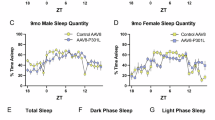
Abstract
Sleep disturbances are associated with the pathogenesis of neurodegenerative diseases such as Alzheimer’s disease and primary tauopathies. Here we demonstrate that administration of the dual orexin receptor antagonist lemborexant in the P301S/E4 mouse model of tauopathy improves tau-associated impairments in sleep–wake behavior. It also protects against chronic reactive microgliosis and brain atrophy in male P301S/E4 mice by preventing abnormal phosphorylation of tau. These neuroprotective effects in males were not observed after administration of the nonorexinergic drug zolpidem that similarly promoted nonrapid eye movement sleep. Furthermore, both genetic ablation of orexin receptor 2 and lemborexant treatment reduced wakefulness and decreased seeding and spreading of phosphorylated tau in the brain of wild-type mice. These findings raise the therapeutic potential of targeting sleep by orexin receptor antagonism to prevent abnormal tau phosphorylation and limit tau-induced damage.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Numerical source data files are available as supplementary data files. RNA-seq data are publicly available from the Gene Expression Omnibus under accession code GSE283736. Source data are provided with this paper.
References
Bubu, O. M. et al. Obstructive sleep apnea, cognition and Alzheimer’s disease: a systematic review integrating three decades of multidisciplinary research. Sleep. Med. Rev. 50, 101250 (2020).
Shi, L. et al. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep. Med. Rev. 40, 4–16 (2018).
Lew, C. H., Petersen, C., Neylan, T. C. & Grinberg, L. T. Tau-driven degeneration of sleep- and wake-regulating neurons in Alzheimer’s disease. Sleep. Med. Rev. 60, 101541 (2021).
Eser, R. A. et al. Selective vulnerability of brainstem nuclei in distinct tauopathies: a postmortem study. J. Neuropathol. Exp. Neurol. 77, 149–161 (2018).
Oh, J. et al. Profound degeneration of wake-promoting neurons in Alzheimer’s disease. Alzheimers Dement. 15, 1253–1263 (2019).
Parhizkar, S. & Holtzman, D. M. The night’s watch: exploring how sleep protects against neurodegeneration. Neuron 113, 817–837 (2025).
Malpetti, M., La Joie, R. & Rabinovici, G. D. Tau beats amyloid in predicting brain atrophy in Alzheimer disease: implications for prognosis and clinical trials. J. Nucl. Med. 63, 830–832 (2022).
Lucey, B. P. et al. Reduced non-rapid eye movement sleep is associated with tau pathology in early Alzheimer’s disease. Sci. Transl. Med. 11, eaau6550 (2019).
Holth, J. K., Mahan, T. E., Robinson, G. O., Rocha, A. & Holtzman, D. M. Altered sleep and EEG power in the P301S Tau transgenic mouse model. Ann. Clin. Transl. Neurol. 4, 180–190 (2017).
Holth, J. K. et al. The sleep–wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 363, 880–884 (2019).
Zhu, Y. et al. Chronic sleep disruption advances the temporal progression of tauopathy in P301S mutant mice. J. Neurosci. 38, 10255–10270 (2018).
Verster, J. C. et al. Residual effects of middle-of-the-night administration of zaleplon and zolpidem on driving ability, memory functions, and psychomotor performance. J. Clin. Psychopharmacol. 22, 576–583 (2002).
Morgan, P. T., Kehne, J. H., Sprenger, K. J. & Malison, R. T. Retrograde effects of triazolam and zolpidem on sleep-dependent motor learning in humans. J. Sleep. Res. 19, 157–164 (2010).
Robbins, R. et al. Sleep medication use and incident dementia in a nationally representative sample of older adults in the US. Sleep. Med. 79, 183–189 (2021).
Donnelly, K., Bracchi, R., Hewitt, J., Routledge, P. A. & Carter, B. Benzodiazepines, Z-drugs and the risk of hip fracture: a systematic review and meta-analysis. PLoS ONE 12, e0174730 (2017).
Sakurai, T. et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585 (1998).
de Lecea, L. et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl Acad. Sci. USA 95, 322–327 (1998).
Herring, W. J. et al. Polysomnographic assessment of suvorexant in patients with probable Alzheimer’s disease dementia and insomnia: a randomized trial. Alzheimers Dement. 16, 541–551 (2020).
Moline, M. et al. Safety and efficacy of lemborexant in patients with irregular sleep–wake rhythm disorder and Alzheimer’s disease dementia: results from a phase 2 randomized clinical trial. J. Prev. Alzheimers Dis. 8, 7–18 (2021).
Lucey, B. P. et al. Suvorexant acutely decreases tau phosphorylation and Aβ in the human CNS. Ann. Neurol. 94, 27–40 (2023).
Beuckmann, C. T., Ueno, T., Nakagawa, M., Suzuki, M. & Akasofu, S. Preclinical in vivo characterization of lemborexant (E2006), a novel dual orexin receptor antagonist for sleep/wake regulation. Sleep 42, zsz076 (2019).
Murphy, P. et al. Lemborexant, a dual orexin receptor antagonist (DORA) for the treatment of insomnia disorder: results from a Bayesian, adaptive, randomized, double-blind, placebo-controlled study. J. Clin. Sleep. Med. 13, 1289–1299 (2017).
Yardley, J. et al. Long-term effectiveness and safety of lemborexant in adults with insomnia disorder: results from a phase 3 randomized clinical trial. Sleep. Med. 80, 333–342 (2021).
Kang, J. E. et al. Amyloid-β dynamics are regulated by orexin and the sleep–wake cycle. Science 326, 1005–1007 (2009).
Roh, J. H. et al. Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer’s disease. J. Exp. Med. 211, 2487–2496 (2014).
Josephs, K. A. et al. β-Amyloid burden is not associated with rates of brain atrophy. Ann. Neurol. 63, 204–212 (2008).
Shi, Y. et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549, 523–527 (2017).
Yoshiyama, Y. et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53, 337–351 (2007).
Shi, Y. et al. Microglia drive APOE-dependent neurodegeneration in a tauopathy mouse model. J. Exp. Med. 216, 2546–2561 (2019).
Mancuso, R. et al. CSF1R inhibitor JNJ-40346527 attenuates microglial proliferation and neurodegeneration in P301S mice. Brain 142, 3243–3264 (2019).
Keenan, R. J. et al. Differential sleep/wake response and sex differences following acute suvorexant, MK-1064 and zolpidem administration in the rTg4510 mouse model of tauopathy. Br. J. Pharmacol. 179, 3403–3417 (2022).
Chen, X. et al. Microglia-mediated T cell infiltration drives neurodegeneration in tauopathy. Nature 615, 668–677 (2023).
Krasemann, S. et al. The TREM2–APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47, 566–581 (2017).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290 (2017).
Yamada, K. et al. Neuronal activity regulates extracellular tau in vivo. J. Exp. Med. 211, 387–393 (2014).
Wu, J. W. et al. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat. Neurosci. 19, 1085–1092 (2016).
Ahmed, Z. et al. A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity. Acta Neuropathol. 127, 667–683 (2014).
Jicha, G. A. et al. cAMP-dependent protein kinase phosphorylations on tau in Alzheimer’s disease. J. Neurosci. 19, 7486–7494 (1999).
Beuckmann, C. T. et al. In vitro and in silico characterization of lemborexant (E2006), a novel dual orexin receptor antagonist. J. Pharmacol. Exp. Ther. 362, 287–295 (2017).
Guo, J. L. et al. Unique pathological tau conformers from Alzheimer’s brains transmit tau pathology in nontransgenic mice. J. Exp. Med. 213, 2635–2654 (2016).
Walsh, C. M. et al. Sleepless night and day, the plight of progressive supranuclear palsy. Sleep 40, zsx154 (2017).
McCurry, S. M. et al. Characteristics of sleep disturbance in community-dwelling Alzheimer’s disease patients. J. Geriatr. Psychiatry Neurol. 12, 53–59 (1999).
Keenan, R. J. et al. Orexin 2 receptor antagonism sex‐dependently improves sleep/wakefulness and cognitive performance in tau transgenic mice. Br. J. Pharmacol. 181, 87–106 (2024).
Gratuze, M. et al. TREM2-independent microgliosis promotes tau-mediated neurodegeneration in the presence of ApoE4. Neuron 111, 202–219 (2023).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Liu, Y. U. et al. Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat. Neurosci. 22, 1771–1781 (2019).
Stowell, R. D. et al. Noradrenergic signaling in the wakeful state inhibits microglial surveillance and synaptic plasticity in the mouse visual cortex. Nat. Neurosci. 22, 1782–1792 (2019).
Small, K. M., Brown, K. M., Forbes, S. L. & Liggett, S. B. Polymorphic deletion of three intracellular acidic residues of the α2B-adrenergic receptor decreases G protein-coupled receptor kinase-mediated phosphorylation and desensitization. J. Biol. Chem. 276, 4917–4922 (2001).
Ijiro, T. et al. Effect of rovatirelin, a novel thyrotropin-releasing hormone analog, on the central noradrenergic system. Eur. J. Pharmacol. 761, 413–422 (2015).
Leonard, C. S. & Kukkonen, J. P. Orexin/hypocretin receptor signalling: a functional perspective. Br. J. Pharmacol. 171, 294–313 (2014).
Kukkonen, J. P. & Leonard, C. S. Orexin/hypocretin receptor signalling cascades. Br. J. Pharmacol. 171, 314–331 (2014).
Chidambaram, H. & Chinnathambi, S. G-Protein coupled receptors and tau-different roles in Alzheimer’s disease. Neuroscience 438, 198–214 (2020).
Scott, C. W. et al. Phosphorylation of recombinant tau by cAMP-dependent protein kinase. Identification of phosphorylation sites and effect on microtubule assembly. J. Biol. Chem. 268, 1166–1173 (1993).
Illenberger, S. et al. The endogenous and cell cycle-dependent phosphorylation of tau protein in living cells: implications for Alzheimer’s disease. Mol. Biol. Cell 9, 1495–1512 (1998).
Liu, F. et al. PKA modulates GSK‐3β‐ and cdk5‐catalyzed phosphorylation of tau in site‐ and kinase‐specific manners. FEBS Lett. 580, 6269–6274 (2006).
Luo, J., Phan, T. X., Yang, Y., Garelick, M. G. & Storm, D. R. Increases in cAMP, MAPK activity, and CREB phosphorylation during REM sleep: implications for REM sleep and memory consolidation. J. Neurosci. 33, 6460–6468 (2013).
Abel, T. et al. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88, 615–626 (1997).
Vecsey, C. G. et al. Sleep deprivation impairs cAMP signalling in the hippocampus. Nature 461, 1122–1125 (2009).
Huynh, T.-P. V. et al. Lack of hepatic apoE does not influence early Aβ deposition: observations from a new APOE knock-in model. Mol. Neurodegener. 14, 37 (2019).
Willie, J. T. et al. Distinct narcolepsy syndromes in orexin receptor-2 and orexin null mice. Neuron 38, 715–730 (2003).
Barger, Z., Frye, C. G., Liu, D., Dan, Y. & Bouchard, K. E. Robust, automated sleep scoring by a compact neural network with distributional shift correction. PLoS ONE 14, e0224642 (2019).
Yaghouby, F., Donohue, K. D., O’Hara, B. F. & Sunderam, S. Noninvasive dissection of mouse sleep using a piezoelectric motion sensor. J. Neurosci. Methods 259, 90–100 (2016).
Acknowledgements
We thank Eisai for providing lemborexant. We would like to thank the Genome Technology Access Center in the Department of Genetics at Washington University School of Medicine for help with genomic analysis. This study was supported by the National Institutes of Health (grants P01NS074969, RF1NS090934 and RF1AG061776 to D.M.H.), the Freedom Together Foundation (to D.M.H.), the Alzheimer’s Association (AARF-21-850865 to S.P.) and the COBRAS Feldman Fellowship (to S.P.).
Author information
Authors and Affiliations
Contributions
S.P. and D.M.H. designed the study. S.P. and X.B. orally gavaged the mice and collected and analyzed the Piezosleep data. S.P., X.B., C.L., G.G. and J.R.S. perfused and collected samples. N.R., W.C., S.P., E.C.L. and M.W. collected and analyzed all EEG/EMG data. Y.C. and S.S. performed the AD-tau extract preparation and intrahippocampal injections. E.T. performed the RNA-sequencing analysis. S.P., X.B., G.G., M.K. and E.F. performed and analyzed the immunohistochemistry, imaging, RNA and protein extraction, qPCR, immunoblotting and ELISA experiments. M.M. and X.B. performed and analyzed the NFL SIMOA data. S.P., X.B., G.G. and M.E.B. maintained the mouse colony. C.M.Y. collected and analyzed all behavioral experiments. S.P. wrote the draft. D.M.H. reviewed and edited the paper. All authors discussed the results and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
D.M.H. is an inventor on a patent licensed by Washington University to C2N Diagnostics on the therapeutic use of anti-tau antibodies; cofounded and is on the scientific advisory board of C2N Diagnostics; is on the scientific advisory board of Denali, Genentech, Cajal Neuroscience and Switch Therapeutics and consults for Pfizer and Roche. The other authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Li Gan, Tara Spires-Jones and Sigrid Veasey for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Lemborexant influences sleep–wake behavior but does not influence reactive microglia or astroglia in non-tau transgenic E4 mice.
a, EEG analyses of percentage time spent in NREM sleep (nE4 = 10 mice/group, nP301S/E4 = 8 mice/group, two-way ANOVA, Fisher’s LSD test), REM sleep (nE4 = 10 mice per group, nP301S/E4 = 8 mice/group, two-way ANOVA, Fisher’s LSD test) and awake (nE4 = 10 mice/group, nP301S/E4 = 8 mice/group; two-way ANOVA and Fisher’s LSD test) in Veh- and Lem-treated E4 and P301S/E4 male mice at ZT13. b, EEG analyses of percentage sleep in the first 4 h after gavage (n = 4 mice/group; two-tailed t test) as well as percentage time spent in NREM sleep (n = 4 mice/group; one-way ANOVA and Dunnett’s post hoc test), REM sleep (n = 4 mice/group; Kruskal–Wallis test) and awake (n = 4 mice/group; one-way ANOVA and Dunnett’s post hoc test) in female P301S/E4 mice. c, Representative confocal images of Iba1 (green), Cd68 (red) and Tmem119 (yellow) stained reactive microglia in Ent/Pcx of Veh or Lem ZT13 treated E4 male mice. Scale bar, 20 μm. d–g, Quantifications as labeled (Iba1: nVeh = 15, nLem ZT13 = 16, two-tailed t test; Cd68: nVeh = 14, nLem ZT13 = 18, two-tailed t test, p = 0.9721; Tmem119: nVeh = 17, nLem ZT13 = 20, two-tailed t test; Gfap: nVeh = 16, nLem ZT13 = 17, two-tailed t test). For detailed statistical information, see Supplementary Table 1. Data represent mean ± s.e.m. of biological replicates; *P < 0.05, *P < 0.01, ***P< 0.001, and ****P<0.0001.
Extended Data Fig. 2 Behavioral assessment across different treatment groups in P301S/E4 mice.
a, Percentage freezing quantified during tone–shock/pairing (males: nVeh = 19, nZol ZT13 = 14, nLem ZT3 = 12, nLem ZT13 = 13, two-way ANOVA, Dunnett’s post hoc test; females: nVeh = 17, nLem ZT3 = 15, nLem ZT13 = 9, two-way ANOVA, Dunnett’s post hoc test); b, contextual conditioning (males: nVeh = 19, nZol ZT13 = 14, nLem ZT3 = 12, nLem ZT13 = 13, two-way ANOVA, Dunnett’s post hoc test; females: nVeh = 17, nLem ZT3 = 15, nLem ZT13 = 9, two-way ANOVA, Dunnett’s post hoc test); c, auditory cue conditioning (males: nVeh = 19, nZol ZT13 = 14, nLem ZT3 = 9, nLem ZT13 = 13, two-way ANOVA, Dunnett’s post hoc test; females: nVeh = 17, nLem ZT3 = 15, nLem ZT13 = 9, two-way ANOVA, Dunnett’s post hoc test). d, Percentage alternation rate quantified in Y-maze alternation test (males: nVeh = 19, nZol ZT13 = 14, nLem ZT3 = 12, nLem ZT13 = 13, one-way ANOVA, Dunnett’s post hoc test; females: nVeh = 17, nLem ZT3 = 14, nLem ZT13 = 9, one-way ANOVA). For detailed statistical information, see Supplementary Table 1. Data represent mean ± s.e.m. in biological replicates. *P < 0.05, and ** P< 0.01.
Extended Data Fig. 3 Orexin receptor antagonism does not influence cAMP/PKA-mediated phosphorylation of tau in P301S/E4 female mice.
a, DAG (nVeh = 15, nLem ZT3 = 15, nLem ZT13 = 12; Kruskal–Wallis and Dunn’s post hoc test); b, cAMP (nVeh = 14, nLem ZT3 = 16, nLem ZT13 = 16; one-way ANOVA and Dunnett’s post hoc test) ELISAs of RIPA-soluble Ent/Pcx extracts. c, Immunoblots of RIPA-soluble Ent/Pcx brain extracts from Veh- and Lem-treated female P301S/E4 mice (n = 3 mice/group). d–h, Quantification of the western blot intensity of protein kinases and cell signaling proteins (for d, e and g—nVeh = 9, nLem ZT3 = 9, nLem ZT13 = 8; for f—nVeh = 9, nLem ZT3 = 9, nLem ZT13 = 7; for h—nVeh = 9, nLem ZT3 = 8, nLem ZT13 = 8). All proteins were first normalized to Gapdh, and the ratio of phosphorylated to their corresponding unphosphorylated form was then calculated. PHF1 was normalized to Gapdh. (d—Kruskal-Wallis test and Dunn’s post hoc test; e—one-way ANOVA, Dunnett’s post hoc test; f—one-way ANOVA, Dunnett’s post hoc test; g—Kruskal–Wallis test and Dunn’s post hoc test; h—one-way ANOVA, Dunnett’s post hoc test). For detailed statistical information, see Supplementary Table 1. Data represent mean ± s.e.m. of biological replicates. *P< 0.05, and ****P < 0.0001.
Extended Data Fig. 4 Gene expression levels of orexin-mediated GPCR signaling molecules.
a–i, Gene expression levels of orexin receptors and downstream effectors in naïve male P301S/E4 mice compared at ZT3 and ZT13 (n = 6 mice/group; a–d, f–i, two-tailed t test; e—Mann–Whitney test), and (j–r) Veh- and Lem-treated male mice (j—nVeh = 9, nLem ZT3 = 7, nLem ZT13 = 7; one-way ANOVA and Dunnett’s post hoc test; k—nVeh = 9, nLem ZT3 = 7, nLem ZT13 = 7; Kruskal–Wallis test and Dunn’s post hoc test; l—nVeh = 8, nLem ZT3 = 7, nLem ZT13 = 7; Kruskal–Wallis test and Dunn’s post hoc test; m—nVeh = 8, nLem ZT3 = 7, nLem ZT13 = 7; one-way ANOVA and Dunnett’s post hoc test; n—nVeh = 9, nLem ZT3 = 7, nLem ZT13 = 7; Kruskal–Wallis test and Dunn’s post hoc test; o—nVeh = 9, nLem ZT3 = 7, nLem ZT13 = 7; one-way ANOVA and Dunnett’s post hoc test; p—nVeh = 9, nLem ZT3 = 7, nLem ZT13 = 7; one-way ANOVA, p = 0.5522; Dunnett’s post hoc test; q—nVeh = 9, nLem ZT3 = 7, nLem ZT13 = 7; Kruskal–Wallis and Dunn’s post hoc test; r—nVeh = 9, nLem ZT3 = 7, nLem ZT13 = 7; one-way ANOVA and Dunnett’s post hoc test). For detailed statistical information, see Supplementary Table 1. Data represent mean ± s.e.m. of biological replicates.
Extended Data Fig. 5 Pharmacological or genetic lack of orexin receptor signaling alters sleep–wake behavior and decreases tau propagation.
a, Sleep–wake behavior quantified by percentage sleep (two-way ANOVA and Sidak’s post hoc test), sleep bout lengths (two-way ANOVA and Sidak’s post hoc test) and wake bout lengths (two-way ANOVA and Sidak’s post hoc test) during the light and dark phase at 7.5 M (nOXR2-WT = 8, nOXR2-WT Lem = 7, nOXR2-KO = 8) male mice. b, Sleep–wake behavior quantified by percentage sleep (two-way ANOVA and Sidak’s post hoc test), sleep bout lengths (two-way ANOVA and Sidak’s post hoc test) and wake bout lengths (two-way ANOVA and Sidak’s post hoc test) during the light and dark phase at 9.5 M (nOXR2-WT = 8, nOXR2-WT Lem = 7, nOXR2-KO = 8) in male mice. c, Percentage sleep in dark phase compared between mice at 7.5 M and 9.5 M (both timepoints: nOXR2-WT = 8, nOXR2-WT Lem = 7, nOXR2-KO = 8; two-way ANOVA and Bonferroni’s post hoc test). d, Wake bout lengths compared between mice at 7.5 M and 9.5 M during dark phase (both timepoints: nOXR2-WT = 8, nOXR2-WT Lem = 7, nOXR2-KO = 8; two-way ANOVA and Bonferroni’s post hoc test). e, Representative images of PG5-stained anterior (ant; top) and posterior (pos; bottom) Hpc. Scale bar, 500 μm. f, Percentage PG5-covered dentate gyrus quantified anterior (nOXR2-WT = 8, nOXR2-WT Lem = 7, nOXR2-KO = 8 male mice; one-way ANOVA and Dunnett’s post hoc test); g, posterior to the seeding site (one-way ANOVA and Dunnett’s post hoc test). h, Representative images of AT8-stained anterior and posterior Hpc. Scale bar, 500 μm. i, Percentage AT8-covered dentate gyrus quantified anterior (nOXR2-WT = 8, nOXR2-WT Lem = 7, nOXR2-KO = 8 male mice; one-way ANOVA and Dunnett’s post hoc test); j, posterior to the seeding site (Kruskal–Wallis test and Dunn’s post hoc test). For detailed statistical information, see Supplementary Table 1. Data represent mean ± s.e.m. in biological replicates. *P< 0.05, **P< 0.01, ***P<0.001, and ****P < 0.0001.
Supplementary information
Supplementary Table 1
Detailed statistical data for Figs. 1–7 and Extended Data Figs. 1–5.
Source data
Source Data Figs. 6e and 7h and Extended Data Fig. 3c
Unprocessed western blots.
Source Data Figs. 1–7 and Extended Data Figs. 1–5
Numerical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parhizkar, S., Bao, X., Chen, W. et al. Lemborexant ameliorates tau-mediated sleep loss and neurodegeneration in males in a mouse model of tauopathy. Nat Neurosci (2025). https://doi.org/10.1038/s41593-025-01966-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41593-025-01966-7