Abstract
The causal relationship between Packed red blood cell (RBC) transfusion and necrotizing enterocolitis (NEC) remains uncertain. This study aims to provide an exploration of transfusion and NEC in very preterm infants. Using data from the Chinese Neonatal Network cohort study between 2019 and 2021, the analysis focused on very preterm infants (with a birth weight of < 1500 g or a gestational age of < 32 weeks) who developed NEC after receiving transfusions. The time interval between the prior transfusion and NEC was analyzed. An uneven distribution of the time interval implies an association of transfusion and NEC. Additionally, multivariable logistic analysis was conducted to detect the prognosis of defined transfusion-associated NEC(TANEC). Of the 16,494 infants received RBC transfusions, NEC was noted in 1281 (7.7%) cases, including 409 occurred after transfusion. Notably, 36.4% (149/409) of post-transfusion NEC occurred within 2 days after transfusion. The time interval distribution showed a non-normal pattern (Shapiro–Wilk test, W = 0.513, P < 0.001), indicating a possible link between transfusion and NEC. TANEC was defined as NEC occurred within 2 days after transfusion. Infants with TANEC had a higher incidence of death (adjusted OR 1.69; 95% CI 1.08 to 2.64), severe bronchopulmonary dysplasia (adjusted OR 2.03; 95% CI 1.41 to 2.91) and late-onset sepsis (adjusted OR 2.06; 95% CI 1.37 to 3.09) compared with infants without NEC after transfusion. Unevenly high number of NEC cases after RBC transfusions implies transfusion is associated with NEC. TANEC is associated with a poor prognosis. Further research is warranted to enhance our understanding of TANEC.
Similar content being viewed by others
Introduction
Necrotizing enterocolitis (NEC) is a frequently encountered gastrointestinal disorder that significantly contributes to adverse outcomes in premature infants. The etiologies of NEC encompass formula feeding, hypoxia, infection1. However, the debate over whether red blood cell (RBC) transfusion is associated with NEC in preterm infants continues to be widely discussed2.
Despite decades of extensive research, the relationship between blood transfusion and NEC remains elusive. Studies have conflicting findings3,4,5,6,7,8,9,10,11, with some suggesting RBC transfusion as a cause of intestinal injury, others proposing it as protective against NEC, and some finding no link. These evidences supporting this association is of lower quality, primarily consisting of case–control studies, with only a limited number of cohort studies conducted thus far. Additionally, the restricted sample sizes across all these studies pose a challenge to definitively clarify the connection between transfusion and NEC. Does transfusion-associated necrotizing enterocolitis (TANEC) exist, and if so, at what critical time point does it occur? Furthermore, what is the prognosis of infants affected by TANEC? A more comprehensive understanding of association between transfusion and NEC is urgently needed and will greatly benefit disease prevention and prognosis.
This study, based on data from the Chinese Neonatal Network (CHNN) cohort study involving very premature infants, seeks to establish a causal link between transfusions and NEC. Additionally, it aims to investigate the prognosis of TANEC infants. The study will provide a more detailed description of TANEC and serve as a basis for disease prevention and treatment.
Methods
Study design
This retrospective study conducts a secondary analysis of the CHNN database. Established in 2018, this collaborative network encompasses 79 (by 2021) neonatal intensive care units (NICUs) across China. The network collects clinical data on infants with a birth weight of < 1500 g or a gestational age of < 32 weeks from member hospitals12. The study was undertaken with the approval from the ethics review board of the Children’s Hospital of Fudan University (2018–296), the central hub of CHNN. Written informed consent was not required for retrospective observational study as per the [Ethics Review Board of the Children’s Hospital of Fudan University]. All methods in this study were performed in accordance with the relevant guidelines and regulations.
Study population
This study utilized the CHNN database to identify all infants who received RBC transfusions between January 2019 and December 2021. Transfusion strategy was implemented in accordance with the "Guideline for Pediatric Transfusion" issued by the National Health Commission of the People's Republic of China13. Infants with major congenital anomalies or missing data regarding NEC or receipt of RBC transfusions were excluded from the analysis.
Definitions
NEC was defined as Bell’s Stage II or greater according to an established criteria14. Spontaneous Intestinal Perforation (SIP) refers to individuals diagnosed intraoperatively or imaging suggestive of intestinal perforation lacking clinical features of necrotizing enterocolitis (NEC) is not included in the NEC group. Infants who received transfusions but did not develop NEC during hospitalization were categorized “No-NEC”. Infants with NEC were segregated into post-transfusion NEC, pre-transfusion NEC and Undefined NEC (Ud-NEC) based on the sequence of NEC and transfusion. Ud-NEC referred to cases where transfusion and NEC occurred on the same day and the onset of NEC (before or after transfusion) was unclear. Among the post-transfusion NEC, NEC that occurred within two calendar days after transfusion exposure was defined as TANEC, NEC cases that occurred beyond 2 days post transfusion, along with pre-transfusion NEC cases, were considered unrelated to transfusion and defined as UNTA-NEC. The time interval between transfusion and NEC refers to the period between the most recent transfusion and the onset of NEC. The accumulated number of transfusions refers to the total count of transfusions administered before TANEC.
Variables
Trained data abstractors obtained variables from the neonate's medical records15. The data were then transmitted electronically to central hub of CHNN, maintaining patient anonymity. At each site, site investigators were accountable for data quality control. Routine data auditing, and periodic feedback were provided to each site to ensure data quality 16.
Clinical characteristics of TANEC infants were detailed including maternal age, cesarean delivery, birth weight, gestational age by best obstetric estimate, sex, small for gestational age(SGA, birth weight < 10th percentile for the gestational age according to the Chinese neonatal birth weight values17), placenta transfusion including delayed cord clamping and umbilical cord milking, low 5-min Apgar score (Apgar score ≤ 7), endotracheal intubation during resuscitation, inborn, placental transfusion, age at the 1st feed and duration of fasting and antibiotic therapy in first seven days of life. Age at transfusion was defined as the postnatal days of life (DOL) and postmenstrual age (PMA).
The outcomes of TANEC infants included cystic periventricular leukomalacia (PVL), severe retinopathy of prematurity (ROP), late-onset sepsis (LOS), severe bronchopulmonary dysplasia (BPD), and mortality. Cystic PVL was defined as the presence of periventricular cysts on cranial ultrasound or magnetic resonance imaging. Severe ROP was defined as ROP stage ≥ 3 according to the International Classification of ROP18. LOS was defined as culture-proven sepsis between 3 and 28 calendar days of life. Severe BPD was defined as nasal continuous positive airway pressure or intermittent positive pressure ventilation or invasive ventilation requirement at 36 weeks of PMA or at discharge, transfer or death if before 36 weeks corrected gestational age19.
Statistical analysis
Data analyses were conducted using SAS version 9.4 (SAS Institute; Cary, NC, USA). Categorical variables were presented as frequencies and percentages, and group comparisons were performed using the chi-square test. Non-normally distributed data were presented as medians and quartiles, and group comparisons were performed using the Wilcoxon test. The normality of the time interval was assessed using the Shapiro–Wilk test. If W = 1 and P > 0.05, it suggests a normal distribution. Otherwise, a non-normal distribution is indicated. Multiple logistic regression analysis was performed to determine the odds ratio (OR) for TANEC for each clinical factor, with adjustment for confounding variables that showed baseline imbalances among the groups. Statistical significance was defined as a two-tailed P < 0.05.
Ethical approval
Ethics review board of the Children’s Hospital of Fudan University (2018-296) and all participating hospitals.
Results
Characteristics of included cases
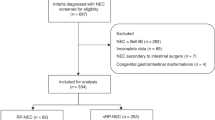
Between Jan 2019 and Dec 2021, a total of 31915 very preterm infants with a gestational age < 32 weeks or a birth weight < 1500 g were identified. Among 16,494 patients who received RBC transfusions during their hospitalization, we recorded a total of 41,973 transfusion episodes and 1281 cases of NEC (7.7% as opposed to 4.9% across all infants) were recorded. Of all NEC cases, there were 535 pre-transfusion NEC, 337 Ud-NEC and 409 post-transfusion NEC (Fig. 1).
Flow chart of preterm infants with transfusion. CHNN Chinese Neonatal Network, NEC necrotizing enterocolitis, No-NEC infants without NEC, UNTA-NEC preterm infants with NEC prior to all transfusion or NEC occurred beyond 2 days after transfusion, TANEC NEC occurred within 2 days after transfusion, Ud-NEC undefined NEC was defined as NEC that transfusion and NEC occurred on the same day and the onset of NEC (before or after transfusion) was ambiguous.
Distribution of time intervals between transfusion and NEC
The distribution curve of time intervals between transfusion and NEC was plotted. Of the 409 post-transfusion NEC cases identified, 149 (36.4%) occurred within 2 days after transfusion, 201 (49.1%) within 3 days, 224 (54.8%) within 4 days, and 282 (68.9%) within one week. With an increase in the time elapsed after transfusion, there was a reduction in the occurrence of NEC (Fig. 2 and Table S1). The time interval between transfusion and the presentation of NEC exhibits a non-normal distribution (Shapiro–Wilk test; W = 0.513, P < 0.001), suggesting an association between transfusion and NEC.
Prognosis of TANEC infants compared with No-NEC or UNTA-NEC infants
Characteristics of infants of TANEC, UNTA-NEC, and No-NEC are shown in Table 1. TANEC primarily occurred between DOL15 to 42 (60.4% of all TANEC, Table S2) or at 31 to 34 weeks of PMA (59.7% of all TANEC, Table S3). By comparing with No-NEC, infants of TANEC exhibited a higher mortality rate (adjusted OR 1.69; 95% CI 1.08 to 2.64; P = 0.022), a higher occurrence of severe BPD (adjusted OR 2.03; 95% CI 1.41 to 2.91; P < 0.001) and more frequent LOS (adjusted OR 2.06; 95% CI 1.37 to 3.09; P < 0.001). When compared with UNTA-NEC, TANEC infants still showed a higher rate of severe BPD (OR 1.76; 95% CI 1.18 to 2.62; P = 0.006) (Fig. 3 and Table S4).
Outcomes of TANEC infants. BPD bronchopulmonary dysplasia, ROP retinopathy of prematurity, PVL periventricular leukomalacia. TANEC vs No-NEC: adjusted for gestational age, placental transfusion, age at first feed in days ≤ 3, PDA; TANEC vs UNTA-NEC: adjusted for gestational age, endotracheal incubation during resuscitation, age at first feed in days ≤ 3, inborn, low 5-min Apgar score ≤ 7, duration of antibiotic therapy in 1st 7 days of life in days > 4.
Discussion
This hospital-based, large-scale, multicenter observational study, featuring the largest sample size to date, unveils that more than one-third of post-transfusion NEC manifest within 2 days following transfusion. Furthermore, TANEC infants are often associated with increased mortality, higher rates of severe BPD and LOS.
The association between RBC transfusion and NEC has consistently been a controversial topic. Some studies assert RBC transfusion as a cause of intestinal injury3,4,5,6, while others have proposed that transfusion may serve as a protective factor against NEC7,8 or has no link with NEC9,10, and that anemia may pose as an independent risk factor for TANEC11. Recent bench studies have uncovered some evidence supporting TANEC. For example, researches have demonstrated that changes in mesenteric blood flow velocity, reoxygenation, and reperfusion after transfusion could incite intestinal oxidative stress injury20,21,22. Furthermore, multiple immune factors such as IL-1β, IL-6, IFN-γ, and ICAM-1 have been shown to increase in circulation following RBC transfusion23,24. Studies have described a murine model of NEC instigated by transfusions after anemia, illustrating typical NEC-like gut injuries in the anemia-transfusion group within 48 h post transfusion due to Toll-like receptor-4-mediated injury and intestinal epithelial barrier dysfunction25,26. Considering our data in tandem with prior animal studies, it is plausible that RBC transfusion is associated with NEC.
Definitive definition of TANEC varied in previous studies, with presenting variable periods ranging from 48 h to 7 days11,27,28. In this study, we observed that 36.9% of post-transfusion NEC cases occurred within the first 2 days after transfusion, with a marked decrease in incidence thereafter. This finding provides more evidence to support the definition of TANEC as NEC occurring within 48 h following RBC transfusion.
To mitigate the occurrence of TANEC, it is crucial to identify the high-risk population and delineate specific transfusion characteristics associated with TANEC. Our study suggests that very preterm infants at 15–42 DOL or at 31–34 weeks may be more susceptible to developing TANEC, and thus could potentially benefit from preventive measures such as considering withholding feeding during transfusions29,30. It is also possible that the underlying cause is the high incidence of anemia during this period, which requires more blood transfusions.
This large retrospective study provides detailed characteristics of transfusions associated with a higher risk of NEC, however, it is important to acknowledge several limitations. First, the retrospective design of the study precluded the analysis of potential factors such as hemoglobin levels, the volume of transfusion, and feeding volume during transfusion. Fortunately, all member units of CHNN adhere to the "Guideline for Pediatric Transfusion" issued by the National Health Commission of the People's Republic of China, thereby effectively mitigating the impact of these factors to the maximum extent possible. Second, the exclusion of transfusion episodes without a definite time interval with Ud-NEC may have resulted in selection bias, although this was necessary for accurate patient grouping. Nevertheless, the study's findings remain robust.
Conclusions
In summary, we have observed an association between RBC transfusion and NEC in very premature infants, as evidenced by a significant increase in NEC occurrence within 2 days post transfusion. TANEC is also associated with higher risks of mortality, BPD and LOS. An in-depth comprehension of the characteristics concerning transfusion episodes linked to NEC may offer a robust theoretical underpinning for the standardization of clinical transfusion practices and the subsequent mitigation of NEC (Supplementary Information).
Data availability
The authors declare that all data supporting the findings of this study are available within the article and its supplementary information files.
References
Hackam, D. J. & Sodhi, C. P. Bench to bedside—New insights into the pathogenesis of necrotizing enterocolitis. Nat. Rev. Gastroenterol. Hepatol. 19, 468 (2022).
Bellach, L. et al. Packed red blood cell transfusion in preterm infants. Lancet Haematol. 9, e615–e626 (2022).
Odom, T. L. et al. Development of necrotizing enterocolitis after blood transfusion in very premature neonates. World J. Pediatrics WJP. 19, 68–75 (2023).
Mohamed, A. & Shah, P. S. Transfusion associated necrotizing enterocolitis: A meta-analysis of observational data. Pediatrics. 129, 529–540 (2012).
Faraday, C. et al. Characteristics and incidence of transfusion-associated necrotizing enterocolitis in the UK. J. Matern.-Fetal Neonatal Med. 33, 398–403 (2020).
Stokes, V., Rajai, A., Mukherjee, D. & Mukherjee, A. Transfusion-associated necrotizing enterocolitis (NEC) in extremely preterm infants: Experience of a tertiary neonatal center in UK. J. Matern. -Fetal Neonatal Med. 35, 1–6 (2021).
Bednarek, F. J. et al. Variations in blood transfusions among newborn intensive care units. SNAP II Study Group. J. Pediatrics 133, 601–607 (1998).
Rai, S. E., Sidhu, A. K. & Krishnan, R. J. Transfusion-associated necrotizing enterocolitis re-evaluated: A systematic review and meta-analysis. J. Perinatal Med. 46, 665–676 (2018).
Janjindamai, W. et al. Risk of necrotizing enterocolitis following packed red blood cell transfusion in very low birth weight infants. Indian J. Pediatrics 86, 347–353 (2019).
Crabtree, C. S., Pakvasa, M., Radmacher, P. G. & Adamkin, D. H. Retrospective case-control study of necrotizing enterocolitis and packed red blood cell transfusions in very low birth weight infants. J. Neonatal-Perinatal Med. 11, 365–370 (2018).
Patel, R. M. et al. Association of red blood cell transfusion, anemia, and necrotizing enterocolitis in very low-birth-weight infants. Jama 315, 889–897 (2016).
Cao, Y. et al. Assessment of neonatal intensive care unit practices, morbidity, and mortality among very preterm infants in China. JAMA Netw. Open 4, e2118904 (2021).
China NHCotPsRo. Guidelines for Pediatric Transfusion. WS/T. 2022. http://www.nhc.gov.cn/wjw/s9493/202202/a180d07419e04584adf80165a33fac57.shtml. Accessed 1 June 2022 (2022).
Bell, M. J. et al. Neonatal necrotizing enterocolitis: Therapeutic decisions based upon clinical staging. Ann. Surg. 187, 1–7 (1978).
Hei, M. et al. Chinese Neonatal Network: A national protocol for collaborative research and quality improvement in neonatal care. BMJ Open 12, e051175 (2022).
Sun, J. et al. Data quality improvement and internal data audit of the Chinese Neonatal Network data collection system. Front. Pediatrics 9, 711200 (2021).
Zhu, L. et al. Chinese neonatal birth weight curve for different gestational age. Zhonghua er ke za zhi Chin. J. Pediatrics 53, 97–103 (2015).
The International Classification of Retinopathy of Prematurity revisited. Arch. Ophthalmol. (Chicago Ill: 1960) 2005(123), 991–999 (1960).
Jobe, A. H. & Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 163, 1723–1729 (2001).
Singh, R., Shah, B. L. & Frantz, I. D. 3rd. Necrotizing enterocolitis and the role of anemia of prematurity. Semin. Perinatol. 36, 277–282 (2012).
Bailey, S. M., Hendricks-Muñoz, K. D. & Mally, P. V. Variability in splanchnic tissue oxygenation during preterm red blood cell transfusion given for symptomatic anaemia may reveal a potential mechanism of transfusion-related acute gut injury. Blood Transfus. Trasfus. Sangue 13, 429–434 (2015).
Balegar, V. K., Jayawardhana, M., Martin, A. J., de Chazal, P. & Nanan, R. K. H. Association of bolus feeding with splanchnic and cerebral oxygen utilization efficiency among premature infants with anemia and after blood transfusion. JAMA Netw. Open 3, e200149 (2020).
Say, B. et al. Interleukin-6 and C-reactive protein load in pre-storage and post-storage white blood cell-filtered red blood cell transfusions in premature infants. Transfus. Med. (Oxford, England). 25, 170–173 (2015).
Dani, C. et al. Red blood cell transfusions can induce proinflammatory cytokines in preterm infants. Transfusion 57, 1304–1310 (2017).
MohanKumar, K. et al. A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nat. Commun. 10, 3494 (2019).
Dang, D. et al. Heme induces intestinal epithelial cell ferroptosis via mitochondrial dysfunction in transfusion-associated necrotizing enterocolitis. Faseb J. 36, e22649 (2022).
Stritzke, A. I., Smyth, J., Synnes, A., Lee, S. K. & Shah, P. S. Transfusion-associated necrotising enterocolitis in neonates. Arch. Dis. Childh. Fetal Neonatal Ed. 98, F10–F14 (2013).
Singh, R. et al. Association of necrotizing enterocolitis with anemia and packed red blood cell transfusions in preterm infants. J. Perinatol. 31, 176–182 (2011).
Jasani, B., Rao, S. & Patole, S. Withholding feeds and transfusion-associated necrotizing enterocolitis in preterm infants: A systematic review. Adv. Nutr. (Bethesda, Md). 8, 764–769 (2017).
Saito-Benz, M. et al. Effects of freshly irradiated vs irradiated and stored red blood cell transfusion on cerebral oxygenation in preterm infants: A randomized clinical trial. JAMA Pediatrics 176, e220152 (2022).
Acknowledgements
We thank the data abstractors from the Chinese Neonatal Network. We thank all the staff at the Chinese Neonatal Network coordinating center for providing organizational support (Lin Yuan, PhD; Tongling Yang, RN; Hao Yuan, RN; Li Wang, RN; Yulan Lu, PhD).
Funding
National Key Research and Development Program of China (2021YFC2701800, 2021YFC2701801); Shanghai Science and Technology Commission's Scientific and Technological Innovation Action Plan (21Y21900800); Canadian Institutes of Health Research (CTP87518); National Natural Science Foundation of China (82271737, 8230195); Jilin Provincial Department of Science and Technology (YDZJ202301ZYTS070).
Author information
Authors and Affiliations
Consortia
Contributions
Dan Dang: performed study concept and design, performed development of methodology, analysis and interpretation of data, writing – original draft, read and approved the final paper. Xinyue Gu: analysis and interpretation of data, statistical analysis, read and approved the final paper. Wenli Li: analysis and interpretation of data, read and approved the final paper Siyuan Jiang: performed study concept and design, provided technical and material support, read and approved the final paper. Wenhao Zhou, Yun Cao and Shoo Kim Lee: Create and maintain CHNN data platform, read and approved the final paper. Hui Wu and Jianguo Zhou: performed study concept and design, project administration, writing – review & editing, read and approved the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dang, D., Gu, X., Jiang, S. et al. RBC transfusion and necrotizing enterocolitis in very preterm infants: a multicenter observational study. Sci Rep 14, 14345 (2024). https://doi.org/10.1038/s41598-024-64923-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64923-7