Abstract
Species with sympatric distribution influence ecosystem dynamics and are impacted by the presence of other co-existing species. Assessing the coexistence and the role of interspecific interactions with the landscape variables is necessary to know the species co-occurrence in space. In the Indian Himalayan region, such studies are completely lacking due to limited efforts being made, mainly because of complex terrains and inaccessible landscape features. We used camera trapping and sign survey in a multi-species occupancy framework to understand the influence of environmental variables on occupancy and detection probability of species-specific and pair-wise interaction of the three ungulates in Uttarkashi. Our results concluded that individual species' occupancy probabilities were related both to the environmental variables and the presence or absence of other interacting species. Our top model showed evidence of interspecific interaction among species pairs, and the occupancy probability of species one varied in the presence or absence of another species. The overall activity patterns were similar among all the three species and were found active throughout the day. The activity overlap between sambar—barking deer (Dhat1 value = 0.85) was considerably higher than barking deer—goral (Dhat1 value = 0.78). The findings of the present study will be useful for the conservation and management of ungulates in the Indian Himalayan and adjoining regions.
Similar content being viewed by others
Introduction
In the Himalayas, the ungulates form the major prey base for the large predators and are good ecological indicators, more prone to anthropogenic disturbance and habitat quality1,2. Conservation strategies are primarily focused on the concept of multiple-species conservation in protecting endangered species and assessing biodiversity within entire communities3,4. Moreover, several studies demonstrated that ecological communities are composed of multiple interacting species, and species occurrence can be influenced by environmental factors and even the presence of other species5,6. Hence understanding those factors (environmental and species interactions) which influence the distribution of species is of fundamental interest in wildlife conservation and management. Multi-species occupancy modeling provides a method for assessing biodiversity while accounting for multiple sources of uncertainty, imperfect detections and sampling designs7,8. However, several studies highlighted that species occupancy probabilities are often influenced by many factors such as environmental factors and the presence or absence of interacting species, e.g. Predator’s presence for prey9,10, or habitat utilization may be impacted by the presence of other species11.
Among the ungulates, Barking deer (Muntiacus vaginalis), Himalayan goral (Naemorhedus goral), and Sambar deer (Rusa unicolor) have sympatric distribution in Himalayan regions and play an essential role in shaping ecosystems by influencing vegetation structure, forming a major prey base for carnivores and act as ecological indicators12,13. However, these species are threatened due to habitat loss, illegal poaching, climate change and interspecific competition14,15,16. Among the three studied species, the barking deer is widely distributed from eastern Pakistan, India, Nepal and up to south-east Asian countries, and mostly prefers high understory dense tropical and sub-tropical forest17,18,19. Barking deer also have a wide elevation distribution (100–3000 m) and are mostly found at the edge of forested habitats20. Whereas the Himalayan goral adapted to high elevation (900 to 2750 m) areas, it is a cliff-dwelling, solitary, sexually monomorphic mountain ungulate. Its distribution encompasses the Himalayan range of India, Nepal and Bhutan with densely forested areas to the sub-alpine scrub, alpine meadow and grassland21,22. The sambar is a large mesoherbivores deer native to South and South-East Asia and distributed in habitats with high tree and shrub densities23.
As per the IUCN Red List, barking deer is listed as ‘Least concern’20, sambar as ‘Vulnerable’23 and himalayan goral as ‘Near threatened’ species24. All three species are listed among the Schedule-III of the Indian Wild-Life (Protection) Act, 1972, and are seriously threatened because of illegal poaching. Since all three species are symmetrically distributed in Himalayan regions, such sympatric distribution of species may face exclusion25, avoidance26 or sometimes extinction due to increasing inter-specific competition27. Furthermore, limited studies on habitat use28, behaviour29, distributions30,31,32,33, genetics22,23 of these ungulates species is available but completely lacking evaluations on their occupancy and species interactions throughout their distribution ranges, including the Himalayan regions.
The Himalayan region is under tremendous pressure because of infrastructural development, climate change and other anthropogenic activities34,35. These events lead to rapid habitat loss, fragmentation, and population decline of various species. Therefore, understanding habitat association, occupancy and interspecies interaction are imperative for conservation and management. A better understanding of the ecology of the ungulate community may allow managers to more fully balance gains against losses when managing the diversity of wildlife35,36. Additionally, our research was motivated by the lack of broad-scale ungulate studies from the study area. This study explores the utility of camera trap surveys to understand species-specific and pair-wise interaction between three ungulate species using multi-species hierarchical models in the Uttarkashi district. These devices are helpful in areas with rugged topography or dense vegetation and, in recent years, have been used to study activity patterns, habitat use, density and occupancy of ungulates37,38,39. Modern cameras record the time of the photo, and researchers have used this to investigate diel activity patterns and compared activity patterns among species to see how overlapping patterns may relate to competition or predation40,41,42,43.
Our specific objectives were to evaluate species-specific responses to environmental variables and pair-wise interaction and the pattern of activity of the three ungulate species in Uttarkashi. We aimed to understand better how the environmental factors and interspecific interaction influence the occupancy of the three ungulates, which is vital for effective conservation and management implications.
Results
Occupancy of three sympatric species
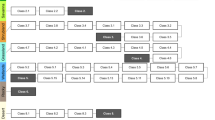
A sampling effort of 2819 camera trap nights across 62 camera sites was achieved in the Uttarkashi district. We obtained 30, 24, 9 detections of barking deer, goral and sambar across 62 sites and recorded 99, 92 and 82 indirect signs (faecal pellets) of each species. Our top model suggests evidence of interspecific dependence of the three species (f12, f13, (f23). The top model assumed the occupancy of the three species at different sites varied as a function of habitat variables ψ (FT 188 + Distance to the village) when detection probability was kept as constant p(.) (Table 1). This model suggests that the mean marginal occupancy probability of barking deer (f1) was positively influenced by distance to the village (β = 2.62 ± 1.13) and negatively influenced by FT 188 (West Himalayan sub-alpine birch/fir forest) (β = − 7.79 ± 0.00) (Table 2). We found that marginal occupancy of goral (f2) exhibits a positive relationship with variable FT 188 (β = 5.76 ± 0.00) and a negative relationship with variable distance to the village (β = − 2.43 ± 1.17). In contrast, marginal occupancy of sambar (f3) was positively influenced by FT 188 (β = 22.80 ± 0.00) and negatively influenced by distance to village (β = − 0.22 ± 0.59) (Table 2). Our top model results also showed evidence of interspecific interaction among species pairs (f12, f13, f23), which makes it very clear that the occupancy probability of species one varied in the presence or absence of another species (Fig. 1). We also found that the occupancy probability that two species occurred together varied as a function of FT 188 (Fig. 1). The influence of variable FT 188 on the co-occurrence of barking deer-goral (f12) and barking deer-sambar (f13) varied markedly. The relationship between the occurrence of barking deer (f1) and variable FT 188 varied markedly depending on whether goral (f2) and sambar (f3) were present. This model suggests that barking deer (f1) was more likely to occur together at sites where sambar (f3) was present (β = 12.80 ± 0.00), having FT 188 (West Himalayan sub-alpine birch/fir forest) as a variable function of occupancy, while both barking deer (f1) and goral (f2) (β = − 13.38 ± 0.00), and goral (f2) and sambar (f3) (β = − 11.05 ± 0.00), are less likely to occur together at sites with FT 188 (Fig. 1). Barking deer and sambar (f13) (β = 34.67 ± 163.39) were more likely to occur together than barking deer and goral (f12) (β = 16.50 ± 188.68) and goral and sambar (f23) (β = − 13.21 ± 188.68). This model also suggests barking deer–goral (f12) is negatively influenced by the variable (FT 188). The FT 188 showed negative influence on co-occurrence of barking deer-goral (f12) (β = − 13.38 ± 0.00) and goral-sambar (f23) (β = − 11.05 ± 0.00), while showed positive influence on occupancy of barking deer-sambar (f13) (β = 12.80 ± 0.00). The model also showed that the probability of two species occupying the same site as a function of covariates provided insight into factors driving marginal occupancy probabilities that might not have been evident otherwise. Our results concluded that individual species' occupancy probabilities were related both to environmental variables and the presence or absence of other interacting species (Fig. 1).
Species-specific and pair-wise interaction showing the influence of top model covariates (Green bars-forest type (West Himalayan Sub-alpine birch/fir Forest (FT 188) and Red bars distance to a village) on the occupancy of barking deer, goral and sambar in Uttarkashi. Microsoft office (MS version 2016) was used to create figures.
Activity pattern
The overall activity patterns were similar among all the three ungulate species. All the species were active throughout the day, with decreased activity between early morning, afternoon and late evening hours. Goral was active throughout the day with decreased activity in the early morning and evening hours (Fig. 2). In contrast, both barking deer and sambar were more active in the late morning and early evening hours (Fig. 2). The overlap of activity between sambar—barking deer (Dhat1 value = 0.85) was considerably higher than barking deer—goral (Dhat1 value = 0.78), and to a very lesser extent than goral—Sambar (Dhat1 = 0.81) (Fig. 2).
Temporal activity pattern of three ungulate species (goral, barking deer and sambar—top row) and overlapping activity pattern of barking deer-goral, Goral-sambar, and sambar-barking deer (Bottom row). Overlap package of R-Software (R.3.4.3, https://www.R-project.org) was used to create the figure.
Discussion
For identifying an area of conservation importance, it is vital to know the spatial distribution of the good quality habitat of the species. The distribution of species in space is influenced by various environmental factors and interactions with other species. Studies have modeled the occupancy of ungulates using the occupancy models15. The recent improvement in analytics has enhanced the capability of ecologists to model the detection/non-detection data for multiple species while incorporating imperfect detection in the data6,44,45,46. In this study, we evaluated the influence of environmental variables on occupancy and detection probability of species-specific (f1, f2, f3) and pair-wise interaction (f12, f13, f23) of three ungulate species in Uttarkashi. The top model assumed the occupancy of the all thethree species at different sites varied as a function of habitat variables ψ (FT 188 + Distance to village) when detection probability was kept as constant p(.) (Table 1). This model suggests that the mean marginal occupancy probability of barking deer (f1) was positively influenced by distance to the village and negatively influenced by FT 188 (West Himalayan sub-alpine birch/fir forest) (Table 2). We found that marginal occupancy of goral (f2) exhibits a positive relationship with variable FT 188 and a negative relationship with variable distance to the village. In contrast, marginal occupancy of sambar (f3) was positively influenced by FT 188 and negatively influenced by distance to the village (Table 2). Our top model results also showed evidence of interspecific interaction among species pairs (f12, f13, f23), which makes it very clear that the occupancy probability of species one varied in the presence or absence of another species (Fig. 1). Our study showed that the two species occupying the same site is a function of covariates that provides insight into factors driving marginal occupancy probabilities that might not have been evident otherwise6. Our results corroborate with the findings of other study6 that the occupancy probabilities of individual species are directly related to the habitat variables as well as the presence or absence of other interacting species.
This is the first attempt to model multi-species occupancy of ungulates from Uttarkashi, Uttarakhand, and the entire Himalayan region. We found evidence for pair-wise interactions (f12, f13, f23) among three ungulate species that varied along with environmental variables in the present study. Nonetheless, we observed the anticipated negative association of FT 188 with pair-wise interaction of f12 (barking deer-goral) and f23 (goral-sambar). However, pair-wise interaction showed; that occupancy of f13 (barking deer-sambar) was positively influenced by FT 188. Of the three species, goral (f2) showed the broadest elevation range covering up to 3600 m. Other studies on the ecology and distribution of goat-antelopes also indicated a broad elevation range for goral2,47,48,49 in western Himalayas. These studies indicated that goral could be found from very low elevation (500 m) to the tree line (4000 m). Since FT 188 is negatively influencing the pair-wise interaction of barking deer-goral and sambar-goral, we suggest that this might be because of resource partitioning, which leads the species to occupy different habitats. Goral inhabits steep mountainous areas and sometimes use forests near cliffs but primarily stays within rugged rocky terrain covering a broad elevation range. Whereas sambar and barking deer are forest dwellers, sambar (f3) prefers hilly areas with high tree density and showed the least affinity towards human settlement, while barking deer prefers plain areas30,50,51.
The model provides the probability of pair-wise interaction that two species occur together as a function of covariates. FT 188 positively influenced the marginal occupancy of goral and sambar and negatively influenced the marginal occupancy of barking deer. Since species-specific occupancy of barking deer (f1) was negatively influenced by FT 188 whereas FT 188 positively influenced the pair-wise interaction of barking deer-sambar (f13) which suggested barking deer (f1) was more likely to occur together at sites where sambar was present. Many species occur in sympatry where intraguild competition is frequent. Further, intraguild competition is an important determinant in structuring ecological communities, as it can lead to spatial or temporal segregation among species. Since we found evidences that the interaction between barking deer and sambar in FT 188 is positive, we suggest that the dietary overlap and resource competition of the two species might be small enough to allow coexistence.
Moreover, our findings were similar to that of52, which suggests barking deer also showed a positive association towards distance to the village, although barking deer (f1) prefers dense forest with high understory53. However, barking deer (f1) are also reported to occupy degraded tracts of forest ranges near human settlements. Their inability to live on rugged steep mountain slopes allows them to occupy forested habitats close to human habitations53. At the same time, goral (f2) and sambar (f3) showed a negative association toward distance to the village. Both the species are shy and mostly solitary thus, avoidance of villages was quite normal, similar to the results of54. The goral (f2) occupancy was associated with a wide variety of habitats throughout the mountain range28, and predominately, it is adapted to steep slopes with rugged mountain terrain50,55,56.
The activity pattern results suggested that the goral (f2) was active throughout the day with decreased activity in the early morning and evening hours (Fig. 2). In contrast, both the barking deer and sambar were more active in the late morning and early evening hours (Fig. 2). The goral was found active during the day due to its cleft dwelling nature, where it escapes from predators and the human presence. The overlap of activity between sambar—barking deer was considerably higher than barking deer—goral, and to a lesser extent, than goral—sambar, possibly due to different habitat preferences (Fig. 2). There is much similarity between all the three species; however, ecological separation nevertheless, the complete absence of sambar above 3200 m and avoidance of sambar to coniferous forests indicates a certain degree of ecological separation between the three species. Goral (f2) is probably associated with more tree cover in winter because of their vulnerability to predation and food availability during heavy snow conditions. Such factors could influence the increased use of tree cover by the goral in summer as the need to find shelter during the monsoon or the need to secure cover for parturition. The biotic and abiotic conditions are essential for understanding the interspecific interactions as they determine the site utilization by the species. Hence, researchers have recognized their imperatives as covariates for modelling the space use, habitat selection57, and distribution58 assessment of a species. Accounting for the interspecific interactions may be necessary when predicting future distributions, predominantly in understanding the impacts of global warming and climate change on the distribution of species and communities. Hence, the present multi-species occupancy model provides a strategy for understanding the interspecific interactions for imperfectly detected species.
Moreover, it may help in evaluating how the interspecific interactions shape habitat selection and species distributions. Among the three ungulate species studied, sambar and goral are threatened according to the IUCN category. Habitat loss, anthropogenic pressure, and wildlife hunting pose a formidable challenge for conserving wildlife58. The long-term survival of wildlife depends on sufficiently large areas of suitable habitat and decreasing anthropogenic pressure. The present findings may be helpful for the better conservation and management of the mountain ungulates in the Indian Himalayan Region and other adjoining areas. Hence, we propose that areas supporting suitable habitats for all three mountain ungulates should seldom have anthropogenic activity for proper management and conservation of the species given the influence of FT 188 on the occupancy of the three species, we would recommend plantation and assisted natural regeneration of the local plant species in suitable gap areas for supporting the ungulates in the landscape.
Complex terrain, inaccessibility and heavy snowfall are the limitations which make it difficult to study the species in the landscape. Due to weather conditions, heavy rainfall, landslides and snowfall during winters, we were not able to do sampling throughout the year, which is the major limitation of the study. Inferences have been made on the basis of short sampling effort, however, long-term study with intensive sampling is required for proper conservation and management of the ungulates. Though our data was collected relatively from a small area, information on the environmental factors governing the distribution of the ungulates would establish the baseline information on the distribution of ungulates in the landscape.
Material and methods
Study area
The present study was conducted in Uttarkashi district, Uttarakhand, located between 38° 28′ to 31°28′ N latitude and 77°49′ to 79°25′ E longitude with an area of about 8016 km2, covering primarily hilly terrain with an altitudinal range of 715–6717 m (Fig. 3). The terrain is mountainous, consisting of undulating hill ranges and narrow valleys with temperate climatic conditions. The district lies in the upper catchment of two major rivers of India, viz., the Ganges (Bhagirathi towards upstream) and the Yamuna. The major vegetation types of the study area are Himalayan moist temperate forest, sub-alpine forest and alpine scrub59. The Uttarkashi district forests are managed under three Forest Divisions viz., (i) Uttarkashi Forest Division (ii) Upper Yamuna Badkot Forest Division and (iii) Tons Forest Division) with two Protected Areas (PAs) (i) Gangotri National Park and (ii) Govind Pashu Vihar National Park. The forested habitats of the study landscape are home to top conservation priority species, including Asiatic Black bear (Ursus thibetanus), Musk deer (Moschus spp.), Common leopard (Panthera pardus), Himalayan brown bear (Ursus arctos isabellinus) and Western Tragopan (Tragopan melanocephalus), Himalayan monal (Lophophorus impejanus). The study was conducted after a study permit issued by the Chief Wildlife Warden, Forest Department, Uttarakhand government, vide letter no. 848/5-6 dated 31/08/2019, we have not handled the species for doing research. Instead, remote camera traps have been used for collecting the data with the permission of the Chief Wildlife Warden, Government of Uttarakhand. Further, informed consent was taken before interviewing the local communities. The data was collected according to the institutional guidelines and approved by the Research Advisory and Monitoring Committee of the Zoological Survey of India.
Map of the study area Uttarkashi, Uttarakhand. ArcGIS 10.6 (ESRI, Redlands, CA) was used to create the map. (Map created using ArcGIS 10.6; http://www.esri.com).
Sampling protocol
The basic sampling protocol and assumptions for multi-species occupancy modelling are identical to the single-species case7. Briefly, a set of 62 intensive sites, were randomly selected, and each site i was surveyed j times. During each survey, detection/non-detection of S focal species was recorded. Additionally, direct or indirect evidences of species presence from the different areas were also recorded.
Data collection
The complete study area was divided into 10 × 10 km grids, consisting of n = 60 grids. Based on the reconnaissance survey, out of these 60 grids, we selected 25 girds that were accessible to conduct the survey and have the species presence. Further, these grids were divided into 2 × 2 km grids to maximize our effort so that all logistically accessible grids could be covered, and we conducted intensive sampling in N = 62 grids after excluding the grids with human settlements. T The field surveys were conducted during 2018–2019, and a team of researchers systematically visited selected grids to collect data on the detection/non-detection of these ungulates. A total of 62 camera traps were deployed in selected grids, and 650 km were traversed, accounting for N = 54 trails in these sampled grids. These camera traps were visited once in every fifteen days for replacing the batteries as well as documenting the presence of the species through the sign surveys. The ultra-compact SPYPOINT FORCE-11D trail camera (SPYPOINT, GG Telecom, Canada, QC) and Browning trail camera (Defender 850, 20 MP, Prometheus Group, LLC Birmingham, Alabama, https://browningtrailcameras.com) camera traps were used to detect the presence/absence of ungulate species. The cameras were mounted 40–60 cm above ground on natural trails without lures.
Data exploration
While deploying camera traps, we also noted habitat variables through on-site observation such as distance to the village and human disturbance. We tested site covariates for collinearity and discarded one of a pair if the Pearson’s correlation was greater than 0.760. Hence, we assumed each of the site covariates could influence the occupancy and detectability of these ungulates.
Covariates
We hypothesized that habitat variables may influence these ungulates' occupancy and detection probability. A total of 21 variables were extracted either from the field or using the ArcGIS v. 10.6 software (ESRI, Redlands, CA), and only 14 were retained after collinearity testing60 (Table 3). These covariates were classified into the following categories (Topographic variables, Habitat variables and anthropogenic variables). The topographic variables (elevation, slope and aspect) were generated using 30× resolution SRTM (Shuttle Radar Topography Mission) image downloaded from EarthExplorer (https://earthexplorer.usgs.gov/). The habitat/ land cover classification was carried out using Landsat 8 satellite imagery (Spatial resolution = 30 m) downloaded from Global Land Cover Facility by following the methodology suggested by61 using the ArcGIS v. 10.6 software (ESRI, Redlands, CA). The study area was classified into nine Land use/land cover (LULC) classes viz., West Himalayan Sub-alpine birch/fir Forest (FT 188), West Himalayan upper oak/fir forest (FT 162), West Himalayan Dry juniper forest (FT 180), Ban oak forest (FT 152), Moist Deodar Forest (FT 155), Western mixed coniferous forest (FT 156), Moist temperate Deciduous Forest (FT 157) which were used for further analysis considering their importance to species ecology and behavior60. The values for all the covariates were extracted at 30 m resolution, and a single value per site was obtained by averaging all the pixel values within each sampling site (camera trap locations).
Occupancy modelling framework
We used multi-species occupancy modelling62 of barking deer, goral and sambar to estimate the probability of the species (s) occurred within the area (i) sampled during our survey period (j), for accounting the imperfect detection of the species8. Distinguishing the true presence/absence of a species from detection/non-detection (i.e., species present and captured or species present but not captured) requires spatially or temporally replicated data. We used camera stations to record the presence/absence of species along with sign survey in all the studied grids. The camera traps were placed along the trail/transects in the studied grids hence each grid needs to be visited once in every fifteen days to check the camera traps as well as to document the presence of the studied species. Therefore, we treated 15 trap nights as one sampling occasion at a particular camera station resulting in ~ 7 sampling occasions per camera station.Our aim was to record the presence/ absence of the species at a particular gird hence we incorporated sign survey data if the species was not detected in camera station but recorded through sign survey. We pooled the presence/absence data in a single sheet of each species following6 and fitted occupancy and detectability models using programme Mark63,64. We model the species (s) presence (ysij = 1) and absence (ysij = 0) at site i during survey j, and the sampling protocol was identical to single species case65, where the Bernoulli random variable was conditional on the presence of species s (Zs = 1) following6
where Psij represents the probability of detecting species S during replicate survey j at site i and Zsi = presence or absence of species s at site i.
Furthermore, we model the latent occupancy state of species s at site i as a multivariate Bernoulli random variable:
where Zi = {Z1i, Z2i….., ZSi} is an S-dimensional vector of 1’s and 0’s denoting the latent occupancy state of all S species and (ψi) is a 2S-dimensional vector denoting the probability of all possible sequences of 1’s and 0’s Zi can attain such that ∑ ψi = 1 with corresponding probability mass function (PMF) adopted from6,64.
The quantity f = log (ψi1/ψi0), is the log odds species S occupies a site often referred to as a ‘natural parameter’.
Since we are modeling three ungulate species (S = 3), 2S = 23 the possible encounter histories included in the dataset were eight, if neither of the two species were detected the value of ‘00’ was assigned; similarly ‘01’ indicates detection of species 1; ‘02’ indicates detection of species 2; ‘03’ indicates detection of both the species; ‘04’ indicates detection of species 3; ‘05’ indicates detection of species 1 and species 3; ‘06’ indicates detection of species 2 and species 3 and ‘07’ indicates detection of all the three species. We modelled constant occupancy and detection probability for each of the three species. Hence, we specified 6 f and p parameters, an intercept (β) for each of one-way f parameter and detection parameter p following64.
We fit a set of models including the detection probability as a constant, p(.), and variable function to occupancy ψ(covariate) for site-specific covariates and models include occupancy as constant ψ(.) and variable function of the detection p(covariates) for the respective site covariates.
As we have assumed the independence among all three species, the model shows marginal occupancy probabilities of species 1, species 2 and species 3 varies as a function of environmental variables. We incorporated site-level characteristics affecting species-specific occurrence (f1: occupancy of species 1, f2: occupancy of species 2, & f3: occupancy of species 3) and detection probabilities using a generalized linear modelling approach42. This requires 9 parameters: an intercept (β1, β3, β5) and slope (β2, β4, β6) coefficient for each 1-way f parameter f1, f2, f3 and an intercept parameter for each detection parameter (β7, β8, β9). Below mentioned is the model for 1-way f parameters.
All covariates were standardized before model fitting. We fitted the most complex model to each species and considered all possible combinations of covariates using the logit link function. Our rationale for including these variables in the occupancy and detectability component of the model was that we expected these variables to influence the occupancy and detectability of the study species.
Since multi-species occupancy simultaneously model environmental variables, & interspecific interactions. Further it also allows to understand the influence of environmental variables on one species occupancy, in the presence or absence of other sympatric species64. Hence, we also modeled two species occur together as a function of covariates. We examined how the variables of each camera site influenced the pair-wise interaction of the three ungulate species. This model assumes that the conditional probability of one species varies in the presence or absence of other species. We assumed f123: co-occurrence of species 1, species 2 & species 3 = 0, hence we did not include higher-order interactions in any of our models, we assumed the conditional probability of 3 species occurred together was purely a function of species-specific (f1, f2, f3) and pair-wise interaction (f12: co-occurrence of species1 & species 2, f13: co-occurrence of species 1 & species 3, f23: co-occurrence of species 2 & species 3) parameters. We modeled pair-wise interaction of species varies as a function of environmental variables keeping detection probability constant. Hence, we specified 15 f and p parameters, an intercept and slope coefficient for each of the one-way (f1, f2, f3) and the two-way f parameters (f12, f13, and f23); as well as an intercept parameter for each of the detection models. The model equation below implies for 2-way f parameters:
We also fitted models including co-occurrence and detection probability of a species varies as a function of environmental variables. Hence, we specified 18 f and p parameters, an intercept and slope coefficient for each of one-way (f1, f2, f3) and two-way f parameters (f12, f13, f23); and an intercept as well as the slope parameters for each of the detection models. The model equation below implies for 2-way f parameters:
A total of 38 models were run to test the influence of environmental variables on occupancy and detection probability of species-specific (f1, f2, f3) and pair-wise interaction of the three ungulate species. The best-supported model was identified by selecting the model with the lowest AICc value and highest model weights66, where higher model weights indicate a better fit of the model to the data. Second-Order Information Criterion (AICc)67 values were used to rank the occupancy models, and all the models whose ΔAICc < 2 were considered as equivalent models. The Akaike weight represents the ratio of ΔAICc values for the whole set of candidate models, providing a strength of evidence for each model. The sign of the logistic coefficient of each variable (positive or negative) was used to determine the direction of influence of the variables on the occupancy and detection probability of the three ungulate species.
Activity pattern
We have compared activity patterns among species to see how overlapping patterns may relate to the competition using the package “Overlap” in R (R Development Core Team). The time and date printed on the photographs have been used to determine the daily activity pattern of individual species68. We used a Daily Activity Index (DAI) of half an hour duration to examine the daily activity. The coefficient of overlap is denoted by “Dhat1” values, ranging between zero (no overlap) and 1.0 (complete overlap).
References
Owen-Smith, N. Assessing the foraging efficiency of a large herbivore, the kudu. S. Afr. J. Wildl. Res. 9(3–4), 102–110 (1979).
Vinod, T. R. & Sathyakumar, S. Ecology and Conservation of Mountain Ungulates in Great Himalayan National Park, Western Himalaya, Final Report (FREEP-GHNP), Vol. 3 (Wildlife Institute of India, 1999).
Barrows, C. W. et al. A framework for monitoring multiple-species conservation plans. J. Wildl. Manag. 69, 1333–1345 (2005).
Zipkin, E. F., Royle, J. A., Dawon, D. K. & Bates, S. Multi-species occurrence models to evaluate the effects of conservation and management actions. Biol. Conserv. 143, 479–484 (2010).
Pollock, L. J. et al. Understanding co-occurrence by modelling species simultaneously with a joint species distribution model (JSDM). Methods Ecol. Evol. 5, 397–406 (2014).
Rota, C. T. et al. A multi-species occupancy model for two or more interacting species. Methods Ecol. Evol. 7(10), 1164–1173 (2016).
MacKenzie, D. I. Occupancy Estimation and Modeling (Academic Press, 2006).
MacKenzie, D. I. et al. Estimating site occupancy rates when detection probabilities are less than one. J. Ecol. 83, 2248–2255 (2002).
Coleman, B. T. & Hill, R. A. Living in a landscape of fear: the impact of predation, resource availability and habitat structure on primate range use. Anim. Behav. 88, 165–173 (2014).
McHugh, D., Goldingay, R. L., Link, J. & Letnic, M. Habitat and introduced predators influence the occupancy of small threatened macropods in subtropical Australia. Ecol. Evol. 9, 6300–6317. https://doi.org/10.1002/ece3.5203 (2019).
Veblen, K. E. Savanna glade hotspots: Plant community development and synergywithlargeherbivores. J. Arid Environ. 78, 119–127 (2012).
McNaughton, S. J. Grazing as an optimization process: Grass-ungulate relationships in the Serengeti. Am. Nat. 113(5), 691–703 (1979).
Bagchi, S. & Ritchie, M. E. Herbivore effects on above-and belowground plant production and soil nitrogen availability in the Trans-Himalayan shrub-steppes. Oecologia 164(4), 1075–1082 (2010).
Kittur, S., Sathyakumar, S. & Rawat, G. S. Assessment of spatial and habitat use overlap between Himalayan tahr and livestock in Kedarnath Wildlife Sanctuary, India. Eur. J. Wildl. Res 56(2), 195–204 (2010).
Bhattacharya, T., Bashir, T., Poudyal, K., Sathyakumar, S. & Saha, G. K. Distribution, occupancy and activity patterns of goral (Nemorhaedus goral) and serow (Capricornis thar) in Khangchendzonga Biosphere Reserve, Sikkim, India. Mammal Study. 37, 173–181 (2012).
Sathyakumar, S., Bhattacharya, T., Bashir, T. & Poudyal, K. Developing Spatial Database on the Mammal Distributions and Monitoring Programme for Large Carnivores, Prey Populations, and their Habitats in Khangchendzonga Biosphere Reserve. Project Final Report. Wildlife Institute of India, Dehradun (2013).
Groves, C. Taxonomy of ungulates of the Indian subcontinent (2003).
Odden, M. & Wegge, P. Predicting spacing behavior and mating systems of solitary cervids: A study of hog deer and Indian muntjac. J. Zool. 110, 261–270. https://doi.org/10.1016/j.zool.2007.03.003 (2007).
Mattioli, S. Family cervidae (deer). In Handbook of the Mammals of the World, Vol. 2, 350–443 (2011).
Timmins, R. J., Steinmetz, R., Samba, K. N., Anwarul, I. M. & Sagar, B. H. Muntiacus vaginalis. The IUCN Red List of Threatened Species (2016).
Sathyakumar, S. Species of the greater Himalaya. ENVIS Bulletin: Wildlife and Protected Areas, 1–6 (2002).
Joshi, B. D. et al. Revisiting taxonomic disparities in the genus Naemorhedus: new insights from Indian Himalayan Region. Mammalia (2022).
Timmins, R. et al. Rusa unicolor (errata version published in 2015). The IUCN Red List of Threatened Species: eT41790A85628124 (2015).
Duckworth, J.W. & MacKinnon J. Naemorhedus goral. IUCN Red List Threat Species 2008 164 eT14296A4430073 (2008).
Steen, D. A. et al. Snake co-occurrence patterns are best explained by habitat and hypothesized effects of interspecific interactions. J. Anim. Ecol. 83(1), 286–295. https://doi.org/10.1111/1365-2656.12121 (2014).
Wisz, M. et al. The role of biotic interactions in shaping distributions and realized assemblages of species: Implications for species distribution modelling. Biol. Rev. Camb. Philos. Soc. 88(1), 1530. https://doi.org/10.1111/j.1469-185X (2013).
Arlettaz, R. Habitat selection as a major resource partitioning mechanism between the two sympatric sibling bat species Myotis myotis and Myotis blythii. J. Anim. Ecol. 68(3), 460–471 (1999).
Nagarkoti, A. & Thapa, T. B. Distribution pattern and habitat preference of barking deer (Muntiacus muntjac Zimmermann) in Nagarjun forest, Kathmandu. Hima J. Sci. 4, 70–74 (2007).
Lovari, S. & Apollonio, M. On the rutting behaviour of the Himalayan goral Nemorhaedus goral (Hardwicke, 1825). J. Etho 12, 25–34 (1994).
Kushwaha, S., Khan, A., Habib, B., Quadri, A. & Singh, A. Evaluation of sambar and muntjak habitats using geostatistical modelling. Curr. Sci. 86(10), 1390–1400 (2004).
Ahmad, S. et al. Using an ensemble modelling approach to predict the potential distribution of Himalayan gray goral (Naemorhedus goral bedfordi) in Pakistan. Glob. Ecol. Conserv. 21, e00845 (2020).
Aryal, A. Status and Conservation of Himalayan Serow (Capricornis sumatraensis. thar) in Annapurna Conservation Area of Nepal. BRTF Nepal: A Report Submitted to the Rufford Small Grant for Nature Conservation, UK and the People’s Trust for Endangered Species, UK (2008).
Bhattacharya, T. & Sathyakumar, S. Abundance, group sizes and habitat use patterns of Himalayan tahr (Hemitragus jemlahicus) and goral (Nemorhaedus goral) in Chenab valley, Chamoli district (Uttarakhand). Indian For. 134(10), 1359–1370 (2008).
Qasim, M., Hubacek, K., Termansen, M. & Fleskens, L. Modelling land use change across elevation gradients in district Swat, Pakistan. Reg. Environ. Chang. 13, 567–581. https://doi.org/10.1007/s10113-012-0395-1 (2013).
Western, D., Russell, S. & Cuthill, I. The status of wildlife in protected areas compared to non-protected areas of Kenya. PLoS ONE 4(7), e6140 (2009).
Zipkin, E. F., DeWan, A. & Royle, J. A. Impacts of forest fragmentation on species richness: A hierarchical approach to community modelling. J. Appl. Ecol. 46, 815–822 (2009).
Tobler, M. W., Carrillo-Percastegui, S. E., Pitman, R. L., Mares, R. & Powell, G. An evaluation of camera traps for inventorying large- and medium-sized terrestrial rainforest mammals. Anim. Conserv. 11, 169–178 (2008).
Rovero, F. & Marshall, A. Camera trapping photographic rate as an index of density in forest ungulates. J. Appl. Ecol. 46, 1011–1017 (2009).
Joshi, B. D. et al. Field testing of different methods for monitoring mammals in Trans-Himalayas: A case study from Lahaul and Spiti. Glob. Ecol. Conserv. 21, e00824 (2019).
Ridout, M. S. & Linkie, M. Estimating overlap of daily activity patterns from camera trap data. J. Agric. Biol. Environ. Stat. 14, 322–337 (2009).
Ramesh, T., Kalle, R., Sankar, K. & Qureshi, Q. Spatio-temporal partitioning among large carnivores in relation to major prey species in Western Ghats. J. Zool. 287, 269–275 (2012).
Ross, J., Hearn, A. J., Johnson, P. J. & Macdonald, D. W. Activity patterns and temporal avoidance by prey in response to Sunda clouded leopard predation risk. J. Zool. 290(2), 96–106 (2013).
Can, Ö. E. et al. Factors affecting the occurrence and activity of clouded leopards, common leopards and leopard cats in the Himalayas. Biodivers. Conserv. 29(3), 839–851 (2020).
MacKenzie, D. I., Bailey, B. L. & Nichols, J. D. Investigating species co-occurrence patterns when species are detected imperfectly. J. Anim. Ecol. 73, 546–555 (2004).
Waddle, J. H. et al. A new parameterization for estimating co-occurrence of interacting species. Ecol. Appl. 20, 1467–1475. https://doi.org/10.1890/09-0850.1 (2010).
Rota, C. T. et al. A two species occupancy model accommodating simultaneous spatial and interspecific dependence. Ecology 97(1), 48–53 (2016).
Green, M.J.B. Aspects of the ecology of the Himalayan musk deer (1985).
Mishra, C. Habitat Use by Goral (Nemorhaedus goral bedfordi) in Mujhatal Harsang Wildlife Sanctuary, Himachal Pradesh, India (Wildlife Institute of India, 1993).
Sathyakumar, S. Habitat Ecology of Major Ungulates in Kedarnath Musk Deer Sanctuary, Western Himalaya. Ph.D Dissertation (Saurashtra University, 1994).
Green, M. J. B. Ecological separation in Himalayan ungulates. J. Zool. 1, 693–719. https://doi.org/10.1111/j.1096-3642.1987.tb00751 (1987).
Haleem, A., Ilyas, O., Syed, Z., Arya, S. K. & Imam, E. Distribution, status and aspects of ecology of mammalian species in Kedarnath Wildlife Sanctuary, Uttarakhand Himalayas, India. J. Mater. Environ. Sci. 5(3), 683–692 (2014).
Oka, G. M. Factors Affecting the Management of Muntjac Deer (Muntiacus muntjak) in Bali Barat National Park, Phd Thesis (University of Western Sydney, 1998).
Paudel, P. K. & Kindlmann, P. Human disturbance is a major determinant of wildlife distribution in Himalayan mid hill landscapes of Nepal. Anim. Conserv. 15, 283–293. https://doi.org/10.1111/j.1469-1795.2011 (2012).
McLoughlin, P. D., Morris, D. W., Fortin, D., Vander Wal, E. & Contasti, A. L. Considering ecological dynamics in resource selection functions. J. Anim. Ecol. 79(1), 4–12 (2010).
Mishra, C. & Johnsingh, A. J. On habitat selection by the goral Nemorhaedus goral bedfordi (Bovidae, Artiodactyla). J. Zool. 240(3), 573–580 (1996).
Godsoe, W. & Harmon, L. J. How do species interactions affect species distribution models?. Ecography 35(9), 811–820 (2012).
Svenning, J. C. et al. The influence of interspecific interactions on species range expansion rates. Ecography 37, 1198–1209 (2014).
Paudel, P. K., Bhattarai, B. P. & Kindlmann, P. An overview of the biodiversity in Nepal. In Himalayan Biodiversity Chang. World (ed. Kindlmann, P.) 1–40 (Springer, 2012).
Champion, H. G. & Seth, S. K. A Revised Survey of the Forest Types of India. Manager of publications (1968).
Sharief, A. et al. Identifying Himalayan brown bear (Ursus arctos isabellinus) conservation areas in Lahaul Valley, Himachal Pradesh. Glob. Ecol. Conserv. 21, e00900 (2020).
Singh, T. P., Singh, G., Roy, P. S. & Rao, B. S. P. Vegetation mapping and characterization in West Siang District of Arunachal Pradesh, India—A satellite remote sensing based approach. Curr. Sci. 83(10), 1221–1230 (2005).
Dorazio, R. M., Royle, J. A., Söderström, B. & Glimskär, A. Estimating species richness and accumulation by modeling species occurrence and detectability. J. Ecol. 87, 842–854 (2006).
White, G. C. & Burnham, K. P. Program MARK: Survival estimation from populations of marked animals. Bird Study 46(Supplement), 120–138 (1999).
Skelly, B. P., Lewis, K., Tyl, R., Dimmig, G. & Rota, C. T. Occupancy models—multi-species. In (eds Cooch, E. G. & White, G. W.) Program MARK: a gentle introduction, 18th ed. 22-1–22-13 (2019).
MacKenzie, D. et al. Occupancy estimation and modelling. Inferring patterns and dynamics of species occurrence. Wildl. Biol. 12, 450 (2006).
Anderson, M. L. Multiple inference and gender differences in the effects of early intervention: A re evaluation of the Abecedarian, Perry Preschool, and early training projects. J. Am. Stat. Assoc. 103, 484. https://doi.org/10.1198/016214508000000841 (2008).
Akaike, H. Second International Symposium on Information Theory, Chapter information theory as an extension of the maximum likelihood principle, 267–281 (Akademiai Kiado, 1973).
Pei, J.-C. An evaluation of using auto-trigger cameras to record activity patterns of wild animals, Taiwan. J. For. Sci. 13, 317–324 (1998).
Grumbine, R. E. & Pandit, M. K. Threats from India’s Himalaya dams. Science 339, 36–37. https://doi.org/10.1126/science.1227211 (2013).
Acknowledgements
We would like to extend our sincere gratitude towards Chief Wildlife Warden, Forest Department Uttarakhand, and Government of Himachal Pradesh for granting us permission for research work Authors also thank to the Director, Head of Office and O/C Technical Section, Zoological Survey of India for their consistent support during the study period. Authors are also thankful to Shree Sandeep Kumar, Divisional Forest Officer (DFO), Uttarkashi Forest Division for his consistent guidance and support during the fieldwork. Authors are extending thanks to DFOs of Tons Forest Division, Purola and Upper Yamuna Forest Division, Badkot, Deputy Director, Govind PashuVihar National Park and Sanctuary, Purola for helping and guiding the field team of ZSI, Kolkata time to time. We are thankful to all the Range Officers and field staff of all three Forest Divisions, National Park for their active support and logistics during the fieldwork. We acknowledge the National Mission for Himalayan Studies, Ministry of Environment, Forest and Climate Change (MoEF&CC) for the funding support under the Grant No. NMHS/2017-18/LG09/02.
Author information
Authors and Affiliations
Contributions
H.S., A.S., and L.K.S. conceptualized and designed the study. H.S., A.S., B.D.J. and T.M. participated in data generation, quality check and primary data analysis. H.S., A.S., V.K., B.D.J., L.K.S., and M.T. wrote the primary draft of the manuscript. H.S., A.S., V.K., B.D.J., T.M., N.B., K.C., M.T., and L.K.S. finalized the manuscript. L.K.S. and N.B. supervised the overall activities and K.C. raised funds for the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Singh, H., Sharief, A., Joshi, B.D. et al. Multi-species occupancy modeling suggests interspecific interaction among the three ungulate species. Sci Rep 12, 17602 (2022). https://doi.org/10.1038/s41598-022-20953-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-20953-7