Abstract
Decisional conflict might occur during shared decision-making (SDM) because immunotherapy is a rather novel treatment option for patients with cancer. To explore the prevalence and severity of physical and psychological symptoms and the effort invested in SDM in relation to decisional conflict among patients with cancer undergoing immunotherapy combined with chemotherapy or targeted therapy. This was a cross-sectional survey study. The SURE version of the Decisional Conflict Scale was used to screen cancer patients’ decisional conflict status. Demographic or clinical characteristics, physical symptoms and psychological distress; efforts invested in the SDM process were also assessed as potential factors related to decisional conflict. One hundred seventeen patients surveyed, the prevalence of fatigue (79.5%), sleep disturbance (78.6%), poor appetite (67.5%), and pain (58.1%) symptoms were high and the severity was at moderate levels. The prevalence of pruritus (40.2%), rash (34.2%), dry skin (41.9%), and diarrhea (17.1%) symptoms were low and the severity was at mild levels. 65.8% of patients reported uncertainty, with mild to moderate levels. Furthermore, 97.4% of the patients made some effort in SDM, and the effort level was moderate (mean: 5.56 ± 2.02). 64.1% of patients were certain that immunotherapy was the best option. Age, uncertainty, and effort in the SDM process were major factors related to decisional conflict. We observed that older patients (age: ≥ 65) and those with higher uncertainty levels and less effort in SDM reported higher levels of decisional conflict. Future studies should explore older patients’ decisional related needs of immunotherapy. Interventions should be designed to reduce the uncertainty experienced by patients with cancer and enhance their understanding of immunotherapy to enable them to take more effort in the SDM process.
Similar content being viewed by others
Introduction
Cancer is among the most life-threatening diseases worldwide1. With advances in medical technology, immunotherapy, which is a newly developed advanced treatment, is currently a crucial cancer treatment option2,3. Although immunotherapy provides hope4 for patients with cancer and an opportunity for them to prolong overall survival5,6, immunotherapy is not usually curative7. This causes the patients to be uncertain about how their disease would respond to immunotherapy, thereby leading to decisional conflict. Decisional conflict is defined in this study as uncertainty during clinical decision-making, which may lead to regret8. In cancer treatment, immunotherapy is often combined with chemotherapy or targeted therapy to enhance the clinical efficacy9,10,11. This may increase or complicate therapy-related adverse effects12,13, which can influence the patient’s decision to undergo immunotherapy. However, few studies have examined the relationships between therapy-related adverse effects and decisional conflict.
According to the Theory of Unpleasant Symptoms, patients with cancer generally have therapy-related adverse effects, including physical and psychological symptoms14, which may influence their decisional conflict. Physical symptoms, such as pain, fatigue, and insomnia, were reported to negatively influence decisional conflict in older patients with cancer undergoing treatment15. Furthermore, psychological distress, such as anxiety and depression, in patients with breast cancer can help predict their decisional conflict after an uninformative BRCA1/2 test16. For patients with cancer receiving therapy, the most common physical symptoms have been identified to include pain, fatigue, insomnia17, and poor appetite, along with those from immune-related adverse events (irAEs), such as pruritus (12.13–23.94%), diarrhea (11.16–27.95%), and rash (11.06–22.43%)13. A qualitative study including 28 patients with metastatic melanoma undergoing immunotherapy reported that uncertainty was the main form of psychological distress due to concerns about immunotherapy’s effectiveness and disease progression18. Uncertainty is an inherent characteristic of decisional conflict8. It can increase the patient’s cognitive load and reduce their ability to deploy the cognitive resources that they have, thereby impairing their cognitive appraisal abilities19. For example, patients may have difficulty determining the meaning of disease-related events when faced with uncertainty20. Thus, the patients might begin to doubt whether their original treatment decision is right, leading to decisional conflict21. Therefore, uncertainty was examined as a factors related to decisional conflict in the present study.
In clinical settings, to avoid decisional conflict, shared decision making (SDM) should be employed. In SDM, both clinicians and patients share their expertise when faced with the task of making decisions, and patients are supported and guided toward considering the range of options available to them to make an informed decision22. SDM is particularly important in clinical settings for achieving patient-centered care communication and thereby facilitating the delivery of high quality cancer care23. In fact, patients prefer to play a collaborative role in decision making24. However, previous studies have shown that only 35 ~ 50% of patients reported to have actually participated in the process of treatment through SDM25,26,27, and decisional conflict might have occurred. Thus, prior to decision making for anti-cancer treatment, patients should be informed of its benefits, risks, limitations, and uncertainties, and their preferences should be considered before making a final decision28, thereby reducing patients’ decisional conflict. SDM has been documented as a major influential factor related to conflict29. Therefore, it is crucial to assess the extent to which efforts have been invested in the SDM process for treatment through immunotherapy, which has not been sufficiently explored in prior research; the current study seeks to address this gap.
To our knowledge, this is the first study to simultaneously examine both major physical and psychological factors related to decisional conflict in patients with cancer undergoing immunotherapy. Hence, we hypothesized that patients with cancer undergoing immunotherapy may suffer from physical and psychological distress, which may influence the levels of decisional conflict, and that efforts invested in SDM could also influence the levels of decisional conflict. We determined the prevalence and severity of physical and psychological symptoms and effort invested in the SDM process in relation to decisional conflict among patients with cancer undergoing immunotherapy combined with chemotherapy or targeted therapy.
The results obtained from this study provide an important reference for health professionals to design specific interventions to decrease immunotherapy-related decisional conflict.
Methods
Study sample
The current study was cross-sectional, descriptive, and correlational in design. Data collection was conducted between February and November 2021. We recruited participants who were diagnosed with cancer and undergoing immunotherapy in inpatient oncology wards and chemotherapy outpatient settings of a medical center in northern Taiwan. Our assistant researcher screened for potential participants from electronic medical records of cancer patients who were receiving immunotherapy at inpatient oncology wards and chemotherapy outpatient settings in a medical center that we collaborated with. The inclusion criteria were as follows: being older than 19 years; having a diagnosis of cancer (stage III–IV); having received information from the physician about immunotherapy, such as it being a relatively new anticancer therapy and its adverse effects, risk, benefits, and financial cost; having received at least one dose of immunotherapy (because the median time of onset of certain irAEs, such as rash and pruritus, is 3 weeks after the first dose of immunotherapy)30; and being able to communicate in Mandarin or Taiwanese dialect. The exclusion criteria for participants were having cognitive functional impairment and the inability to respond to questions. Of the 131 potentially eligible patients, 5 were too tired, 2 had severe adverse effects, and 7 refused to participate because of concerns regarding the COVID-19 pandemic. Eventually, data from 117 patients were included in the final analysis (response rate = 89.3%).
Data collection
The SURE version of the decisional conflict scale (SURE)
Patients’ level of decisional conflict was detected by the SURE version of the Decisional Conflict Scale, which has four items and has been designed for quickly identifying patients with clinically significant decisional conflict31. SURE is the acronym derived from four dimensions measured in this 4-item questionnaire (e.g., Sure of myself, Understand information, Risk–benefit ratio, and Encouragement). If the answer to an item is yes, the score is 1; if the answer is no, the score is 0. Thus, the four responses are added together to obtain a score ranging from 0 to 4, with a lower score indicating a higher degree of clinically significant decision making conflict; a total score of 4 indicates no decisional conflict. The SURE scale has acceptable internal consistency (Kuder-Richardson-20, coefficient = 0.7)31,32; its internal consistency (Cronbach’s alpha) in this study was 0.94.
Physical symptoms and psychological distress
Patients’ severity of physical symptoms and psychological distress were assessed using the 11-point numerical rating scale (NRS), ranging from 0 (“no pain”) to 10 (“the worst pain you can imagine”)33. The NRS is an acceptable response scale for assessing patient-reported outcomes34. For physical symptoms, we measured pain, fatigue, sleep disturbance, and poor appetite as the most common cancer-related symptoms17 and pruritus, rash, dry skin and diarrhea as the most common immunotherapy-related symptoms13. For the psychological aspect, we assessed the level of patients’ uncertainty in the same way. The internal consistency (Cronbach’s alpha) of the physical symptoms aspect in this study was 0.74, while that of the psychological distress (uncertainty) aspect was not presented due to our consideration of only one uncertainty item.
CollaboRate
Efforts toward facilitating the SDM process for immunotherapy was assessed using CollaboRate, a tool developed to measure efforts invested in the SDM process, and one that has demonstrated good psychometric properties35. We used CollaboRate based on the patient reports to revise three items to make it more specific to immunotherapy-related decision-making issues. For example, “How much effort was made to help you understand your health issues?” was revised to “How much effort was made to help you understand immunotherapy?” This item was scored on a 10-point anchored scale ranging from 0 (“no effort was made”) to 9 (“every effort was made”), with higher scores representing greater effort taken by patients to understand what immunotherapy is in the SDM process35. The internal consistency (Cronbach’s alpha) of the CollaboRate tool in this study was 0.87.
Sociodemographic and clinical characteristics
Patients’ sociodemographic characteristics included gender, age categorized based on the definition of elderly or not (≥ 65 or < 65)36, education in number of years classified according to education at or below the college or university level37, and marital status. Patients’ clinical characteristics included time since diagnosis, cancer type, treatment modules, treatment expenses (self-funded or non-self-funded, such as participation in clinical trials or insurance payments) and performance status, which was assessed by the Eastern Cooperative Oncology Group (ECOG) Performance Status Scale, with a score ranging from 0 (fully active) to 5 (dead)38.
Statistical analysis
Data analysis was performed using SPSS version 24.0 software. Descriptive statistics including percentage, mean, and standard deviation [SD] were used to present participants’ sociodemographic and clinical characteristics, and the current status of physical symptoms (pain, fatigue, poor appetite, and sleep disturbance), psychological distress (uncertainty), efforts invested in the SDM process and decisional conflict (aim 1). Since some scores of measures were not normally distributed, we employed nonparametric analysis methods to present the inference statistics. The levels of decisional conflict were dichotomized as non-conflict cases (the SURE total score was 4) versus cases having some level of conflict (the SURE total score was 0, 1, 2, or 3). Before conducting logistic regression, the Mann–Whitney U test was performed to compare the differences of potential factors (independent variables) in the levels of decisional conflict. Further, Spearman’s tests were conducted to determine the potential factors correlated with decisional conflict. All the significant potential factors were included in a logistic regression model39 to determine the robust factors related to decisional conflict (aim 2).
Sample size
We strived to have sufficient samples by using G-Power with alpha = 0.05 and power = 0.8 in a two-sided test and adopted a giving odds ratio (OR) of 2.09 based on previous studies40 in conducting the logistic regression, to reasonably detect an effect in the current study41. The minimum sample size of 101 was suggested. Thus, 117 participants were considered adequate to conduct the logistic regression in this study.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki to follow the principles of Ethical Considerations. This study was approved by the Institutional Review Board of National Taiwan University Hospital, Taipei, Taiwan (approval no. 201812191RINB). The participants were informed about the study purpose, after informed consent was obtained from all subjects; subsequently, paper-based questionnaires were distributed.
Consent to participate
All participants provided written informed consent prior to enrolment in the study.
Results
Patients’ sociodemographic and clinical characteristics and their relationships with decisional conflict
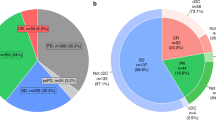
Most of the patients were male (78.6%). The average age of patients was 56.1 (SD = 11.2) years and the average education years was 11.9 (SD = 3.9). Most patients were married (76.9%) and more than half of them (59.0%) were diagnosed with cancer, for more than or equal to 12 months. Furthermore, 51.3% of the patients were diagnosed with head and neck cancer while other cancer types were very diverse (48.7%), such as lung cancer, hepatocellular carcinoma (HCC), gastrointestinal stromal tumor (GIST), breast cancer, and colon cancer. Moreover, 76.9% of the cancer patients who were receiving immunotherapy reported receiving treatment combined with chemotherapy or targeted therapy. 61.5% of patients reported self-funding for immunotherapy and the mean performance status (ECOG) for patients was 1.43 (SD = 0.83) (Table 1).
We found the differences in the levels of decisional conflict among patients’ age (≥ 65 vs. < 65) (Z = − 3.73, p < 0.01) and education (college or university level vs. below college) (Z = − 3.18, p < 0.01). This indicated that older patients (aged ≥ 65 years) and those having lower level of education reported higher levels of decisional conflict than younger patients (aged < 65 years) and those with college or university level of education. Patients’ performance status were negatively associated with decisional conflict (r = − 0.22, p < 0.05) (Table 1).
Prevalence and severity of physical symptoms and psychological distress and efforts invested in the SDM process: correlations with decisional conflict
With respect to the prevalence of patients’ physical symptoms, the prevalence of immunotherapy-related symptoms such as for pruritus, rash, dry skin, and diarrhea were 40.2%, 34.2%, 41.9%, and 17.1%, respectively, and for cancer-related symptoms such as pain, fatigue, poor appetite, and sleep disturbance were 58.1%, 79.5%, 67.5%, and 78.6%, respectively. Furthermore, the mean scores for pruritus, rash, dry skin, diarrhea, pain, fatigue, poor appetite, and sleep disturbance were 1.95 (SD = 2.61), 1.03 (SD = 2.04), 1.09 (SD = 2.05), 1.17 (SD = 2.11), 3.12 (SD = 3.31), 4.06 (SD = 3.01), 3.27 (SD = 3.03), and 4.05 (SD = 3.10), respectively. The prevalence of patients’ uncertainty was 65.8%, and the mean uncertainty levels was mild (2.86; SD, 2.73). Moreover, 97.4% of the participants made some effort in SDM, with the mean effort levels being moderate (5.56; SD, 2.02) (Table 2).
Only cancer-related symptoms such as pain (r = − 0.21, p < 0.05), fatigue (r = − 0.30, p < 0.01), and poor appetite (r = − 0.29, p < 0.01) were negatively associated with decisional conflict. Patients’ uncertainty (r = − 0.39, p < 0.01) was negatively associated with decisional conflict as well. Moreover, patients’ efforts invested in the SDM process (r = 0.20, p < 0.05) was positively associated with decisional conflict (Table 2).
Status of immunotherapy-related decisional conflict
For the status of decisional conflict, 64.1% of patients reported that they felt sure about immunotherapy being the best choice (i.e., response on the Sure of myself item); the response rates for the other 3 items of the SURE scale about Understand information, Risk–benefit ratio, and Encouragement were 71.8%, 70.9%, and 60.7%, respectively. Results revealed that 80.3% of patients experienced clinically significant decisional conflict (total scores less than 4) (Table 3).
Factors related to decisional conflict
For the decisional conflict model, significant factors related to conflict included age, uncertainty, and the effort invested in the SDM process (Table 4). The OR for conflict risk was 4.82 times higher (95% CI 1.48–15.69) in older patients than in younger patients. Moreover, the OR for conflict risk was higher if the patients had higher levels of uncertainty (95% CI 1.04–1.52) and lower if they spent more effort investing in the SDM process (95% CI 0.58–0.96; Table 4).
Discussion
In this cross-sectional study, 76.9% of the cancer patients who were receiving immunotherapy reported receiving treatment combined with chemotherapy or targeted anti-cancer agents, an approach supported by recent clinical trials for improved clinical efficacy9,10,11. Thus, patients with cancer might experience a variety of physical symptoms from a combination of different therapies. In the current study, approximately 80% of patients experienced fatigue (79.5%) and sleep disturbance (78.6%) symptoms, and over half of the patients had poor appetite (67.5%) and pain (58.1%) symptoms. In an earlier systematic review of patients who were undergoing surgery, chemotherapy, or radiotherapy, the prevalence of physical symptoms varied across different studies examining fatigue (38.9–82.1%), sleep disturbance (14–72.2%), poor appetite (2.0–76.2%), and pain (17.4–61.8%)13. One point of clarification: this review study did not include patients who had undergone immunotherapy. The review study included patients with all stages of cancer who had received surgery, chemotherapy or radiotherapy. One noteworthy finding was that patients with cancer stages III and IV, especially those undergoing chemotherapy, exhibited a higher prevalence of physical symptoms. The lower range data is attributed to those patients with cancer stages I and II. Moreover, both the systematic review and the current study indicate similarities in which patients with cancer stage III or IV have a higher prevalence of physical symptoms. However, in the current study, only patients with cancer stages III and IV were observed. In closing, clinicians should exercise caution when caring for these patients in particular. Presently, there is an inadequate amount of study exploring whether or not immunotherapy (as only treatment or in combination with other treatment options) leads to a higher prevalence of physical symptoms compared to those patients who do not receive immunotherapy. Thus, future studies are recommended to focus on these issues.
Moreover, over one-third of the patients reported pruritus (40.2%), rash (34.2%), and dry skin (41.9%) symptoms, and approximately one-fifth reported diarrhea (17.1%). These prevalence are higher than those reported by a past systematic and meta-analysis study13 examining the immune-related side effects of pruritus (12.13–23.94%), rash (11.06–22.43%), dry skin (5.42%), and diarrhea (11.16–27.95%)13. The difference may be because, in the current study, most (76.9%) of the patients were undergoing immunotherapy combined with targeted therapy and those symptoms were also more common in patients with targeted therapy, with over 50% of patients experiencing skin toxicities (pruritus, rash, and dry skin)42 and diarrhea43. Some similarities in side effects can be observed between immunotherapy and targeted therapy. Although we found that treatment modalities were not associated with decisional conflict in this study, health-care professionals should pay more attention to such a high prevalence of symptoms in patients with cancer and provide appropriate and adequate treatment. The management of targeted therapy and immunotherapy does not involve the same measures. Using sunscreen with a sun protection factor (SPF) of at least 15 to protect the skin from the sun’s harmful rays, a mild soap for managing skin problems44, and drinking enough water to prevent dehydration45 are common recommendations for patients. Furthermore, diarrhea may also result from chemotherapy. However, according to the management of immunotherapy guidelines, corticosteroids may routinely be used to manage irAEs46. Thus, cancer patients demonstrating any symptoms would benefit from engaging in discussions with the health care team to distinguish possible causes of the problem. Furthermore, regarding symptom severity, health providers should pay more attention when patients report more than moderate levels of symptoms, since most of the patients experience intolerable moderate and severe symptoms47, especially related to fatigue and sleep disturbance.
We also found that approximately 70% of patients knew the benefits and risks of immunotherapy, indicating that they were aware of their treatment choice as it was described to them. Even though 64.1% of patients reported feeling that immunotherapy was the best choice, the implementation of SDM still requires more efforts. Approximately 60% of the patients in this study reported having enough support and advice to make an informed choice, consistent with several studies reporting that 35–50% of patients were involved in treatment-related SDM25,26. However, this SURE item merely indicates the patient’s subjective perception of having received sufficient support and advice in SDM. More research is required on specific items that are insufficient.
Furthermore, three factors associated with immunotherapy-related decisional conflict were found: age, uncertainty, and effort in the SDM process. We found that patients ≥ 65 years showed a high risk of decisional conflict. Most older patients may experience mild age-associated changes in cognition, which is a normal part of aging process but affects thinking speed and attention48. This may cause them to take longer to respond to or understand immunotherapy to avoid or reduce decisional conflict. Further, elder people tend to seek less information when making decisions49, especially if they are both physically and mentally affected by cancer. Although health-care providers should actively provide information on immunotherapy to older patients and give them sufficient time to understand the information provided, the context of the SDM must be considered28. The patients should first be asked about the depth of information that they seek. If they deliberately choose not to be informed or to be minimally informed, health-care providers must consider the patients’ preferences while still ensuring that patients make informed decisions.
Our finding on higher levels of uncertainty presenting a higher risk of conflict was consistent with a prior report which documented that unaddressed uncertainty can result in treatment-related decisional conflict21. Moreover, a study indicated that since most medical decisions are complicated by a lack of evidence about risk/benefit information, decision satisfaction is related to the uncertainty50. Thus, in patients facing new immunotherapy, uncertainty may persist in their minds regardless of increased medical knowledge51. Indeed, our study provided quantitative results of the high prevalence (65.8%) of uncertainty, which was reported by cancer patients undergoing immunotherapy and was supported by a previous qualitative study18. Although the severity level of uncertainty was mild in this study, it is one of the major factors in decisional conflict. Therefore, understanding the reasons for uncertainty and assessing the needs of patients are recommended steps in the SDM process to help them reduce or cope better with their feelings of uncertainty.
Furthermore, in our study, patients reported that they invested moderate levels of effort trying to understand immunotherapy. In addition, while they invested more effort in understanding immunotherapy, a higher efforts invested in the SDM process among patients resulted in lower risk of decisional conflict in this study. This result is consistent with a previous study29 which showed that patients who perceived that they were more involved in the SDM process were significantly negatively correlated with decisional conflict. Thus, since a high prevalence (80.3%) of immunotherapy-related decisional conflict was found in this study, it is strongly recommended that health professionals should design specific interventions to decrease patients’ uncertainty and encourage them to involve in the discussion of immunotherapy for reducing decisional conflict, which would reflect better communication in patient-centered care and the performance of SDM23.
Lastly, some interesting findings from this study were that several factors in the correlation analysis, including severe physical performance, severe pain, fatigue, and poor appetite, that were associated with greater immunotherapy-related decisional conflict. These factors were newly explored in this study, especially for physical symptoms related to decisional conflict, supported by our hypothesis. However, these factors were not related to immunotherapy-related decisional conflict. Uncertainty as a factor of psychological distress in this study was related to decisional conflict. Some factors can explain these results. Most cancer patients experience physical symptoms (such as pain, fatigue, and poor appetite) for a long time during their anti-cancer therapies12,13 that could be managed in clinical settings52, so that they could tolerate these symptoms. In addition, immunotherapy is commonly used as a second-line or later treatment modality, and thus it can provide renewed hope for patients4. At that point, they would focus on the efficacy of treatment and how long they will survive, and these issues are uncertain7. Thus, for avoiding decisional conflict, uncertainty has to be managed in ways such as collaborating with doctors to provide the knowledge to increase immunotherapy-related information and invite patients to participate more in the SDM process, which reflects another important factor related to decisional conflict in this study.
Limitations
This study has some limitations. First, only 23.1% of the patients received immunotherapy in this study, and most of them received immunotherapy combined with chemotherapy or targeted therapy. Thus, a further analysis of decisional conflict-related factors in different immunotherapy modalities could be considered using larger sample sizes. Second, men were overrepresented in our cohort and most patients had head or neck cancer, thus precluding the generalization of these findings to patients with other cancers, such as breast cancer. Third, we collected data on the most common immunotherapy-related adverse effects, namely, pruritus, rash, dry skin, and diarrhea; however, we did not have an “others” option. Thus, we could not evaluate other immunotherapy-related adverse effects, such as cardiovascular, hematologic, renal, and neurological symptoms53,54. Four, we could assess only the level of decisional conflict in the current study. A qualitative study is warranted on how the patient’s immunotherapy experience affects decisional conflict. Finally, self-reported CollaboRATE may have social desirability bias55 and a priori dichotomization of age needed to consider the estimated risk factor effect was biased56 in the current study.
Conclusion
Immunotherapy is a newly developed anti-cancer therapy. The present study examined the factors associated with immunotherapy-related decisional conflict. Results revealed that age, uncertainty, and effort invested in the SDM process were major factors related to decisional conflict. It is strongly recommended to design interventions that can support cancer patients to reduce or cope better with uncertainty, and invite patients and encourage them to spend more time and efforts in the SDM process. Further, proactive care should be taken for cancer patients ≥ 65 years, who may experience more decisional conflict and need more assistance from health professionals for reducing the same.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
World Health Organization. Cancer. https://www.who.int/health-topics/cancer#tab=tab_1. (2021).
Kruger, S. et al. Advances in cancer immunotherapy 2019-latest trends. J. Exp. Clin. Cancer Res. 38, 268. https://doi.org/10.1186/s13046-019-1266-0 (2019).
Akinleye, A. & Rasool, Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J. Hematol. Oncol. 12, 92. https://doi.org/10.1186/s13045-019-0779-5 (2019).
Nouri Rouzbahani, F. et al. Immunotherapy a New Hope for cancer treatment: A review. Pak. J. Biol. Sci. 21, 135–150. https://doi.org/10.3923/pjbs.2018.135.150 (2018).
Khan, M. et al. Comparative analysis of immune checkpoint inhibitors and chemotherapy in the treatment of advanced non-small cell lung cancer: A meta-analysis of randomized controlled trials. Med. (Baltim) 97, e11936. https://doi.org/10.1097/MD.0000000000011936 (2018).
Lau, A., Yang, W. F., Li, K. Y. & Su, Y. X. Systemic therapy in recurrent or metastatic head and neck squamous cell carcinoma-A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 153, 102984. https://doi.org/10.1016/j.critrevonc.2020.102984 (2020).
Borcoman, E. et al. Novel patterns of response under immunotherapy. Ann. Oncol. 30, 385–396. https://doi.org/10.1093/annonc/mdz003 (2019).
LeBlanc, A., Kenny, D. A., O’Connor, A. M. & Légaré, F. Decisional conflict in patients and their physicians: A dyadic approach to shared decision making. Med. Decis. Mak. 29, 61–68. https://doi.org/10.1177/0272989X08327067 (2009).
Galluzzi, L., Buqué, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 28, 690–714. https://doi.org/10.1016/j.ccell.2015.10.012 (2015).
Gandhi, L. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 378, 2078–2092. https://doi.org/10.1056/NEJMoa1801005 (2018).
Robert, L., Ribas, A. & Hu-Lieskovan, S. Combining targeted therapy with immunotherapy. Can 1+1 equal more than 2?. Semin. Immunol. 28, 73–80. https://doi.org/10.1016/j.smim.2016.01.001 (2016).
Anand, U. et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 10, 1367–1401. https://doi.org/10.1016/j.gendis.2022.02.007 (2023).
Xing, P. et al. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO+IPI: A systematic review and meta-analysis. J. Immunother. Cancer 7, 341. https://doi.org/10.1186/s40425-019-0779-6 (2019).
Lenz, E. R., Pugh, L. C., Milligan, R. A., Gift, A. & Suppe, F. The middle-range theory of unpleasant symptoms: An update. ANS Adv. Nurs. Sci. 19, 14–27. https://doi.org/10.1097/00012272-199703000-00003 (1997).
Kates, J. M. Treatment-related decisional conflict, quality of life, and comorbidity in older adults with cancer. Asia Pac. J. Oncol. Nurs. 5, 421–429. https://doi.org/10.4103/apjon.apjon_32_18 (2018).
Rini, C. et al. Cognitive and emotional factors predicting decisional conflict among high-risk breast cancer patients who receive uninformative BRCA1/2 results. Health Psychol. 28, 569–578. https://doi.org/10.1037/a0015205 (2009).
Kokkonen, K. et al. Cancer patients’ symptom burden and health-related quality of life (HRQoL) at tertiary cancer center from 2006 to 2013: A cross-sectional study. Anticancer. Res. 39, 271–277. https://doi.org/10.21873/anticanres.13107 (2019).
Levy, D. et al. Certainty within uncertainty: A qualitative study of the experience of metastatic melanoma patients undergoing pembrolizumab immunotherapy. Support Care Cancer 27, 1845–1852. https://doi.org/10.1007/s00520-018-4443-3 (2019).
Mishel, M. H. Uncertainty in illness. Image J. Nurs. Sch. 20, 225–232. https://doi.org/10.1111/j.1547-5069.1988.tb00082.x (1988).
Coutinho, M. V. et al. The interplay between uncertainty monitoring and working memory: Can metacognition become automatic?. Mem. Cognit. 43, 990–1006. https://doi.org/10.3758/s13421-015-0527-1 (2015).
Gustafson, A. Reducing patient uncertainty: implementation of a shared decision-making process enhances treatment quality and provider communication. Clin. J. Oncol. Nurs. 21, 113–115. https://doi.org/10.1188/17.CJON.113-115 (2017).
Elwyn, G. et al. Implementing shared decision making in the NHS. BMJ 341, c5146. https://doi.org/10.1136/bmj.c5146 (2010).
Committee on improving the quality of cancer care: Addressing the challenges of an aging, P., S. Board on Health Care, and M. Institute of, in Delivering high-quality cancer care: charting a new course for a system in crisis, (eds Levit, L.) (National Academies Press, 2013). All rights reserved: Washington (DC). https://www.ncbi.nlm.nih.gov/books/NBK202153/.
Ihrig, A. et al. The treatment decision-making preferences of patients with prostate cancer should be recorded in research and clinical routine: A pooled analysis of four survey studies with 7169 patients. J. Cancer Educ. 37, 675–682. https://doi.org/10.1007/s13187-020-01867-2 (2022).
Keating, N. L., Guadagnoli, E., Landrum, M. B., Borbas, C. & Weeks, J. C. Treatment decision making in early-stage breast cancer: should surgeons match patients’ desired level of involvement?. J. Clin. Oncol. 20, 1473–1479. https://doi.org/10.1200/JCO.2002.20.6.1473 (2002).
Zhang, J. et al. The willingness and actual situation of Chinese cancer patients and their family members participating in medical decision-making. Psychooncology 24, 1663–1669. https://doi.org/10.1002/pon.3835 (2015).
Hack, T. F. et al. Predictors of distress and quality of life in patients undergoing cancer therapy: Impact of treatment type and decisional role. Psycho. Oncol. 19, 606–616. https://doi.org/10.1002/pon.1590 (2010).
Fischhoff, B. & Broomell, S. B. Judgment and decision making. Annu. Rev. Psychol. 71, 331–355. https://doi.org/10.1146/annurev-psych-010419-050747 (2020).
Graham, M. E. et al. Shared decision making and decisional conflict in the management of vestibular schwannoma: A prospective cohort study. J. Otolaryngol. Head Neck Surg. 47, 52. https://doi.org/10.1186/s40463-018-0297-4 (2018).
Villadolid, J. & Amin, A. Immune checkpoint inhibitors in clinical practice: Update on management of immune-related toxicities. Transl. Lung Cancer Res. 4(5), 560. https://doi.org/10.3978/j.issn.2218-6751.2015.06.06 (2015).
Légaré, F. et al. Are you SURE?: Assessing patient decisional conflict with a 4-item screening test. Can. Fam. Physician 56, e308–e314 (2010).
Ferron Parayre, A., Labrecque, M., Rousseau, M., Turcotte, S. & Légaré, F. Validation of SURE, a four-item clinical checklist for detecting decisional conflict in patients. Med. Decis. Mak. 34, 54–62. https://doi.org/10.1177/0272989X13491463 (2014).
Streiner, D. L. & Norman, G. R. Health measurement scales: A practical guide to their development and use 4th edn. (Oxford University Press, 2008).
Gries, K. et al. Literature review to assemble the evidence for response scales used in patient-reported outcome measures. J. Patient Rep. Outcomes 2, 41. https://doi.org/10.1186/s41687-018-0056-3 (2017).
Barr, P. J. et al. The psychometric properties of CollaboRATE: A fast and frugal patient-reported measure of the shared decision-making process. J. Med. Internet Res. 16, e2. https://doi.org/10.2196/jmir.3085 (2014).
Orimo, H. [Reviewing the definition of elderly]. Nihon Ronen Igakkai Zasshi 43, 27–34 (2006). https://onlinelibrary.wiley.com/doi/full/https://doi.org/10.1111/j.1447-0594.2006.00341.x.
Dictionary, C. Meaning of higher education. https://dictionary.cambridge.org/dictionary/english/higher-education (2022).
Oken, M. M. et al. Toxicity and response criteria of the Eastern cooperative oncology group. Am. J. Clin. Oncol. 5, 649–655. https://doi.org/10.1097/00000421-198212000-00014 (1982).
Kleinbaum, D. G. & Klein, M. Logistic regression. Statistics for biology and health. Ordinal logistic regression (Springer, 2010).
Mandelblatt, J., Kreling, B., Figeuriedo, M. & Feng, S. What is the impact of shared decision making on treatment and outcomes for older women with breast cancer?. J. Clin. Oncol. 24, 4908–4913. https://doi.org/10.1200/JCO.2006.07.1159 (2006).
Hsieh, F. Y., Bloch, D. A. & Larsen, M. D. A simple method of sample size calculation for linear and logistic regression. Stat. Med. 17, 1623–1634. https://doi.org/10.1002/(sici)1097-0258(19980730)17:14%3c1623::aid-sim871%3e3.0.co;2-s (1998).
Yu, C. C. et al. Reaffirming adverse events related to lung cancer survivors’ target therapies and their apparent effects on fear of cancer progression, anxiety, and depression. Cancer Nurs. https://doi.org/10.1097/ncc.0000000000001147 (2022).
Pessi, M. A. et al. Targeted therapy-induced diarrhea: A review of the literature. Crit. Rev. Oncol. Hematol. 90, 165–179. https://doi.org/10.1016/j.critrevonc.2013.11.008 (2014).
ASCO. Skin reactions to targeted therapy and immunotherapy. (Accessed 24 March 2022). https://www.cancer.net/coping-with-cancer/physical-emotional-and-social-effects-cancer/managing-physical-side-effects/skin-reactions-targeted-therapy-and-immunotherapy (2022).
ASCO. Diarrhea. (Accessed 24 March 2022). https://www.cancer.net/coping-with-cancer/physical-emotional-and-social-effects-cancer/managing-physical-side-effects/diarrhea (2022).
Schneider, B. J. et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J. Clin. Oncol. 39, 4073–4126. https://doi.org/10.1200/JCO.21.01440 (2021).
Serlin, R. C., Mendoza, T. R., Nakamura, Y., Edwards, K. R. & Cleeland, C. S. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain 61, 277–284. https://doi.org/10.1016/0304-3959(94)00178-H (1995).
American Psychological Association. Older adults’ health and age-related changes. Accessed 22 Jun 2022 (2022).
Löckenhoff, C. E. Aging and decision-making: A conceptual framework for future research-a mini-review. Gerontology 64, 140–148. https://doi.org/10.1159/000485247 (2018).
Politi, M. C., Clark, M. A., Ombao, H., Dizon, D. & Elwyn, G. Communicating uncertainty can lead to less decision satisfaction: a necessary cost of involving patients in shared decision making?. Health Expect. 14, 84–91. https://doi.org/10.1111/j.1369-7625.2010.00626.x (2011).
West, A. F. & West, R. R. Clinical decision-making: Coping with uncertainty. Postgrad. Med. J. 78, 319–321. https://doi.org/10.1136/pmj.78.920.319 (2002).
Kwekkeboom, K. L., Wieben, A., Stevens, J., Tostrud, L. & Montgomery, K. Guideline-recommended symptom management strategies that cross over two or more cancer symptoms. Oncol. Nurs. Forum 47, 498–511. https://doi.org/10.1188/20.ONF.498-511 (2020).
Park, J. J., Arafath, S., Kumar, S. T., Sharma, R. & Dixit, D. Managing toxicities associated with immune checkpoint inhibitors. JAAPA 34(6), 32–39. https://doi.org/10.1097/01.JAA.0000735760.65235.3c (2021).
Brigida, M. et al. Management of Immunotherapy adverse events in oncological patients: Anti-CTLA-4, Anti-PD-1/PD-L1. Rev. Recent Clin. Trials 15, 339–346. https://doi.org/10.2174/1574887115666200622161418 (2020).
Grimm, P. Social desirability bias. Wiley international encyclopedia of marketing (2010).https://doi.org/10.1002/9781444316568.wiem02057
Chen, H., Cohen, P. & Chen, S. Biased odds ratios from dichotomization of age. Stat. Med. 26(18), 3487–3497. https://doi.org/10.1002/sim.2737 (2007).
Acknowledgements
We are grateful to all the participants for their willingness to engage in this study.
Funding
This study was supported by a Grant from the Ministry of Science and Technology (MOST 108-2635-B-002-003).
Author information
Authors and Affiliations
Contributions
Y.H.L. designed and directed the project; C.C.H. helped to screen potential participants and Z.X.G. collected the data; Y.H.L. and X.Y.C. analyzed and discussed the final data; Y.H.L. and Y.H.L. were involved in planning and supervising the work; Y.H.L. drafted the manuscript and C.T.H. contributed to review and editing. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, YH., Chou, XY., Lai, YH. et al. Decisional conflict and its determinants among patients with cancer undergoing immunotherapy combined with chemotherapy or targeted therapy: a cross-sectional study. Sci Rep 13, 12715 (2023). https://doi.org/10.1038/s41598-023-39280-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-39280-6
This article is cited by
-
Culturally Adapted Shared Decision-Making Tool for Breast Cancer Clinical Trials in China: A Nurse-Led Approach
Journal of Cancer Education (2025)
-
People living with chronic pain in Canada face difficult decisions and decisional conflict concerning their care: data from the national DECIDE-PAIN survey
BMC Primary Care (2024)