Representative plots showing fold changes in interferon-γ (IFN-γ) spot-forming units at weeks 2 and/or 6 compared to baseline in clinical responders and b, clinical non-responders. Significance increases were seen at the following timepoints in the following patients: Due to sample limitations, co-culture using neopeptide-primed monocyte-derived DCs with autologous T cells from each patient at each time point was conducted once without replicates.
Extended Data Fig. 5 No change was found in the tertiary lymphoid structure (TLS) density from pre- to post-treatment in non-responding patients following treatment.
a, Representative whole slide multiplex immunofluorescence images from a patient who did not respond to treatment, demonstrating lack of increase of TLS in the post-treatment samples. b, TLS density at baseline did not predict pathologic response to combined oncolytic immunotherapy and immune checkpoint inhibitor (n = 16; p = 0.594). Two-sided p values were calculated by Wilcoxon’s signed-rank test. Boxes represent median (center) and first/third quartile (bottom and top, respectively) values; Tukey whiskers represent the ± 1.5 interquartile range (IQR); individual data points are shown in dots colored by the response (responder = blue; non-responder = gray).
Extended Data Fig. 6 Features of mature vs. immature tertiary lymphoid structure (TLS).
a, TLS were scored according to morphological features on multiplex immunofluorescence. Immature TLS consisted of general clustering of CD20 + B cells with unclear shape and structure. Mature TLS consisted of large, oval or circular shaped conglomeration of CD20 + B cells. As expected, mature TLS were marked by higher surface areas (***p < 0.001) (b) and cellular density (**p = 0.00652) (c). Two-sided p values were calculated by Wilcoxon’s signed-rank test. Boxes represent median (center) and first/third quartile (bottom and top, respectively) values; Tukey whiskers represent the ± 1.5 interquartile range (IQR).
Extended Data Fig. 7 Heightened antibody response post-treatment is directed against tumor cells.
Of all pathologic complete responders, one patient exhibited downstaging of disease (cTis) on the post-treatment biopsy prior to attaining pathologic complete response at the time of radical cystectomy. On multiplex immunofluorescence staining of the post-treatment biopsy specimen, IgG was observed to coat tumor cells, suggesting specific anti-tumor humoral response.
Supplementary information
Supplementary Information
Supplemental Tables 1–3 and study protocol.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, R., Villa, N.Y., Yu, X. et al. Oncolytic immunotherapy with nivolumab in muscle-invasive bladder cancer: a phase 1b trial. Nat Med 31, 176–188 (2025). https://doi.org/10.1038/s41591-024-03324-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-024-03324-9
This article is cited by
-
CDCA3-MYC positive feedback loop promotes bladder cancer progression via ENO1-mediated glycolysis
Journal of Experimental & Clinical Cancer Research (2025)
-
Neoadjuvant therapy in MIBC
Nature Reviews Urology (2025)