Abstract
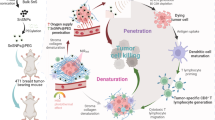
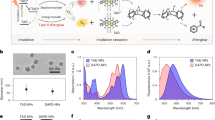
Optical imaging of tumor biomarkers provides key diagnostic information about tumor status. Light-induced afterglow (photoafterglow) imaging provides a higher signal to background ratio than typical fluorescence imaging; however, both modalities face challenges in detecting biomarkers in deep tissues owing to the limited penetration depth of light. Here we provide instructions for synthesizing ultrasound-induced afterglow (sonoafterglow) nanoprobes (SNAP) for the deep-tissue imaging of peroxynitrite (ONOO−), a biomarker specific for M1 macrophages and a proinflammatory tumor microenvironment. SNAPs are coassembled from initiators, afterglow substrates and amphiphilic polymers via the film rehydration method, a generic and facile approach that enables their rapid nanoconstruction (in 10 min), with high reproducibility, while also providing control over the nanoprobe concentration, which overcomes limitations of traditional nanoconstruction methods including solvent injection and emulsion–solvent evaporation. Following ultrasound stimulation, SNAPs emit sonoafterglow with bright near-infrared emission (peaking at 780 nm), with a long half-life (~2 min), and can be detected through biological tissues twice deeper than photoafterglow. We further develop SNAP into SNAP-M, which can be switched on only in the presence of ONOO−, allowing the real-time in vivo imaging of a proinflammatory tumor microenvironment at an unprecedented tissue depth for optical imaging. This Protocol can be implemented by users with expertise in material science in 1 week for nanoprobe construction and characterization, 1–2 week for cell assays and 3–4 weeks for animal experiments.
Key points
-
The Protocol uses the film rehydration method for the formation of a thin film containing the sonoafterglow initiator, substrate and amphiphilic stabilizer, which are rehydrated to a monodispersed nanoparticle solution. The nanoparticles produced emit long-lasting luminescence following stimulation with ultrasound for the detection of deep-tissue peroxynitrite.
-
Alternative methods are time-consuming and suffer from interbatch variations, whereas the film rehydration method is efficient, reproducible and applicable to various solvents.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All relevant data supporting the findings of this study are available within the article and its supplementary information or from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Michalet, X. et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307, 538–544 (2005).
Gao, X. H., Cui, Y. Y., Levenson, R. M., Chung, L. W. K. & Nie, S. M. Cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 22, 969–976 (2004).
Jiang, Y. Y. & Pu, K. Y. Molecular probes for autofluorescence-free optical imaging. Chem. Rev. 121, 13086–13131 (2021).
Miao, Q. Q. et al. Molecular afterglow imaging with bright, biodegradable polymer nanoparticles. Nat. Biotechnol. 35, 1102–1110 (2017).
Jiang, Y. Y. et al. A generic approach towards afterglow luminescent nanoparticles for ultrasensitive in vivo imaging. Nat. Commun. 10, 2064 (2019).
Xie, C., Zhen, X., Miao, Q. Q., Lyu, Y. & Pu, K. Y. Self-assembled semiconducting polymer nanoparticles for ultrasensitive near-infrared afterglow imaging of metastatic tumors. Adv. Mater. 30, 1801331 (2018).
Maldiney, T. et al. The in vivo activation of persistent nanophosphors for optical imaging of vascularization, tumors and grafted cells. Nat. Mater. 13, 418–426 (2014).
Wei, X. et al. Highly bright near-infrared chemiluminescent probes for cancer imaging and laparotomy. Angew. Chem. Int. Ed. Engl. 62, e202213791 (2023).
Lin, Y. et al. Highly photoreactive semiconducting polymers with cascade intramolecular singlet oxygen and energy transfer for cancer-specific afterglow theranostics. J. Am. Chem. Soc. 147, 2597–2606 (2025).
Zhu, J., Zhao, L., An, W. & Miao, Q. Recent advances and design strategies for organic afterglow agents to enhance autofluorescence-free imaging performance. Chem. Soc. Rev. 54, 1429–1452 (2025).
Qu, R., Jiang, X. & Zhen, X. Light/X-ray/ultrasound activated delayed photon emission of organic molecular probes for optical imaging: mechanisms, design strategies, and biomedical applications. Chem. Soc. Rev. 53, 10970–11003 (2024).
Canavese, G. et al. Nanoparticle-assisted ultrasound: a special focus on sonodynamic therapy against cancer. Chem. Eng. J. 340, 155–172 (2018).
Ouyang, J. et al. Ultrasound mediated therapy: recent progress and challenges in nanoscience. Nano Today 35, 100949 (2020).
Zhang, Z. et al. Ultrasound-chargeable persistent luminescence nanoparticles to generate self-propelled motion and photothermal/NO therapy for synergistic tumor treatment. ACS Nano 17, 16089–16106 (2023).
Mohammadpour, R., Dobrovolskaia, M. A., Cheney, D. L., Greish, K. F. & Ghandehari, H. Subchronic and chronic toxicity evaluation of inorganic nanoparticles for delivery applications. Adv. Drug Deliv. Rev. 144, 112–132 (2019).
Soenen, S. J. et al. Cellular toxicity of inorganic nanoparticles: common aspects and guidelines for improved nanotoxicity evaluation. Nano Today 6, 446–465 (2011).
Xu, C. & Pu, K. Illuminating cancer with sonoafterglow. Nat. Photon. 18, 301–302 (2024).
Szabo, C., Ischiropoulos, H. & Radi, R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 6, 662–680 (2007).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Wynn, T. A., Chawla, A. & Pollard, J. W. Macrophage biology in development, homeostasis and disease. Nature 496, 445–455 (2013).
Cheng, D. et al. Selective visualization of the endogenous peroxynitrite in an inflamed mouse model by a mitochondria-targetable two-photon ratiometric fluorescent probe. J. Am. Chem. Soc. 139, 285–292 (2017).
Wu, L. et al. Fluorescent probe for the imaging of superoxide and peroxynitrite during drug-induced liver injury. Chem. Sci. 12, 3921–3928 (2021).
Wang, W. et al. Activatable two-photon near-infrared fluorescent probe tailored toward peroxynitrite in vivo imaging in tumors. Anal. Chem. 92, 13305–13312 (2020).
Chen, C. et al. Amplification of activated near-infrared afterglow luminescence by introducing twisted molecular geometry for understanding neutrophil-involved diseases. J. Am. Chem. Soc. 144, 3429–3441 (2022).
Gao, Z. et al. An activatable near-infrared afterglow theranostic prodrug with self-sustainable magnification effect of immunogenic cell death. Angew. Chem. Int. Ed. Engl. 61, e202209793 (2022).
Xu, C. et al. Nanoparticles with ultrasound-induced afterglow luminescence for tumor-specific theranostics. Nat. Biomed. Eng. 7, 298–312 (2023).
Ni, X. et al. Near-infrared afterglow luminescent aggregation-induced emission dots with ultrahigh tumor-to-liver signal ratio for promoted image-guided cancer surgery. Nano Lett. 19, 318–330 (2019).
Chen, C., Zhang, X., Gao, Z., Feng, G. & Ding, D. Preparation of AIEgen-based near-infrared afterglow luminescence nanoprobes for tumor imaging and image-guided tumor resection. Nat. Protoc. 19, 1–27 (2024).
Jiang, Y. et al. Acidity-activatable upconversion afterglow luminescence cocktail nanoparticles for ultrasensitive in vivo imaging. Nat. Commun. 15, 2124 (2024).
Wu, L. et al. H2S-activatable near-infrared afterglow luminescent probes for sensitive molecular imaging in vivo. Nat. Commun. 11, 446 (2020).
Dasgupta, A., Sofias, A. M., Kiessling, F. & Lammers, T. Nanoparticle delivery to tumors: from EPR and ATR mechanisms to clinical impact. Nat. Rev. Bioeng. 2, 714–716 (2024).
Jiang, Y., Huang, J., Xu, C. & Pu, K. Activatable polymer nanoagonist for second near-infrared photothermal immunotherapy of cancer. Nat. Commun. 12, 742 (2021).
Xu, C., Jiang, Y., Han, Y., Pu, K. & Zhang, R. A polymer multicellular nanoengager for synergistic NIR-II photothermal immunotherapy. Adv. Mater. 33, e2008061 (2021).
Cavallaro, G. et al. PHEA–PLA biocompatible nanoparticles by technique of solvent evaporation from multiple emulsions. Int. J. Pharm. 495, 719–727 (2015).
Schaeffel, D. et al. Fluorescence correlation spectroscopy directly monitors coalescence during nanoparticle preparation. Nano Lett. 12, 6012–6017 (2012).
Huang, J. et al. Molecular radio afterglow probes for cancer radiodynamic theranostics. Nat. Mater. 22, 1421–1429 (2023).
Xu, C. et al. A cascade X-ray energy converting approach toward radio-afterglow cancer theranostics. Nat. Nanotechnol. 20, 286–295 (2025).
Wells, P. N. Review: absorption and dispersion of ultrasound in biological tissue. Ultrasound Med. Biol. 1, 369–376 (1975).
Xu, C. et al. Activatable sonoafterglow nanoprobes for T-cell imaging. Adv. Mater. 35, e2211651 (2023).
Wei, X. et al. Leveraging long-distance singlet-oxygen transfer for bienzyme-locked afterglow imaging of intratumoral granule enzymes. J. Am. Chem. Soc. 146, 17393–17403 (2024).
Huang, W. et al. Ratiometric afterglow luminescent imaging of matrix metalloproteinase-2 activity via an energy diversion process. Angew. Chem. Int. Ed. Engl. 63, e202404244 (2024).
Liu, Y. et al. Ratiometric afterglow luminescent nanoplatform enables reliable quantification and molecular imaging. Nat. Commun. 13, 2216 (2022).
Chen, F. et al. Ultrasmall targeted nanoparticles with engineered antibody fragments for imaging detection of HER2-overexpressing breast cancer. Nat. Commun. 9, 4141 (2018).
Graf, N. et al. α(V)β(3) integrin-targeted PLGA–PEG nanoparticles for enhanced anti-tumor efficacy of a Pt(IV) prodrug. ACS Nano 6, 4530–4539 (2012).
Rao, L., Yuan, Y., Shen, X., Yu, G. & Chen, X. Designing nanotheranostics with machine learning. Nat. Nanotechnol. 19, 1769–1781 (2024).
Adir, O. et al. Integrating artificial intelligence and nanotechnology for precision cancer meldicine. Adv. Mater. 32, e1901989 (2020).
Cheong, J. et al. Engineered nanoparticles for clinical assays. Nat. Rev. Bioeng. 2, 887–905 (2024).
Xu, M. et al. Sonodynamic cytokine nanocomplexes with specific stimulation towards effector T cell for combination cancer immunotherapy. Angew. Chem. Int. Ed. Engl. 62, e202308362 (2023).
Yu, J. et al. Polymeric STING pro-agonists for tumor-specific sonodynamic immunotherapy. Angew. Chem. Int. Ed. Engl. 62, e202307272 (2023).
He, S. et al. A semiconducting iron-chelating nano-immunomodulator for specific and sensitized sono-metallo-immunotherapy of cancer. Angew. Chem. Int. Ed. Engl. 62, e202310178 (2023).
Yang, F. et al. Principles and applications of sono-optogenetics. Adv. Drug Deliv. Rev. 194, 114711 (2023).
Huang, J., Jiang, Y., Li, J., Huang, J. & Pu, K. Molecular chemiluminescent probes with a very long near-infrared emission wavelength for in vivo imaging. Angew. Chem. Int. Ed. Engl. 60, 3999–4003 (2021).
Su, X. et al. Interferon-gamma regulates cellular metabolism and mRNA translation to potentiate macrophage activation. Nat. Immunol. 16, 838–849 (2015).
Acknowledgements
K.P. thanks the Singapore National Research Foundation (grant no. NRF-NRFI07-2021-0005) and the Singapore Ministry of Education Academic Research Fund Tier2 (grant nos. MOE-T2EP30220-0010 and MOE-T2EP30221-0004) for financial support.
Author information
Authors and Affiliations
Contributions
K.P. and C.X. conceived and developed the Protocol. C.X. conducted in vitro and in vivo assays and drafted the manuscript. J.H. conducted the synthesis. K.P. contributed to the discussion and manuscript review. All authors contributed to the writing of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Hak Soo Choi, Cyrille Richard and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key references
Xu, C. et al. Nat. Biomed. Eng. 7, 298–312 (2023): https://doi.org/10.1038/s41551-022-00978-z
Xu, C. et al. Adv. Mater. 35, e2211651 (2023): https://doi.org/10.1002/adma.202211651
Supplementary information
Supplementary Information
Supplementary Figs. 1–5 and methods
Source data
Source Data Fig. 6
Statistical Source data
Source Data Fig. 7
Statistical Source data
Source Data Fig. 8
Statistical Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, C., Huang, J. & Pu, K. Sonoafterglow nanoprobes for deep-tissue imaging of peroxynitrite. Nat Protoc (2025). https://doi.org/10.1038/s41596-025-01202-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41596-025-01202-3