Abstract
Multiple linear stapler firings is a risk factor for anastomotic leakage (AL) in laparoscopic low anterior resection (LAR) using double stapling technique (DST) anastomosis. In this study, our objective was to establish the risk factors for ≥ 3 linear stapler firings, and to create and validate a predictive model for ≥ 3 linear stapler firings in laparoscopic LAR using DST anastomosis. We retrospectively enrolled 328 mid–low rectal cancer patients undergoing laparoscopic LAR using DST anastomosis. With a split ratio of 4:1, patients were randomly divided into 2 sets: the training set (n = 260) and the testing set (n = 68). A clinical predictive model of ≥ 3 linear stapler firings was constructed by binary logistic regression. Based on three-dimensional convolutional networks, we built an image model using only magnetic resonance (MR) images segmented by Mask region-based convolutional neural network, and an integrated model based on both MR images and clinical variables. Area under the curve (AUC), sensitivity, specificity, accuracy, positive predictive value (PPV), and Youden index were calculated for each model. And the three models were validated by an independent cohort of 128 patients. There were 17.7% (58/328) patients received ≥ 3 linear stapler firings. Tumor size ≥ 5 cm (odds ratio (OR) = 2.54, 95% confidence interval (CI) = 1.15–5.60, p = 0.021) and preoperative carcinoma embryonic antigen (CEA) level > 5 ng/mL [OR = 2.20, 95% CI = 1.20–4.04, p = 0.011] were independent risk factors associated with ≥ 3 linear stapler firings. The integrated model (AUC = 0.88, accuracy = 94.1%) performed better on predicting ≥ 3 linear stapler firings than the clinical model (AUC = 0.72, accuracy = 86.7%) and the image model (AUC = 0.81, accuracy = 91.2%). Similarly, in the validation set, the integrated model (AUC = 0.84, accuracy = 93.8%) performed better than the clinical model (AUC = 0.65, accuracy = 65.6%) and the image model (AUC = 0.75, accuracy = 92.1%). Our deep-learning model based on pelvic MR can help predict the high-risk population with ≥ 3 linear stapler firings in laparoscopic LAR using DST anastomosis. This model might assist in determining preoperatively the anastomotic technique for mid–low rectal cancer patients.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the third most prevalent cancer worldwide with high rate of cancer mortality, while approximately one third of all CRCs occur in rectum1. Total mesorectal excision (TME) is the primary treatment for localized rectal cancer patients, and has high rate of cancer control2,3. It is a widely applied surgical technique to perform laparoscopic low anterior resection (LAR) using double-stapling technique (DST) anastomosis4,5.
In mid–low rectal cancer patients, anastomotic leakage (AL) is the most common postoperative complication after LAR6. In addition to increasing the incidence of reoperation and mortality, AL may even negatively affect long-term survival7,8,9. Despite advances in surgical techniques and anastomotic equipment over the past few decades10,11,12, there has been no significant decrease in the incidence of AL after LAR13.
DST simplifies colorectal reconstruction in LAR, especially in laparoscopic surgery, but the incidence of postoperative AL was not reduced by the application of this technique14,15. Several studies have proved that ≥ 3 linear stapler firings in laparoscopic LAR is an independent risk factor for AL16,17,18. Thus, the Chinese expert consensus statement proposes ≤ 2 linear stapler firings in LAR surgery19. To avoid multiple linear stapler firings, a predictive model should be created to predict ≥ 3 linear stapler firings in anastomosis, and alternative techniques could be considered.
Several studies have shown that pelvic anatomical features (such as the anteroposterior diameter and the transverse diameter of the pelvic outlet, the anteroposterior diameter of the pelvic inlet) are related to surgical difficulty and the number of the linear stapler firings20,21,22. Those studies mainly measured pelvic parameters, but did not include the influence of rectal and mesenteric conditions. How to effectively integrate above parameters to predict ≥ 3 linear stapler firing needs further research.
With the advancement of imaging technology, pelvic magnetic resonance imaging (MRI) is a preferred tool for local staging of mid-low rectal cancer before surgery23,24. MRI can obtain relevant parameters of tumor, meso-rectum and pelvis comprehensively and accurately. Mask region-based convolutional neural network (Mask R-CNN)25 and three-dimensional convolutional network (C3D)26 image recognition are current techniques for advanced-recognition artificial intelligence (AI), and have been applied in various medical fields27,28,29. With advanced deep-learning technology, pelvic MRIs’ complex data can be identified, extracted, analyzed, and integrated efficiently, and we can create a predictive model to screen out high-risk patients with ≥ 3 linear stapler firings based on the database.
In this study, we aimed to establish the risk factors for ≥ 3 linear stapler firings in laparoscopic LAR using DST anastomosis, and to create and validate a predictive model for ≥ 3 linear stapler firings with deep-learning technology based on MRI.
Methods
Patients
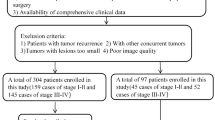
A total of 328 mid–low rectal cancer patients who underwent laparoscopic LAR at Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine, China, between January 2016 and June 2021 were retrospectively analyzed as the deep-learning set. Clinical data were obtained from Ruijin hospital database and medical records. All methods were performed in accordance with the relevant guidelines and regulations, and the study was approved by the Medical Ethics Committee of Ruijin Hospital (No. 2019-82). The need for informed consent was waived by Ethics Committee of Ruijin Hospital. With a split ratio of 4:1, 260 patients were divided into the training set and 68 patients were divided into the testing set on the basis of an unbiased random sampling method. The prospective validation set comprised 128 patients from an independent clinical trial in our institution (Transanal versus laparoscopic total mesorectal excision for rectal cancer, ClinicalTrials.gov Identifier: NCT03359616).
The inclusion criteria were: (1) histopathologically confirmed rectal cancer; (2) pelvic MRI examination within 14 days before surgery; (3) tumor distance from the anal verge ≤ 10 cm; and (4) laparoscopic LAR with DST anastomosis. The exclusion criteria were: (1) LAR without anastomosis (e.g., Hartmann’s operation); (2) anastomotic techniques rather than DST (e.g., manual rectal anastomosis through the anus); (3) number of linear stapler firings was not recorded on surgical reports; and (4) robotic surgery.
Surgical procedure
Laparoscopic LAR was performed by the gastrointestinal surgery team with experience in completing more than 100 rectal cancer operations every year. The laparoscopic LAR surgical procedure was carried out in strict accordance with the national guidelines for laparoscopic radical resection of CRC (2018 edition). During dissection of the distal rectum, the surgeon manually fired endoscopic linear staplers (Endo-GIA™ Ultra Universal Stapler Reload with Tri-stapler™ Technology; Covidien LLC), which was loaded with 60- or 45-mm staple cartridges which have three types of heights: 3.0, 3.5 and 4.0 mm.
Data collection and model building
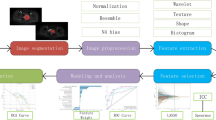
We collected clinical data that potentially correlated with the number of linear staplers used in surgery, including baseline characteristics, including age, gender and body mass index (BMI); biochemical data, including hemoglobin, albumin, and carcinoma embryonic antigen (CEA); and tumor characteristics, including tumor distance from the anal verge, tumor stage, tumor size and circumferential resection margin (CRM). A predictive model of ≥ 3 linear stapler firings was constructed by binary logistic regression. The variables of the clinical model included: gender, BMI, serum CEA level (> or ≤ 5 ng/mL), tumor distance from the anal verge, tumor size and CRM.
MRI and target area labeling
During MRI, patients were in the supine position and scanned using a Philips INGENIA™ scanner with 3.0 T field strength. The pelvic phased-array surface coil covered from the aortic bifurcation to the anal verge. The scanning parameters were: layer thickness 5 mm; field of view 250 × 340 × 166 mm; echo time 80 ms; repetition time 3565 ms; and image matrix 312 × 357.
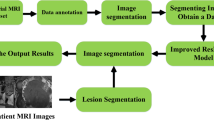
With the Picture Archiving and Communication System, fat-suppressed fast spin-echo (FSE) T2-weighted sequences in the axial plane of the pelvis were used for image segmentation. Then, pelvic MRI specialists who have > 10 years of experience built an image database by an online annotation tool called Labelme (labelme.csail.mit.edu/)30, and labeled three kinds of target area on each of the T2-weighted images (tumor body, mesorectum, and pelvis represented by green, yellow, and drab, respectively, Fig. 1A,B). Then, all data were transformed into a COCO dataset for segmentation experiments31.
Segmentation model based on Mask R-CNN
As an effective two-stage detection and segmentation algorithm, Mask R-CNN was adopted to identify and segment the three kinds of target areas in the image (Fig. 1C,D). In the first stage, the ResNet-101-FPN network served as the backbone to extract multiscale and discriminative feature maps. The Region Proposal Network scanned the feature map in a sliding-window and selected the rough detection rectangle that contained the object. After the regions of interest alignment process, the candidate regions entered the next stage. This consisted of three functional branches: classification, detection and segmentation, based on fully connected layers and convolutional layers. We trained the Mask R-CNN network on the training set for 200 epochs, and evaluated the performance of the testing set with standard COCO metrics. We evaluated the trained Mask R-CNN model, obtained average precision (AP) by calculating the precision–recall curve under different intersection-over-union thresholds, and then calculated the three types of targets. The respective AP values of the regions were weighted to obtain the class-wide mean average precision (mAP). mAP > 50 indicated that the model performed well32.
Deep-learning model based on C3D
We used C3D networks to address 24 images of a case simultaneously and learn 3D spatial features. The C3D network consisted of eight 3D-convolution layers, a softmax layer, two fully connected layers and five pooling layers. It took the entire image of the case as input and output the probability of ≥ 3 linear stapler firings, and the sample with probability > 50% (empirical value) was judged as positive. The C3D network was trained until convergence (~ 1000 epochs) and evaluated the performance of the deep-learning model. In the training set, two C3D-based models were trained, including an image model using only MR images and an integrated model based on both MR images and the six clinical variables in clinical model. In the testing set, the clinical model, image model and integrated model were examined, and receiver operating characteristic (ROC) curves were plotted. Area under the curve (AUC), sensitivity, specificity, accuracy, positive predictive value (PPV), and Youden index were calculated.
Prospective validation
We used clinical data and T2-weighted images of patients from the validation set to validate the predictive performance of the above three models. ROC curves were plotted, and sensitivity, specificity, accuracy, Youden index, PPV, and AUC were calculated. The flow chart of the design is shown in Fig. 2.
Statistical analysis
Statistical analysis was performed using SPSS (version 25.0). Categorical variables were analyzed by Fisher’s exact test or Pearson’s chi-square. In our binary logistic regression models, only factors with a P value < 0.10 in the univariate analysis were entered into the multivariate analysis. All tests were two-sided, and differences were considered statistically significant at p < 0.05.
Results
Clinical characteristics of patients in deep-learning set
The 328 patients had a median age of 63 (24–87) years, including 227 men and 101 women. The proportion of patients who received ≥ 3 linear stapler firings was 17.7% (58/328). Clinical characteristics of patients with ≥ 3 firings of the linear stapler and those with ≤ 2 firings in the deep-learning set were compared (Table 1). There were no significant differences in age, gender, BMI, diabetes mellitus, neoadjuvant chemoradiotherapy ratio, operation time, hemoglobin, albumin, tumor distance from the anal verge, T stage and N stage between the two groups. Patients with ≥ 3 firings showed significantly higher incidence of AL than those with ≤ 2 firings (p = 0.021), and patients with ≥ 3 firings of the linear stapler showed significantly higher CEA level (p = 0.007), larger tumor size (p = 0.004) and higher rate of positive CRM (p = 0.014). In univariate and multivariate analyses, tumor size ≥ 5 cm (odds ratio (OR) = 2.54, 95% confidence interval (CI) = 1.15–5.60, p = 0.021) and serum CEA > 5 ng/mL [OR = 2.20, 95% CI = 1.20–4.04, p = 0.011] were independent risk factors associated with ≥ 3 linear stapler firings (Table 2).
Predicting performance in testing set
The AUCs of the clinical, imaging and integrated models were obtained as 0.72, 0.81 and 0.88, respectively (Fig. 3A–C). The sensitivity, specificity, accuracy, PPV and Youden index of the clinical model were: 70.0%, 81.0%, 79.4%, 38.9%, and 0.51, respectively. The relevant indicators of the image model were: 50.0%, 98.3%, 91.2%, 83.3%, and 0.48, respectively. The relevant indicators of the integrated model were: 70.0%, 98.3%, 94.1%, 87.5%, and 0.68, respectively.
Receiver operating characteristic curves of the predictive models. (A) clinical model in deep-learning set; (B) image model in deep-learning set; (C) integrated model in deep-learning set; (D) clinical model in validation set; (E) image model in validation set; (F) integrated model in validation set.
Prospective validation
In the validation set, the proportion of patients who received ≥ 3 linear stapler firings was 12.5% (16/128). All related clinical characteristics between the deep-learning and validation sets were comparable (Table 3). The AUCs of the clinical, imaging and integrated models were obtained as 0.65, 0.75 and 0.84, respectively (Fig. 3D–F).
The sensitivity, specificity, accuracy, PPV and Youden index of the clinical model were: 62.5%, 66.1%, 65.6%, 21.0%, and 0.29, respectively. The relevant indicators of the image model were: 68.8%, 95.5%, 92.1%, 68.8%, and 0.64, respectively. The relevant indicators of the integrated model were: 68.8%, 97.3%, 93.8%, 78.5%, and 0.66, respectively.
Discussion
In this study, we built an MRI-based deep-learning model to predict ≥ 3 linear stapler firings in LAR using DST anastomosis. This model aimed to help determine the surgical strategy for mid–low rectal cancer patients by predicting the probability of ≥ 3 firings of the linear stapler before surgery. Thus, we can reduce the occurrence of AL by using other more suitable anastomosis techniques. Our findings suggest that clinical information alone may not be sufficient to predict cases with ≥ 3 firings of the linear stapler. Compared with the clinical or image model, the integrated model that combined clinical information with pelvic MR images achieved better AUC and higher PPV.
LAR using DST anastomosis is currently a widely applied surgical technique for mid–low rectal cancer, a series of high-quality randomized controlled trials has confirmed its feasibility and safety5,33. The technique greatly reduces the difficulty of reconstruction of the digestive tract. However, some studies have reported that multiple linear stapler firings is closely related to AL17,34, and AL is more likely to occur at the intersection of two staples35. In some cases, due to the limitation of the pelvic space or thickness of the rectum, the surgeons have to trigger more linear stapler firings during rectal dissection36.
In the high-risk populations with ≥ 3 linear stapler firings, other anastomosis techniques rather than DST anastomosis could be considered, such as transanal anastomosis after transanal transection of the rectum37 and manual purse-string suture after endoluminal transection of the rectum (e.g., Transanal total mesorectal excision)38. Although some studies have shown that the above techniques do not reduce the incidence of AL39, these techniques can minimize the anastomotic difficulty in patients with a narrow pelvis and avoid excessive use of linear stapler firings.
Akiyoshi et al. used clinical data and pelvic parameters to predict surgical difficulty and the incidence of AL in patients undergoing LAR using DST anastomosis. They found that tumor distance from the anal verge, BMI, pelvic outlet, and tumor invasive depth were independent predictors of operation time and occurrence of AL10. Foo et al. reported a predictive model for predicting ≥ 3 linear stapler firings. The model included the following parameters: tumor distance from the anal verge, gender, pelvic entrance, internodal distance and interspinous distance40. Compared with the above two studies, our predictive model has several advantages. (1) Using AI-based image segmentation, pelvic measurements can be identified comprehensively, rather than obtaining certain pelvic parameters separately. Thus, all anatomical features of the pelvis can be entirely integrated into the image model. (2) Clinically, the space between the pelvis, mesorectum and tumor mass affects the number of linear stapler firings. Our model takes into account not only pelvic parameters, but also the influence of meso-rectal factors and tumor conditions. (3) The predicting time of this AI-based warning model is only 100 ms. It greatly reduces the time and labor of manual measurement.
It should be noted that our study has some limitations. First, the cohort of 328 patients was too small for training the deep-learning model, further study with larger sample size is needed. Second, other technical factors that were difficult to quantify can also affect the number of linear stapler firings, such as the correct angle between the stapler and rectum and precompression before stapler firings35,36. Therefore, no 100% accuracy were achieved in our three models. Third, the number of linear stapler firings was just one of anastomotic factors related to AL, the circular end-to-end anastomosis, intersections of staple lines41, and the distance between the linear staple line36 were also risk factors for AL. Finally, our deep-learning traning is only performed on T2-weighted MR sequences. Other MR sequences or contrast-enhanced MRI could be investigated in future studies.
In conclusion, the pelvic MR-based deep-learning model can help identify the high-risk population with ≥ 3 linear stapler firings in laparoscopic LAR surgery. It might help determine the anastomotic technique for mid–low rectal cancer patients preoperatively. However, it is still necessary to verify its value through clinical application.
Data availability
The datasets used during the current study available from the corresponding author on reasonable request.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68(6), 394–424. https://doi.org/10.3322/caac.21492 (2018).
MacFarlane, J., Ryall, R. & Heald, R. Mesorectal excision for rectal cancer. Lancet 341(8843), 457–460. https://doi.org/10.1016/0140-6736(93)90207-w (1993).
Rullier, E. et al. Organ preservation for rectal cancer (GRECCAR 2): A prospective, randomised, open-label, multicentre, phase 3 trial. Lancet 390(10093), 469–479. https://doi.org/10.1016/s0140-6736(17)31056-5 (2017).
Griffen, F., Knight, C., Whitaker, J. & Knight, C. The double stapling technique for low anterior resection. Results, modifications, and observations. Ann. Surg. 211(6), 745–751. https://doi.org/10.1097/00000658-199006000-00014 (1990) (discussion 51-2).
Shrikhande, S. V. et al. Outcomes of resection for rectal cancer in India: The impact of the double stapling technique. World J. Surg. Oncol. 5(1), 35. https://doi.org/10.1186/1477-7819-5-35 (2007).
Climent-Agustín, M. & Martin, S. T. Complications of laparoscopic rectal cancer surgery. Mini-invasive Surg. 2, 45. https://doi.org/10.20517/2574-1225.2018.62 (2018).
Koedam, T. et al. Oncological outcomes after anastomotic leakage after surgery for colon or rectal cancer: Increased risk of local recurrence. Ann. Surg. 275(2), e420–e427. https://doi.org/10.1097/sla.0000000000003889 (2020).
Kverneng Hultberg, D. et al. The impact of anastomotic leakage on long-term function after anterior resection for rectal cancer. Dis. Colon Rectum 63(5), 619–628. https://doi.org/10.1097/dcr.0000000000001613 (2020).
Taflampas, P., Christodoulakis, M. & Tsiftsis, D. Anastomotic leakage after low anterior resection for rectal cancer: Facts, obscurity, and fiction. Surg. Today 39(3), 183–188. https://doi.org/10.1007/s00595-008-3835-2 (2009).
Akiyoshi, T. et al. Factors affecting the difficulty of laparoscopic total mesorectal excision with double stapling technique anastomosis for low rectal cancer. Surgery 146(3), 483–489. https://doi.org/10.1016/j.surg.2009.03.030 (2009).
Huang, B. Y., Yang, L. C. & Ding-Li, M. Modified rectocolon end-to-side anastomosis for middle and low rectal cancer after anterior resection. J. Mod. Oncol. 11(2), 118–119 (2003).
Ishii, Y., Hasegawa, H., Nishibori, H., Endo, T. & Kitajima, M. The application of a new stapling device for open surgery (Contour Curved Cutter Stapler) in the laparoscopic resection of rectal cancer. Surg. Endosc. 20(8), 1329–1331. https://doi.org/10.1007/s00464-005-0633-4 (2006).
Shearer, R., Gale, M., Aly, O. E. & Aly, E. H. Have early postoperative complications from laparoscopic rectal cancer surgery improved over the past 20 years?. Colorectal Dis. 15(10), 1211–1226. https://doi.org/10.1111/codi.12302 (2013).
Bernhard, D. et al. Anastomotic leakage after low anterior resection for rectal cancer: Comparison of stapled versus compression anastomosis. Langenbeck’s Arch. Surg. 398(7), 957–964. https://doi.org/10.1007/s00423-013-1103-4 (2013).
Kawada, K. & Sakai, Y. Preoperative, intraoperative and postoperative risk factors for anastomotic leakage after laparoscopic low anterior resection with double stapling technique anastomosis. World J. Gastroenterol. 22(25), 5718–5727. https://doi.org/10.3748/wjg.v22.i25.5718 (2016).
Balciscueta, Z. et al. Impact of the number of stapler firings on anastomotic leakage in laparoscopic rectal surgery: A systematic review and meta-analysis. Tech. Coloproctol. 24(9), 919–925. https://doi.org/10.1007/s10151-020-02240-7 (2020).
Park, J. S. et al. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: The Korean laparoscopic colorectal surgery study group. Ann. Surg. 257(4), 665–671. https://doi.org/10.1097/SLA.0b013e31827b8ed9 (2013).
Ito, M. et al. Relationship between multiple numbers of stapler firings during rectal division and anastomotic leakage after laparoscopic rectal resection. Int. J. Colorectal Dis. 23(7), 703–707. https://doi.org/10.1007/s00384-008-0470-8 (2008).
Zhang, Z. T. Chinese expert consensus statement on the diagnostic, prevention and treation of the anastomotic leakage for rectal cancer (article in Chinese). Zhonghua Wei Chang Wai Ke Za Zhi 22(3), 201–206. https://doi.org/10.3760/cma.j.issn.1671-0274.2019.03.001 (2019).
Bertani, E. et al. The impact of pelvimetry on anastomotic leakage in a consecutive series of open, laparoscopic and robotic low anterior resections with total mesorectal excision for rectal cancer. Hepatogastroenterology 61(134), 1574–1581. https://doi.org/10.5754/hge13724 (2014).
Killeen, T. et al. Magnetic resonance (MR) pelvimetry as a predictor of difficulty in laparoscopic operations for rectal cancer. Surg. Endosc. 24(12), 2974–2979. https://doi.org/10.1007/s00464-010-1075-1 (2010).
Zhou, X. et al. CT pelvimetry and clinicopathological parameters in evaluation of the technical difficulties in performing open rectal surgery for mid-low rectal cancer. Oncol. Lett. 11(1), 31–38. https://doi.org/10.3892/ol.2015.3827 (2016).
Balyasnikova, S. & Brown, G. Optimal imaging strategies for rectal cancer staging and ongoing management. Curr. Treat. Options Oncol. 17(6), 32. https://doi.org/10.1007/s11864-016-0403-7 (2016).
Jhaveri, K. S. & Sadaf, A. Role of MRI for staging of rectal cancer. Expert Rev. Anticancer Ther. 9(4), 469–481. https://doi.org/10.1586/era.09.13 (2009).
Rajjak, S. & Kureshi, A. K. Multiple-object detection and segmentation based on deep learning in high-resolution video using Mask-RCNN. Int. J. Pattern Recogn. Artif. Intell. https://doi.org/10.1142/S0218001421500385 (2021).
Tran, D., Bourdev, L., Fergus, R., Torresani, L. & Paluri, M. Learning spatiotemporal features with 3D convolutional networks. In IEEE International Conference on Computer Vision, 4489–4497 https://doi.org/10.1109/ICCV.2015.510 (2015).
Li, W., Wang, G., Fidon, L., Ourselin, S. & Cardoso, M. J. Vercauteren T (2017) On the Compactness, Efficiency, and Representation of 3D Convolutional Networks: Brain Parcellation as a Pretext Task Vol. 10265, 348–360 (Springer, 2017). https://doi.org/10.1007/978-3-319-59050-9_28.
Meng, J., Xue, L., Chang, Y., Zhang, J. & Yang, K. Automatic detection and segmentation of adenomatous colorectal polyps during colonoscopy using Mask R-CNN. Open Life Sci. 15(1), 588–596. https://doi.org/10.1515/biol-2020-0055 (2020).
Qadir, H. A., Shin, Y., Solhusvik, J., Bergsland, J. & Balasingham, I. Polyp detection and segmentation using Mask R-CNN: Does a deeper feature extractor CNN always perform better? In 2019 13th International Symposium on Medical Information and Communication Technology (ISMICT), 1–6 https://doi.org/10.1109/ISMICT.2019.8743694 (2019) .
Russell, B. C., Torralba, A., Murphy, K. P. & Freeman, W. T. LabelMe: A database and web-based tool for image annotation. Int. J. Comput. Vis. 77(1–3), 157–173. https://doi.org/10.1007/s11263-007-0090-8 (2008).
Lin, T. Y., Maire, M., Belongie, S., Hays, J. & Zitnick, C. L. Microsoft COCO: Common Objects in Context (Springer International Publishing, 2014). https://doi.org/10.1007/978-3-319-10602-1_48.
Hu, J (2013). Proceedings of the 2013 IEEE International Conference on Computer Vision. IEEE International Conference on Computer Vision.
Huang, M. J. et al. Laparoscopic-assisted versus open surgery for rectal cancer: A meta-analysis of randomized controlled trials on oncologic adequacy of resection and long-term oncologic outcomes. Int. J. Colorectal Dis. 26(4), 415. https://doi.org/10.1007/s00384-010-1091-6 (2011).
Kim, C. et al. Anastomotic leakage after low anterior resection for rectal cancer is different between minimally invasive surgery and open surgery. Ann. Surg. 263(1), 130–137. https://doi.org/10.1097/sla.0000000000001157 (2016).
Kawada, K. et al. Risk factors for anastomotic leakage after laparoscopic low anterior resection with DST anastomosis. Surg. Endosc. 28(10), 2988–2995. https://doi.org/10.1007/s00464-014-3564-0 (2014).
Kuroyanagi, H. et al. Standardized technique of laparoscopic intracorporeal rectal transection and anastomosis for low anterior resection. Surg. Endosc. 22(2), 557–561. https://doi.org/10.1007/s00464-007-9626-9 (2008).
Nakagoe, T. et al. Oncological outcome of ultra-low anterior resection with total mesorectal excision for carcinoma of the lower third of the rectum: Comparison of intrapelvic double-stapled anastomosis and transanal coloanal anastomosis. Hepato-gastroenterology 52(66), 1692–1697. https://doi.org/10.1246/bcsj.40.2063 (2005).
Spinelli, A. et al. Transanal Transection and Single-Stapled Anastomosis (TTSS): A comparison of anastomotic leak rates with the double-stapled technique and with transanal total mesorectal excision (TaTME) for rectal cancer. Eur. J. Surg. Oncol. 47(12), 3123–3129. https://doi.org/10.1016/j.ejso.2021.08.002 (2021).
Penna, M. et al. Incidence and risk factors for anastomotic failure in 1594 patients treated by transanal total mesorectal excision: Results From the International TaTME Registry. Ann. Surg. 269(4), 700–711. https://doi.org/10.1097/sla.0000000000002653 (2019).
Foo, C., Hung, H., Ho, Y., Lam, W. & Law, W. Predicting the level of difficulty of the double-stapling technique in laparoscopic total mesorectal excision. Surg. Endosc. 34(8), 3382–3387. https://doi.org/10.1007/s00464-019-07112-2 (2020).
Lee, S., Ahn, B. & Lee, S. The relationship between the number of intersections of staple lines and anastomotic leakage after the use of a double stapling technique in laparoscopic colorectal surgery. Surg. Laparosc. Endosc. Percutaneous Tech. 27(4), 273–281. https://doi.org/10.1097/sle.0000000000000422 (2017).
Funding
This study was funded by Shanghai Jiaotong University [grant number YG2019QNB24 to J.M.].
Author information
Authors and Affiliations
Contributions
Z.F., S.L. and Z.C. wrote the main manuscript text and prepared figures. J.M., L.Z. and F.D. enrolled the participating patients . All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fu, Z., Li, S., Zang, L. et al. Predicting multiple linear stapler firings in double stapling technique with an MRI-based deep-learning model. Sci Rep 13, 18906 (2023). https://doi.org/10.1038/s41598-023-46225-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-46225-6