Abstract
Social status has a profound impact on mental health and propensity towards drug addiction. However, the neural mechanisms underlying the effects of social rank on drug-seeking behavior remain unclear. Here we found that dominant male rodents (based on the tube test) had denser mesocortical dopaminergic projections and were more resistant to methamphetamine (METH)-seeking, whereas subordinates had heightened dopaminergic function in the mesolimbic pathway and were more vulnerable to METH seeking. Optogenetic activation of the mesocortical dopaminergic pathway promoted winning and suppressed METH seeking in subordinates, whereas lesions of the mesocortical pathway increased METH seeking in dominants. Elevation of social rank with forced win training in subordinates led to remodeling of the dopaminergic system and prevented METH-seeking behavior. In females, however, both ranks were susceptible to METH seeking, with mesocorticolimbic pathways comparable to those in subordinate males. These results provide a framework for understanding the neural basis of the impact of social status on drug-seeking.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data generated or analyzed in this article are available in the Source data. Source data are provided with this paper.
Code availability
No unique code was generated in this paper.
References
United Nations. World Drug Report 2023. www.unodc.org/unodc/en/data-and-analysis/world-drug-report-2023.html (2023).
Swendsen, J. & Le Moal, M. Individual vulnerability to addiction. Ann. N. Y. Acad. Sci. 1216, 73–85 (2011).
Nestler, E. J. & Lüscher, C. The molecular basis of drug addiction: linking epigenetic to synaptic and circuit mechanisms. Neuron 102, 48–59 (2019).
Morgan, D. et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat. Neurosci. 5, 169–174 (2002).
Scott, K. M. et al. Associations between subjective social status and DSM-IV mental disorders: results from the World Mental Health surveys. JAMA Psychiatry 71, 1400–1408 (2014).
Langlois, S. et al. Subjective social status, objective social status, and substance use among individuals with serious mental illnesses. Psychiatry Res. 293, 113352 (2020).
Finch, K. A., Ramo, D. E., Delucchi, K. L., Liu, H. & Prochaska, J. J. Subjective social status and substance use severity in a young adult sample. Psychol. Addict. Behav. 27, 901 (2013).
Stelly, C. E., Pomrenze, M. B., Cook, J. B. & Morikawa, H. Repeated social defeat stress enhances glutamatergic synaptic plasticity in the VTA and cocaine place conditioning. eLife 5, e15448 (2016).
Rodriguez-Arias, M. et al. Social defeat in adolescent mice increases vulnerability to alcohol consumption. Addict. Biol. 21, 87–97 (2016).
Li, R. et al. Associations of socioeconomic status and healthy lifestyle with incident early-onset and late-onset dementia: a prospective cohort study. Lancet Healthy Longev. 4, e693–e702 (2023).
Demakakos, P., Biddulph, J. P., de Oliveira, C., Tsakos, G. & Marmot, M. G. Subjective social status and mortality: the English Longitudinal Study of Ageing. Eur. J. Epidemiol. 33, 729–739 (2018).
Snyder-Mackler, N. et al. Social status alters immune regulation and response to infection in macaques. Science 354, 1041–1045 (2016).
Belmi, P., Neale, M. A., Reiff, D. & Ulfe, R. The social advantage of miscalibrated individuals: the relationship between social class and overconfidence and its implications for class-based inequality. J. Personal. Soc. Psychol. 118, 254 (2020).
Korous, K. M., Causadias, J. M., Bradley, R. H., Luthar, S. S. & Levy, R. A systematic overview of meta-analyses on socioeconomic status, cognitive ability, and achievement: the need to focus on specific pathways. Psychol. Rep. 125, 55–97 (2022).
Dohrenwend, B. P. et al. Socioeconomic status and psychiatric disorders: the causation-selection issue. Science 255, 946–952 (1992).
Murphy, J. M. et al. Depression and anxiety in relation to social status: a prospective epidemiologic study. Arch. Gen. Psychiatry 48, 223–229 (1991).
Teles, M. C., Dahlbom, S. J., Winberg, S. & Oliveira, R. F. Social modulation of brain monoamine levels in zebrafish. Behav. Brain Res. 253, 17–24 (2013).
Winberg, S., Nilsson, G. E. & Olsén, K. H. Social rank and brain levels of monoamines and monoamine metabolites in Arctic charr, Salvelinus alpinus (L.). J. Comp. Physiol. A 168, 241–246 (1991).
Cheng, H. G. et al. Change in social rank and brain dopamine levels: findings from a novel pig model. Preprint at bioRxiv https://doi.org/10.1101/2020.08.06.239780 (2020).
Martinez, D. et al. Dopamine type 2/3 receptor availability in the striatum and social status in human volunteers. Biol. Psychiatry 67, 275–278 (2010).
Wiers, C. E. et al. Socioeconomic status is associated with striatal dopamine D2/D3 receptors in healthy volunteers but not in cocaine abusers. Neurosci. Lett. 617, 27–31 (2016).
Juarez, B. & Han, M.-H. Diversity of dopaminergic neural circuits in response to drug exposure. Neuropsychopharmacology 41, 2424–2446 (2016).
Pascoli, V., Terrier, J., Hiver, A. & Lüscher, C. Sufficiency of mesolimbic dopamine neuron stimulation for the progression to addiction. Neuron 88, 1054–1066 (2015).
Shan, Q., Hu, Y., Chen, S. & Tian, Y. Nucleus accumbens dichotomically controls social dominance in male mice. Neuropsychopharmacology 47, 776–787 (2022).
Hollis, F. et al. Mitochondrial function in the brain links anxiety with social subordination. Proc. Natl Acad. Sci. USA 112, 15486–15491 (2015).
Wang, F. et al. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science 334, 693–697 (2011).
Zhou, T. et al. History of winning remodels thalamo-PFC circuit to reinforce social dominance. Science 357, 162–168 (2017).
Vander Weele, C. M. et al. Dopamine enhances signal-to-noise ratio in cortical-brainstem encoding of aversive stimuli. Nature 563, 397–401 (2018).
Beyer, C. E. & Steketee, J. D. Dopamine depletion in the medial prefrontal cortex induces sensitized-like behavioral and neurochemical responses to cocaine. Brain Res. 833, 133–141 (1999).
Schenk, S., Horger, B. A., Peltier, R. & Shelton, K. Supersensitivity to the reinforcing effects of cocaine following 6-hydroxydopamine lesions to the medial prefrontal cortex in rats. Brain Res. 543, 227–235 (1991).
Antinori, S. et al. Levodopa prevents the reinstatement of cocaine self‐administration in rats via potentiation of dopamine release in the medial prefrontal cortex. Addict. Biol. 23, 556–568 (2018).
Sorg, B. A., Li, N. & Wu, W.-R. Dopamine D1 receptor activation in the medial prefrontal cortex prevents the expression of cocaine sensitization. J. Pharmacol. Exp. Ther. 297, 501–508 (2001).
Lammel, S., Ion, D. I., Roeper, J. & Malenka, R. C. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron 70, 855–862 (2011).
Ventura, R. et al. Dopamine in the medial prefrontal cortex controls genotype-dependent effects of amphetamine on mesoaccumbens dopamine release and locomotion. Neuropsychopharmacology 29, 72–80 (2004).
Xu, F. et al. High-throughput mapping of a whole rhesus monkey brain at micrometer resolution. Nat. Biotechnol. 39, 1521–1528 (2021).
Fan, Z. et al. Neural mechanism underlying depressive-like state associated with social status loss. Cell 186, 560–576 (2023).
Sulzer, D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 69, 628–649 (2011).
Sun, F. et al. Next-generation GRAB sensors for monitoring dopaminergic activity in vivo. Nat. Methods 17, 1156–1166 (2020).
Lammel, S. et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature 491, 212–217 (2012).
Dunkley, P. R. & Dickson, P. W. Tyrosine hydroxylase phosphorylation in vivo. J. Neurochem. 149, 706–728 (2019).
Guillot, T. S. & Miller, G. W. Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons. Mol. Neurobiol. 39, 149–170 (2009).
Calipari, E. S. et al. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat. Commun. 8, 13877 (2017).
Foster, J. D. & Vaughan, R. A. Phosphorylation mechanisms in dopamine transporter regulation. J. Chem. Neuroanat. 83, 10–18 (2017).
Matthews, G. A. et al. Dorsal raphe dopamine neurons represent the experience of social isolation. Cell 164, 617–631 (2016).
Sun, P. et al. Recombinase system-dependent copackaging strategy for highly efficient neurocircuit tracing. Preprint at bioRxiv https://doi.org/10.1101/705772 (2019).
Challasivakanaka, S., Smith, M., Foster, J. & Vaughan, R. Post-phosphorylation control of dopamine transporter by peptidyl prolyl cis–trans isomerase PIN1 (803.6). FASEB J. 28, 803.6 (2014).
Kokane, S. S. & Perrotti, L. I. Sex differences and the role of estradiol in mesolimbic reward circuits and vulnerability to cocaine and opiate addiction. Front. Behav. Neurosci. 14, 74 (2020).
Zakiniaeiz, Y. et al. Sex differences in amphetamine-induced dopamine release in the dorsolateral prefrontal cortex of tobacco smokers. Neuropsychopharmacology 44, 2205–2211 (2019).
Gërxhani, K., Brandts, J. & Schram, A. Competition and gender inequality: a comprehensive analysis of effects and mechanisms. Am. J. Sociol. 129, 715–752 (2023).
Zilkha, N. et al. Sex-dependent control of pheromones on social organization within groups of wild house mice. Curr. Biol. 33, 1407–1420 (2023).
Xing, B., Mack, N. R., Zhang, Y.-X., McEachern, E. P. & Gao, W.-J. Distinct roles for prefrontal dopamine D1 and D2 neurons in social hierarchy. J. Neurosci. 42, 313–324 (2022).
Diana, M. et al. Rehabilitating the addicted brain with transcranial magnetic stimulation. Nat. Rev. Neurosci. 18, 685–693 (2017).
Acknowledgements
We thank E. Neher, X. Chen and M. Han for critical comments and suggestions, G. Bi and F. Xu for assistance in VISoR experiments, F.Q. Xu (Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences) for providing sparse labeling virus, and Y. Sun and C. Jiang for preliminary explorations. We also acknowledge Q. Luo, Z.Y. Yang and H. Gu for their help with mass spectrometry analysis. This work was supported by the Major Project of the Science and Technology Innovation 2030 of China (2021ZD0202103 and 2021ZD0203500), Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0930000), the National Natural Science Foundation of China (82425023, 82171492, 32300846, 82101569 and 32400840), Guangdong Basic and Applied Basic Research Foundation (2023B1515040009 and 2023A1515012122), the Technology and Innovation Commission of Shenzhen (RCJC20200714114556103, ZDSYS20190902093601675, JCYJ20210324141201003, KCXFZ20211020164543007 and KCXFZ20230731100901004), the Shenzhen Medical Research Funding (SMRFA2303034) and the Yunnan Technological Innovation Centre of Drug Addiction Medicine (202305AK340001). We also thank the support from the Innovative Research Team of High-level Local Universities in Shanghai.
Author information
Authors and Affiliations
Contributions
Y.Z. and X.D. conceptualized the project. X.D., Y.L., G.C. and K.S. developed the methodology. X.D., W.X., Y.L., H.J., J.Z., K.S., W.C., S.J., G.C., Y.Z., R.Z., L.X., X.W. and B.Z. conducted the investigation. Y.Z., X.D., W.X., Y.L. and G.C. acquired funding. Y.Z. supervised the project. Y.Z. and X.D. wrote the original draft of the paper. Y.Z., X.D., W.X. and Y.L. contributed to writing, reviewing and editing of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Neir Eshel, Kay Tye and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Other behavioral and physiological results.
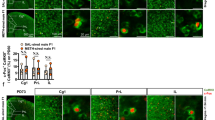
a,b, Schematic and behavioral outcomes of the agonistic behavior assay (a) and the warm spot test (b) in male mice (n = 18 pairs). Paired t test. c, Average futile pokes during the last three sessions of METH self-administration for the dominant (n = 13) and subordinate (n = 13) rats. Paired t test. Futile pokes = active pokes − infusions. d, Winning probability across trials and total wins in mice (n = 28 pairs). Paired t test. e, Schematic of METH self-administration in mice. f–h, Nose pokes (f) and infusions (g) across session and their average number (h) during the last three sessions in the dominant (n = 11) and subordinate (n = 12) mice. f,g, Two-way ANOVA; h, paired t test. i, Hyperlocomotion induced by 2.5 mg kg−1 METH injection in mice (n = 8 pairs). Two-way ANOVA followed by Sidak’s test. j, Morphine CPP scores in mice (n = 5 pairs). Unpaired t test. k, Body weight change. Two-way ANOVA. l, Distance traveled and central time during open-field test in rats (n = 11 pairs). Unpaired t test. m–o, Immobility time in the forced swimming test (m) and the tail suspension test (n) and preference ratio (o) in the sucrose preference test in mice (n = 12 pairs). Paired t test. p, Serum corticosterone levels in rats (sub, n = 10; dom, n = 9). Unpaired t test. q, Serum testosterone levels in rats (female sub, n = 5; female dom, n = 4; male sub, n = 10; male dom, n = 8). Two-way ANOVA. r, Serum estrogen levels in rats (females, n = 4/group; male sub, n = 5; male dom, n = 4). Two-way ANOVA. s, Timeline of the mass spectrometry analysis. t,u, Serum concentrations (t) and half-life (u) of METH following METH injection in rats (n = 5 pairs). x, two-way ANOVA; y, paired t test. Data are represented as mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.01, ****P < 0.0001. Detailed statistical information is available in Supplementary Table 1.
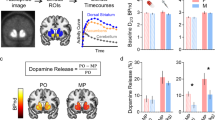
Extended Data Fig. 2 Other fiber photometry results for DA release in the NAc and mPFC.
a,b, Schematics of viral expression and optic fiber placement in the NAc shell (a) and core (b) for fiber photometry. c–g, Representative image of viral expression in the NAc shell (c) and core (e). Scale bar = 0.5 mm. DA release in the NAc shell (d) and core (f) and AUC comparison (g) after METH (0.1 mg kg−1) infusion in mice (shell: sub, n = 9; dom: n = 10; core: sub, n = 7; dom, n = 6). Two-way ANOVA followed by Sidak’s post-hoc test, *P < 0.05, **P < 0.01. h–k, Schematics for viral expression and optic fiber placement in the NAc (h) and mPFC (j) for fiber photometry experiments. Saline-induced DA release in the NAc (i) and mPFC (k) in mice (NAc: n = 13/group; mPFC: n = 9/group). Two-sided unpaired t test. l, Timeline and schematic showing the METH self-administration with simultaneous fiber photometry recording. m,n, Schematics showing viral expression and optic fiber placement in the NAc (m) and mPFC (n) for fiber photometry in a free-moving self-administration. o,p, DA release in the NAc (o) and mPFC (p) following active and inactive pokes in a free-moving METH self-administration (NAc: n = 10 per group; mPFC: sub, n = 8; dom, n = 9). Two-sided unpaired t test, *P < 0.05, **P < 0.01. Each row in the heatmap represents averaged DA dynamics over trials from individual mouse (ranked by AUC). Data are represented as mean ± s.e.m. Detailed statistical information is available in Supplementary Table 1.
Extended Data Fig. 3 Activity of VTADA neurons following METH infusion between dominants and subordinates.
a, Schematic of the virus strategy and a representative image showing GCaMP expression and optic fiber placement (top). Scale bar = 200 µm. Diagrams showing viral expression and optic fiber ___location in the VTA for the fiber photometry experiments (bottom). b–e, Activity of VTADA neurons following i.v. injection of saline (b), 0.1 mg kg−1 (c) and 0.3 mg kg−1 METH (d) and sucrose licking (e) in mice (saline and 0.1 METH: n = 5/group; 0.3 METH: sub, n = 4; dom, n = 3; sucrose: sub, n = 5; dom, n = 4). Mann–Whitney test. f, Schematic of the viral strategy to specifically record the NAc-projecting VTA neurons and a representative image (top). Scale bar = 200 µm. Diagrams showing viral expression and optic fiber ___location in the VTA for the fiber photometry experiments (bottom). g,h, Activity of NAc-projecting VTADA neurons following i.v. injection of 0.1 mg kg−1 (g) and 0.3 (h) mg kg−1 METH in mice (sub, n = 5; dom, n = 6). Two-sided unpaired t test. Each row in the heatmap represents averaged DA dynamics over trials from individual mouse (ranked by AUC). Data are represented as mean ± s.e.m. Detailed statistical information is available in Supplementary Table 1.
Extended Data Fig. 4 DA pathways from the VTA projecting to mPFC and NAc are structurally and functionally distinct.
a, Schematic showing the CTB-mediated retrograde tracing experiment in DAT::Ai14 mice (top). Representative images of CTB488 in the mPFC (bottom left) and CTB647 in the NAc (bottom right). Scale bar = 0.5 mm. b, Representative images showing DA neurons, mPFC-projecting and NAc-projecting neurons in the VTA. Scale bar = 0.2 mm. c, Venn diagram illustrating the overlap of CTB-labeled VTADA neuron projecting to the mPFC and NAc (n = 5 mice). d, Distribution of CTB-labeled mPFC-projecting and NAc-projecting VTADA neurons across different anteroposterior coordinates. e,f, Optogenetically activation of the mesolimbic (e) and mesocortical (f) DA pathway in mice during a real-time place preference experiment (EYFP, n = 5; ChR2, n = 10). Two-sided unpaired t test, **P < 0.01. Data are represented as mean ± s.e.m. Detailed statistical information is available in Supplementary Table 1.
Extended Data Fig. 5 Additional DA-related protein and immunofluorescence data.
a, Overlay of images of DA terminals in the NAc (n = 9 mouse pairs). Scale bar = 400 μm. b, Schematic of patch clamp. c, Representative traces showing action potential firing of VTADA neurons. d, Intrinsic excitability of VTADA neurons from dominant (n = 4 cells) and subordinate (n = 3 cells) mice. Two-way ANOVA. APS, action potentials. e,f, Representative western blot images (e) and relative levels (f) of ERK/pERK in the NAc (n = 8 rat pairs). Two-sided paired t test, *P < 0.05. g, Overlay of images showing TH-positive terminals in the mPFC (n = 9 mouse pairs). Scale bar = 400 μm. h, The percentage of the PrL area covered by DA terminals across different layers. Two-sided paired t test, *P < 0.05, **P < 0.01. i, Representative images (i) and quantification (j) of DA varicosities in the PrL layer 2–5 (n = 18 slices from four mouse pairs) and along DA axon branches (n = 26 axon branches from three mouse pairs). Two-sided unpaired t test, ***P < 0.001. k, Schematic showing sparsely labeling DA neurons in the VTA. l, Representative sections showing VTADA neurons, and DA terminals in the NAc and mPFC by VISoR. Scale bar = 2 mm. m, Overlay of seven reconstructed mPFC-projecting neurons (one neuron from a subordinate; six neurons from four dominants) registered to a reference brain. n, Number of labeled VTADA neurons in the subordinate (n = 4) and dominant (n = 3) mice. Two-sided unpaired t test. o, Proportion of mPFC-projecting VTADA neurons in the dominant (n = 3 mice) and subordinate (n = 4 mice) mice. Two-sided unpaired t test, *P < 0.05. p,q, GFP-labeled fluorescence in the NAc (p) and mPFC (q) in the dominant (n = 5) and subordinate (n = 5) mice. Two-sided unpaired t test, **P < 0.01. Data are represented as mean ± s.e.m. Detailed statistical information is available in Supplementary Table 1.
Extended Data Fig. 6 Additional results of pharmacological and optogenetic manipulation in the mesolimbic pathway.
a, Timeline for intracranial injections followed by behavioral assessments (top). Schematic showing intracranial infusions of GDC/API-1 into the NAc followed by METH CPP (bottom). b, CPP scores for subordinate mice with infusion of GDC (n = 6) and vehicle (n = 6). Two-sided unpaired t test, *P < 0.05. c, CPP scores for dominant mice with infusion of API-1 (n = 6) and vehicle (n = 6). Two-sided unpaired t test. d, Changes of social rank before and after intracranial infusion of GDC in subordinates (d) and API-1 in dominants (e) into the NAc (GDC, n = 6; API-1, n = 8; vehicle, n = 6). Two-sided unpaired t test. f, Timeline for mesolimbic activation experiments. g, Schematic of the viral strategy and diagram of optogenetic stimulation during the tube test (left). Representative image in the NAc (right). Scale bar = 200 µm. h, Confirmed positions of optic fibers in the NAc. i,j, Changes of social rank during and after mesolimbic activation in dominant mice; individual data (i) and averaged data (j; ChR2, n = 6; EYFP, n = 6). Two-sided unpaired t test. k, Schematic illustrating mesolimbic activation during METH self-administration in dominant mice. Light was delivered at 20 Hz for 1 s, following an active nose poke. l–n, Nose pokes (l) and infusions (m) across ten sessions and their average number during the last three sessions (n) in the dominant + ChR2 (n = 9) and dominant + EYFP (n = 9) mice. l, Two-way ANOVA, ****P < 0.0001, followed by Tukey’s post-hoc test, ****P < 0.0001; m, ****P < 0.0001; n, two-sided unpaired t test, ****P < 0.0001. Data are represented as mean ± s.e.m. Detailed statistical information is available in Supplementary Table 1.
Extended Data Fig. 7 Activating the VTA–PFC pathway alone is not sufficient to change drug-seeking behavior unless it is mediated by social winning.
a, Confirmed positions of optic fibers used for optogenetic mesocortical activation. b, Schematic showing the viral strategy to record DA release during optogenetic mesocortical activation. c, DA dynamics (left) and AUC of DA release (right) in the mPFC induced by optogenetic stimulation of the mesocortical pathway at different frequencies (n = 4 mice). Friedman test, ***P < 0.001. d, Infusions across 12 METH sessions in the control subordinate (n = 9), the unchange (n = 5) and the partial win + full win (n = 5) mice. Two-way ANOVA, *P < 0.05, followed by Tukey’s test, ***P < 0.001, ****P < 0.0001. e, Correlation between the number of METH infusions and the winning probability after 4 days of optogenetic stimulation (n = 10 mice). Spearman’s correlation, r = −0.6922, *P < 0.05. f, Timeline for VTA→mPFC optogenetic stimulation experiments in subordinates. g, Rank changes in the subordinate + ChR2 (n = 7) group induced by optogenetic stimulation of the VTA→mPFC pathway. h, Comparison of winning probability before and after optogenetic stimulation in the Subordinate + ChR2 (n = 7) group. Two-sided paired t test. i–k, Active/inactive pokes (i) and infusions (j) across sessions and average number (k) during the last three sessions in the subordinate + EYFP (n = 13) and subordinate + ChR2 (n = 7) groups across ten sessions of METH self-administration. i, Two-way ANOVA, **P < 0.01, followed by Tukey’s post-hoc test; j, P > 0.05; k, two-sided unpaired t test. Data are represented as mean ± s.e.m. Detailed statistical information is available in Supplementary Table 1.
Extended Data Fig. 8 Repeated loss experiences facilitate METH-seeking behavior in dominants resistant to forced loss.
a, Timeline for forced loss procedure followed by depressive assays. b–d, Immobility time in the forced swimming test (b) and the tail suspension test (c) and preference ratio in the sucrose preference test (d) between native control (n = 16), natural loss (n = 10) and forced loss (n = 8) mice. One-way ANOVA followed by Tukey’s test, *P < 0.05, **P < 0.01. e, Rank change across sessions for subordinates resistant to forced win (defined as S-to-S, n = 8 rats). f–h, Active/inactive pokes (f) and infusions (g) across sessions and average number (h) during the last three sessions in subordinate (n = 10) and S-to-S (n = 7) rats. f, Two-way ANOVA, ***P < 0.001, followed by Tukey’s test; g, P > 0.05; h, two-sided unpaired t test. i, Rank change across sessions for dominants resistant to forced loss (defined as D-to-D, n = 12 rats). j–l, Active/inactive pokes (j) and infusions (k) across sessions and average number (l) during the last three sessions in the dominant (n = 10) and D-to-D (n = 7) rats. j, Two-way ANOVA, ****P < 0.0001, followed by Tukey’s test, ****P < 0.0001; k, **P < 0.01; l, two-sided unpaired t test, **P < 0.01. m,n, Verification of viral expression and optic fiber placement for fiber photometry in the NAc (m) and mPFC (n). o, pERK expression in the NAc before and after forced win/loss procedure, normalized to matched subordinates (n = 6 rats/groups). Two-sided unpaired t test, **P < 0.01. Data are represented as mean ± s.e.m. Detailed statistical information is available in Supplementary Table 1.
Extended Data Fig. 9 Both female groups exhibited high METH-seeking behavior.
a, Timeline and schematics showing the tube test and METH self-administration in female rats. b, Winning probability across trials and total wins in female rats (n = 8 pairs). Two-sided paired t test, ****P < 0.0001. c,d, Behavioral outcome in the warm spot test (c) and agonistic behavior (d) for females (n = 12 mice per group). Two-sided paired t test, *P < 0.05. e–g, Nose pokes (e) and infusions (f) across sessions and average number (g) during the last three days in female (n = 5 per group) and male rats (n = 10 per group). e, Two-way ANOVA, *P < 0.05, followed by Tukey’s test; f, **P < 0.01, followed by Tukey’s test, ****P < 0.0001. g, One-way ANOVA, **P < 0.01, followed by Sidak’s test, ***P < 0.001. h, Timeline of the fiber photometry recordings in females. i,j, Schematic (i) and verification (j) of viral injections and optic fiber placement in the mPFC. Scale bar = 0.5 mm. k,l, DA release in the mPFC following 0.1 mg kg−1 (k) and 0.3 mg kg−1 (l) METH infusion in female (0.1 METH: sub, n = 9; dom, n = 8; 0.3 METH: n = 8/group) and male (0.1 METH: sub, n = 9; dom, n = 8; 0.3 METH: sub, n = 9; dom, n = 10) mice. Two-sided unpaired t test, *P < 0.05, **P < 0.01. m, Schematic (m) and verification (n) of viral injections and optic fiber placement in the NAc. Scale bar = 0.5 mm. o,p, DA release in the NAc following 0.1 mg kg−1 (o) and 0.3 mg kg−1 (p) METH infusion in female (0.1 METH: sub, n = 9; dom, n = 8; 0.3 METH: n = 8 per group) and male (0.1 METH: sub, n = 13; dom, n = 14; 0.3 METH: sub, n = 9; dom, n = 8) mice. Two-sided unpaired t test, *P < 0.05, **P < 0.01. Each row in the heatmap represents averaged DA dynamics over trials from individual mouse (ranked by AUC). Data are represented as mean ± s.e.m. Detailed statistical information is available in Supplementary Table 1.
Extended Data Fig. 10 Both female groups showed higher pDAT in the NAc and lower mesocortical projection density in the mPFC layer 2/3 compared to male dominants.
a, Timeline for the immunofluorescence analysis. b, Representative images of TH-positive terminals in the mPFC for female mice. Scale bar = 400 μm. c, Relative fluorescence density of TH-positive signals in the PrL, ACC, IL and the entire mPFC, normalized to paired subordinate (n = 7 pairs of female mice). Two-sided paired t test, *P < 0.05, **P < 0.01. d, Percentage of PrL covered by TH-positive terminals for male (n = 9 pairs) and female (n = 7 pairs) mice. Unpaired t test, *P < 0.05. e, Representative images of TH-positive terminals in the PrL regions across different cell layers for female mice. Scale bar = 200 μm. f, Percentage of the PrL area covered by TH-positive terminals across different layers for male (n = 9 pairs) and female (n = 7 pairs) mice. Two-way ANOVA, **P < 0.01, followed by Tukey’s test, *P < 0.05, ***P < 0.001. g, Timeline for the protein dectection. h, Representative western blot image showing expression of DAT, pDAT and GAPDH from NAc tissue samples of female rats. i, Relative expression levels of proteins in the NAc of female rats, normalized to paired subordinate (n = 8 pairs). j, Relative expression levels of pDAT in the NAc for male (n = 8 per group) and female (n = 6 per group) rats, normalized to GAPDH. Unpaired t test, ***P < 0.001. Data are represented as mean ± s.e.m. Detailed statistical information is available in Supplementary Table 1.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Note (protocol).
Supplementary Table 1
Statistical analysis of Figs. 1–7 and Extended Data Figs. 1–10.
Source data
Source Data Figs. 1–7 and Extended Data Figs. 1–10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deng, X., Xu, W., Liu, Y. et al. Social rank modulates methamphetamine-seeking in dominant and subordinate male rodents via distinct dopaminergic pathways. Nat Neurosci 28, 1268–1279 (2025). https://doi.org/10.1038/s41593-025-01951-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-025-01951-0