Abstract
This study investigated the three-dimensional (3D) cellular interactions and tunneling nanotubes (TNTs) during fetal mouse skin regeneration on embryonic days 13 (E13) and 15 (E15). We aimed to understand spatial relationships among cell types involved in skin regeneration and assess the potential role of TNTs. Full-thickness skin incisions were performed in E13 and E15 embryos. Wound sites were collected, embedded in epoxy resin, processed for 3D reconstruction (1 μm thickness sections), and subjected to whole-mount immunostaining. We conducted in vitro co-culture experiments with fetal macrophages and fibroblasts to observe TNT formation. To assess the effect of TNTs on skin regeneration, an inhibiting agent (cytochalasin B) was administered to amniotic fluid. Results revealed that E13 epidermal keratinocytes interacted with dermal fibroblasts and macrophages, facilitating skin regrowth. TNT structures were observed at the E13-cell wound sites, among macrophages, and between macrophages and fibroblasts, confirmed through in vitro co-culture experiments. In vitro and utero cytochalasin B administration hindered those formation and inefficient skin texture regeneration at E13 wound sites. This emphasizes the necessity of 3D cellular interactions between epidermal and dermal cells during skin regeneration in mouse embryos at E13. The prevalence of TNT structures indicated their involvement in achieving complete skin texture restoration.
Similar content being viewed by others
Introduction
In mammals, the majority of organs, excluding the liver, cannot fully regenerate damaged tissues; instead, they undergo repair processes, resulting in scar formation1. In 1954, Hess observed the rapid healing of wounds in fetal guinea pigs, indicating remarkable regenerative abilities2. In the 1970s, it was discovered that fetal sheep could regenerate skin wounds during early development, without scarring. Similar regenerative capacities have been demonstrated in numerous animal species, including sheep, rats, mice, pigs, monkeys, and humans3. This indicates that understanding the differences in wound healing mechanisms between fetal and adult skin may be crucial for achieving scarless skin regeneration in adults4.
Skin scar tissue is characterized by the absence of cutaneous appendages, loss of skin texture, and dermal fibrosis. In our research laboratory, we developed a mouse fetal skin wound healing model that induced wounds at various developmental stages in mouse embryos. During the healing process, as development progressed, we observed variations in the timing of skin regeneration, including changes in the three aforementioned parameters. We discovered instances in which the skin, including skin texture, was fully regenerated, whereas at other stages, dermal regeneration occurred without skin texture restoration. Finally, scar tissue, analogous to adults, is formed with visible scars1,5,6,7,8.
We observed complete regeneration of wounds formed due to skin injuries, including skin texture restoration in fetal mouse skin on embryonic day 13 (E13). However, beyond this stage, skin texture regeneration over the wound area failed. The underlying mechanism of this phenomenon revealed that during the healing process at E13, the peripheral actin cables of the epidermis consistently facilitated interactions between the epidermis and dermis, thereby ensuring wound closure. In subsequent stages, the absence of actin cable formation results in transient interactions between the epidermis and loose fascia tissue, compromising skin texture regeneration8.
In this study, we aimed to enhance the 3D cellular interactions that have been previously identified in various mouse fetal developmental stages. To accomplish this objective, we induced wounds in mouse fetal skin at various developmental stages and acquired thick semi-thin consecutive tissue Sects. (1 μm thickness each) from the wound sites. Subsequently, these sections were overlaid and reconstructed in 3D to analyze the spatial relationships between cells. Additionally, we performed whole-mount immunostaining on thicker tissue sections using confocal microscopy to examine the 3D microstructures of the cells relevant to wound healing. Based on these observations, we made a novel discovery at E13, with the formation of dense tunneling nanotubes (TNT), specifically among macrophages and between macrophages and fibroblasts. We demonstrated inhibition of skin texture regeneration by administering a inhibiting agent of actin polymerization, including TNTs (cytochalasin B). This groundbreaking discovery aligns with the results of this present study.
Results
3D visualization of spatial relationship between the epidermis and subcutaneous tissue
Paraffin sections were 7 μm in thick, whereas resin-embedded sections were 1 μm in thick. Resin-embedded sections offer higher resolution than paraffin sections, enabling the observation of intricate structures [Fig. 1A]. Resin embedding is a conventional method used for electron microscopy. The epoxy resin-embedded samples have been used to cut consecutive sections and to make 3D reconstructed images in transmission electron microscopy. These sections have enabled comprehensive detailed information retrieval (Supplementary Video 1). The acquired data were processed, including contrast normalization, grayscale conversion, and transformation/alignment, and saved as consecutive images. To render these images as volume-rendered images in 3D reconstruction, we used INTAGE Expert medical imaging software. For E13 and E15 at 24, 48, and 72 h, we reconstructed 3D images, enabling reproduction of the actual wound sites under a microscope [Fig. 1B]. These reconstructed images were highly manipulated within the software, as illustrated in the video, facilitating in-depth examination of the wound site. Furthermore, using the endoscopy mode of the software, we explored the intravascular space. Proceeding through the blood vessels in this data suggests that each section has been seamlessly reconstructed, allowing for valid observation of the three-dimensional structure. (Supplementary Video 2).
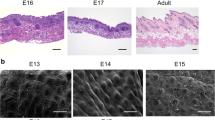
(A): Skin wounds of mouse fetuses at E15-24 h. left: H&E staining (7 μm). right: Toluidine blue staining (1 μm). Comparison between 7 and 1 μm images; the 1 μm image offers distinctive cell boundaries. Scale bar = 100 µm. (B): 3D reconstructed images in the z-axis direction of skin wounds at E13-24, 48, and 72 h and E15. (C): Comparison of epidermis-dermis interactions in consecutive section alignment images at E13-24 h and E15-24 h. Each layer was shown in pseudocolor. The epidermis at E13-24 h contracts and closes via actin cables, while at E15-24 h, the epidermal cells proliferate and thicken as they close. This difference indicates that at E15-24 h, the thickened part of the epidermis comes into contact with the deep layer dermis.
At 24 h of incision of E13 embryo (E13-24 h), the epidermis consisted of approximately two layers, with the majority of flattened epidermal cells. In contrast, at E15-24 h, the epidermis had approximately four layers. The basal layer cells had cuboidal shape and the superficial keratinocytes appeared flattened, resembling those of adult skin. At E13-24 h, the epidermis closes via actin cables, but at E15-24 h, wound closure progresses with the epidermis thickening due to cell division at the wound margin. Fundamentally, the epidermis contacted the deep dermis layer in consecutive images [Fig. 1C, Supplementary Video 3]. This interaction between the epidermis and dermis differed from that observed at E13-24 h, indicating its role in disrupting skin texture regeneration.
In this study, macrophages accumulated in the wound area, even at E13, and were present in normal skin. When examined using a confocal microscope, these cells displayed numerous thin, elongated processes, forming a network of intercellular interactions [Fig. 2A,B].
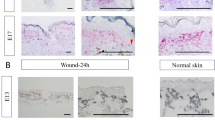
(A): Observation of wound-site macrophages. (a): Immunostaining on E13-24 h. Green (F4/80), red (CD31), and blue (4′,6-diamidino-2-phenylindole [DAPI]) staining. Scale bar = 100 µm. (b): Enlarged image showing the F4/80-positive linear structure. Yellow arrows (wound ridge) and white arrows (tunneling nanotube [TNT]). Scale bar = 100 µm. (B): Macrophage-phagocytic enlarged images at the wound center. This image shows F4/80-positive macrophages engulfing multiple cells. Scale bar = 50 μm. (C): Macrophage phagocytosis after toluidine blue staining on E13-24 h. White arrows indicate phagocytic cells. Scale bar = 100 µm. (D): Overview of the images at each time point. The number of phagocytic cells per 10 slices (1 μm/slice) around the wound center is measured. Scale bar = 100 µm.
Furthermore, at E13-24 h, a substantial number of phagocytosis-involved macrophages were observed near the wound area and those number is decreased after 48 h. However, at E15-24 h, a limited number of macrophages with similar phagocytic morphology were observed. Additionally, even after 72 h, active macrophages engaged in phagocytosis within the loose fascia were sporadically observed in the E15 group. There was a statistically significant difference (P = 0.005) between E13-24 h and E13-48 h. In the E15 groups, a difference (P = 0.006) was observed between 48 and 72 h, as determined by a t-test with a p-value (P < 0.05) [Fig. 2C,D].
Tunneling nanotubes of macrophages
To elucidate the spatial arrangement and fine structure of macrophage clusters within the wound area, whole-mount immunostaining was performed, followed by 3D observation using a confocal microscope [Fig. 3A]. Observation of the E13-24 h wound site revealed the accumulation of numerous macrophages spanning the entire wound area. Moreover, these macrophages exhibited linear protrusions with F4/80 staining, exhibiting interconnections with themselves and possibly with the neighboring fibroblasts. These structures, in terms of morphology and thickness, were analogous to previously reported TNT structures [Fig. 3B, Supplementary Video 4].
(A): 3D image of whole-mount staining on E13-24 h and E15-24 h. Green (F4/80), and blue (DAPI). Numerous F4/80-positive tunneling nanotube (TNT) structures are observed along the wound ridge. Scale bar = 100 μm. (B): The image of zoom in wound sites. White dotted lines indicates the wound. TNTs also located in the both wound. Scale bar = 100 μm. (C): Similarly, at E14 and E16, whole-mount staining. Green (F4/80). TNT structures are observed among macrophages at developmental stage. Scale bar = 50 μm.
In comparison to E15-24 h, E13-24 h exhibited a higher density of macrophages at the wound center. However, both conditions resulted in elongated TNT structures along the periphery of the wound. Conversely, there was no local density of TNT-like structures in normal skin areas at E13-24 h and E15-24 h (corresponding to E14 and E16, each) [Fig. 3C].
Transfer of signaling molecules through TNTs and cell culture model
Using macrophages from the fetal stage, we investigated the potential for TNT formation in vitro, similar to previous reports9,10. The F4/80-positive macrophages were isolated from E13 fetal trunk skin using MACS and subjected to co-cultivation with dermal fibroblasts from the corresponding period. The presence or absence of TNTs was observed using SEM. Those structures of the macrophage were observed to be interconnected with another macrophage.
and/or with fibroblasts [Fig. 4]. To confirm the material transfer within TNTs, live observations were performed using time-lapse imaging. We co-cultured macrophages stained with DiI, a hydrophobic near-infrared fluorescent cyanine dye that infiltrates cell membranes. These observations revealed active extensions and contractions of the TNTs, demonstrating the transfer of dye through these extensions. This indicates the transmission of information from the cell membrane surface [Supplementary Video 5].
The effect of TNT inhibitors on fetal skin regeneration
At the wound site of mouse E13, with fully regenerated skin, we observed a distinct accumulation of macrophages and TNT formation between the macrophages and neighboring cells [Figs. 3,4]. Therefore, the potential involvement of TNTs in complete skin regeneration was considered. Tnfaip2 reported in Adult macrophages was not predominant in fetal macrophages [Fig. 5A].
(A): Immunohistochemistry images of E13 macrophages and adult macrophages. Green (F4/80), red (Tnfaip2), and blue (DAPI) staining. In adult macrophages, F4/80 and Tnfaip2 merge, but this is not observed in E13. Scale Bar = 20 μm. (B): Immunostaining after administration of cytochalasin (B) to fibroblast cultures at different concentrations (Control, 1 μg/mL or 10 μg/mL). Green (phalloidin), red (αSMA), and blue (DAPI) staining. Scale bar = 100 µm. (C): Inhibition of TNT formation in E13 macrophages with Cytochalasin B. In the control, TNT structures were observed as indicated by white arrowheads. Red (Cell membrane). Scale Bar = 20 μm. (D): Observation of actin polymerization inhibition in the skin wound site under Cytochalasin B administration. Green(Phalloidin)and bule (DAPI). At E15-24 h, many cells at the wound margin exhibit a rounded morphology due to Cytochalasin B, but after 72 h, the fibers of actin polymerization can be clearly observed. Scale Bar = 50 μm.
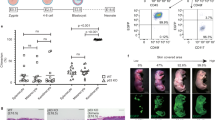
To investigate this further, we observed skin wound healing by administering Cytochalasin B, which inhibits actin polymerization11, including TNTs [Fig. 5B–D, Fig. 6A].
(A): Cytochalasin B administration to fetuses and whole-mount staining of 3D images at E13-24 h and E15-24 h. Green (F4/80), red (CD31), and blue (DAPI) staining. Scale bar = 100 µm. (B): Comparison between the cytochalasin B-treated and control groups after 24 h. The cytochalasin B-treated group shows a reduction in macrophage density within the wound and TNT formation. The zoom shows that macrophage cells were detected with surface mode by Imaris. Scale bar = 20 µm. (C): Comparison among the number of F4/80-positive tunneling nanotubes in the wounds. Measurements are performed at three different locations. P < 0.05. (D): Microscopic images taken 72 h after cytochalasin B administration to fetal wound sites. The administered group exhibits the disappearance of skin texture at E13-72 h, which typically undergoes complete regeneration. E15-72 h showed delayed wound healing. Scale bar = 1000 µm. E: H&E staining 72 h after cytochalasin B administration depicts delayed re-epithelialization at E15-72 h compared to the control. Scale bar = 100 µm. F: Whole-mount staining after cytochalasin B administration on E13-72 hand E15-72 h. Green (F4/80), red (CD31), and blue (DAPI) staining. Scale bar = 100 µm.
After 24-h application of cytochalasin B whole-mount staining, we observed a reduction in the F4/80-positive linear structures compared to the control group [Fig. 6B]. There was statistically significant difference in E13 (P = 0.004) and E15 (P = 0.046) (t-test, P < 0.05) [Fig. 6C].
Furthermore, despite wound closure at E13-72 h, the normal skin texture that usually regenerates disappeared in cytochalasin B group [Fig. 6D]. At E15, we observed a delay in re-epithelialization compared with normal E15 wound healing [Fig. 6E]. Based on these findings, we suggest that early macrophage TNTs are essential for complete regeneration and rapid wound closure [Fig. 6F].
Discussion
In a previous study, we reported that in mouse embryos, the skin, encompassing its texture, can fully regenerate when a wound is induced at E13, owing to the constant interaction between the epidermis and dermis during wound closure. However, from E14, a temporary failure of epidermal adhesion to the dermis occurs, with an alternative adherence to the fascial tissue above, resulting in a lack of morphogenesis and failure of skin texture regeneration5. In this study, we aimed to further investigate the relationship between various cells and their intricate structures and observe the spatial relationships among cell populations beneath the wound site. To minimize tissue damage, we used dual fixation and epoxy resin embedding, a reliable technique that ensures consistent collection of continuous sections with minimal risk of cutting errors or missed sections.
Moreover, for a comprehensive understanding of complex structures, such as cell interactions in 3D, it is generally deemed highly considerable to observe them in full color and 3D, rather than as monochromatic 2D flat images. To achieve this, we employed medical imaging software for whole-slide imaging using color coding to distinguish and visualize the features12.
We observed the aggregation of macrophages at the wound site, despite the relatively low-inflammatory conditions in fetal wounds, such as those at E13, when skin regeneration is complete. Although further analysis is required to determine their specific roles, we observed that macrophages are involved in activities, such as phagocytosis of necrotic tissue, indicating their significance in wound healing during full skin regeneration.
As reported previously, macrophages inhabit the skin during the developmental transition from yolk sac-derived to liver-derived macrophages, eventually progressing to bone marrow-derived macrophages. According to these reports, it is probable that in E13 mouse wounds, the majority of macrophages are yolk sac-derived, with a subsequent transition to monocyte-derived macrophages, potentially liver-derived, in the later stages13,14,15. This transition in macrophage origin corresponds to variations in their functions during wound healing and the timing of cessation of skin texture regeneration. Therefore, yolk sac-derived macrophages may affect skin regeneration.
In summary, our findings indicate that, at E13, the epidermis adheres to the dermis without cross-sectional thickening. Instead, it traverses the dermal surface to communicate with the opposing epidermis. Macrophage-formed TNTs are not confined to the loose fascia and dermis; rather, they traverse the interface between these tissues and potentially promote connective tissue regeneration. In contrast, E15 wounds showed early thickening of the epidermal edge due to cell division, leading to the coverage of the dermal cut edge that attached to the panniculus carnosus muscle. Furthermore, the cut edges of the dermis were also fragmented. Therefore, connective tissue regeneration in the dermis depends solely on the information provided by dermal macrophages. The difference in wound healing between E13 and E15 indicates healing diversity [Fig. 7, Supplementary Video 3].
Cytochalasin B is an actin polymerization inhibitor and affects cell morphology11.
Additionally, the dynamics of actin directly regulate fibrosis16.
Since the specific factor for TNT formation in fetal macrophages has not been identified, this experiment included inhibition of actin polymerization among other approaches17. In the fetal skin wound model, inhibition of those formation resulted in a considerable reduction of TNTs at the 24-h time point, hindering skin texture regeneration at E13-72 h, indicating the involvement of those structures in skin texture regeneration. However, the molecular mechanisms and the substances involved in this process require further investigation. Additionally, it is essential to elucidate the contributions of macrophages during the developmental stages, differences between superficial and deep fibroblast subtypes, spatial and temporal molecular interactions among inflammatory cells, and the functions of TNTs associated with these processes.
Material and method
We consulted the ARRIVE guideline (https://arriveguidelines.org) to ensure proper reporting of animal experiments. The research protocol was approved by the Institutional Animal Care and Use Committee of Keio University School of Medicine (Approval Number: A2023-006). All experiments were followed with the Facility Guidelines.
Surgical procedure for murine fetal skin
This experiment used Pregnant Slc:ICR mice obtained from Sankyo Labo Service Corporation (Tokyo, Japan). Embryos were labeled E0 when vaginal plugs were observed. Fetal surgeries were performed at E13 and E15, with a minimum of four mothers undergoing surgery at each time point.
Pregnant mice were anesthetized using 3–5% isoflurane inhalation and their abdominal walls were incised to expose the pregnant uterus. Under the microscope, during E13 surgeries, an incision was made in the uterine muscle layer covering the fetus. Subsequently, the amniotic sac and yolk sac were exposed, and a small incision was made in the amniotic sac to partially expose the fetal skin. Using micro scissors, a full-thickness incision of approximately 1 mm in length was made on the fetal lateral chest. Following wound creation at E13, only the amniotic and yolk sacs were sutured with 9–0 nylon, leaving the uterine muscle layer open. The fetus remaining within the amniotic sac and uncovered by the uterine muscle layer, was returned to the abdominal cavity before suturing the abdomen.
For E15 wounds, after opening the abdominal cavity, a small incision was made from the top of the uterus to the amniotic and yolk sacs. Following the fetal wound creation, only the uterine muscle layer was sutured with 9–0 nylon, the uterus was returned to the abdominal cavity and sutured. Prior to abdominal suturing, a tocolytic agent, ritodrine hydrochloride (FUJIFILM Wako Pure Chemical Co., Ltd., Osaka, Japan), was intraperitoneally administered at a rate of 1 μg/g body weight. The abdominal wall and skin were sutured using 5–0 nylon sutures. For fetal specimens collected 48 and 72 h postoperatively, the wound site was identified by labeling with DiI (0.25%) dissolved in 1% ethanol in phosphate-buffered saline (PBS) for visualization. At various time points following wound creation, the mothers were euthanized through cervical dislocation, and fetal wounds were obtained at 24, 48, and 72 h post-wounding, with a minimum of four fetuses per time point undergoing wound creation.
Procedure of embedding in epoxy resin, making semi-thin sections and staining
Fetal trunk skin wound regions were collected at each time point under a stereomicroscope and subjected to the fixation and embedding procedures described below. The samples were pre-fixed with 2.5% glutaraldehyde (TAAB Laboratories Equipment, UK) and 2% paraformaldehyde (PFA) (Nakalai Tesque, Japan) in 0.1 M phosphate buffer (pH7.4) (PB) for 2 h at room temperature (RT). After washing in PB for 10 min × 3 times at RT, the samples were post-fixed with 1% OsO4 (TAAB Laboratories Equipment, UK) in PB for 1 h on ice. They were washed in cold distilled water for 10 min × 3 times. The samples were dehydrated with a graded series of ethanol (50, 60, 70, 80, 90, 95, and 99.5% for 10 min each and 3 times in 100% for 10 min each) and propylene oxide (twice for 15 min each), then followed by infiltration and embedding in Epon 812 (TAAB Laboratories Equipment, UK). Samples were degassed using an aspirator at RT. They were polymerized at 60 °C for 48 h. Semi-thin Sects. (1 μm thickness) (800 to 1,000 sections from each sample) were cut with a diamond knife (DiATOME, Switzerland) equipped with an ultramicrotome ULTRACUT R (Leica Biosystems, Germany) and collected on slide glass CREST (Matsunami, Osaka, Japan) (about 15 sections on each slide glass). After drying the sections for 24 h on a heater at 60 ℃, they were stained with 0.5% toluidine blue (Merck, Germany). Finally, images were captured using a NanoZoomer (Hamamatsu Photonics, Hamamatsu, Japan) with a 40 × objective lens and saved in a TIFF format.
Image processing for 3D reconstruction
The images were processed using a free software, GNU Image Manipulation Program (Gimp 2.10.14). The following procedures were done using free-software, ImageJ 2.1.0/1.53t. The image set consisted of about 1000 images from each sample were arranged in order. After performing color adjustment and contrast enhancing, the images were converted to 8-bit grayscale images, inverted. The region of interest was trimmed from each image. Then, they were aligned. 3D volume images were reconstructed using the medical imaging software, Expert INTAGE V1.1.2.
(Cybernet, Tokyo, Japan, https://www.cybernet.co.jp/medical-imaging/products/expertintage/overview.html).
Histology and immunohistochemistry
The skin wound sections were fixed by immersion in PFA (4%) for 24 h. For histological examination, fixed tissues were embedded in paraffin and cut into sections of 7 µm thickness. Deparaffinization was accomplished using xylene and ethanol, followed by hematoxylin and eosin (H&E) staining using hematoxylin and eosin stain (Muto Pure Chemicals Co., Ltd., Tokyo, Japan).
Frozen sections were used for immunostaining. Tissues were first fixed in PFA (4%), immersed in sucrose solutions (20 and 40%) in PBS, embedded in optimal cutting temperature (OCT) compound (Sakura Finetek Japan Co., Ltd., Tokyo, Japan), and frozen at − 80 °C. Sections of 10 µm thickness were obtained using a cryostat (Leica Biosystems, Wetzlar, Germany).
The immunostaining procedure involved the following steps. Sections were washed with PBS, ethanol, and PBST (PBS containing 0.2% Triton X-100), followed by blocking with 5% bovine serum albumin (BSA) in PBST. Primary antibodies, rat anti-F4/80 (BioLegend, CA, USA; 1:500), hamster anti-CD31 antibody (Proteintech, IL, USA; 1:200) and anti-TNFa-IP2 antibody (Santa Cruz, TX, USA; 1:200) diluted in blocking solution, were added to the sections and incubated at 4 °C overnight. After antigen–antibody reactions, the sections were washed thrice with PBST. Secondary antibodies, Alexa Fluor series (Invitrogen, Waltham, MA, USA; Alexa Fluor 488, 555, or 647; 1:500) diluted in PBS, were added, and the membranes were incubated for 1 h. Sections were washed and mounted on glass slides using ProLong™ Gold Antifade Mountant (Thermo Fisher Scientific, MA, USA).
For whole-mount samples measuring 20 × 15 mm, the following steps were performed:
The sections were washed thrice for 10 min each with PBST. The sections were blocked with BSA (3%) in PBS solution at RT for 1 h. The primary and secondary antibodies used for frozen sections were applied to the whole-mount samples. The samples were observed and imaged using a confocal laser-scanning microscope (FLUO-VIEW FV3000, Evident Co., Ltd., Japan). The captured data were analyzed using either the viewer provided by the confocal microscope or Imaris 9.8.2 (Oxford Instruments, UK, https://imaris.oxinst.com/products/image-analysis-software/).
Observation of primary macrophages in cell culture
Skin samples were collected from the trunks of E13 fetuses after euthanizing two Slc: ICR mice. The collected samples, weighing approximately 1 mg, were dissected on ice using scissors and a No. 15 blade. The dissected tissue was subjected to a reaction in a mixture of Dispase II (GIBCO, Thermo Fisher Scientific, Waltham, MA, USA), Collagenase type IV (in PBST: PBS with 0.1% Triton X-100), and DNase (Takara Bio Inc., Shiga, Japan) for 30 min at 37 °C with agitation. Following the reaction, the tissue was neutralized using RPMI 1640 with GlutaMAX supplement (GIBCO, Thermo Fisher Scientific, Waltham, MA, USA) and 5 mL of 10% fetal bovine serum (FBS) buffer. Subsequently, it was strained using a cell strainer (70 μm), followed by centrifugation (4 °C, 5 min, 500 g). A 40 μm cell strainer was used, and the tissue was incubated in lysis buffer (BD Pharm Lyse, BD Bioscience, NJ, USA) for 3 min at 37 °C, followed by centrifugation (21 °C, 5 min, 500 g). The tissue was washed twice with buffer, and the cell suspension was adjusted in PBS (pH 7.2) containing 0.5% BSA and 2 mM ethylenediaminetetraacetic acid (EDTA) magnetic-activated cell sorting (MACS) buffer. The tissue was further processed by incubating with Anti-F4/80 MicroBeads UltraPure (Miltenyi Biotec, Germany) at a 1:10 dilution in 100 μL for 15 min at 4 °C. Subsequently, MACS buffer (900 μL) was added, followed by centrifugation (4 °C, 5 min, 300 g). A Miltenyi magnetic separation (MS) column was prepared in MACS MultiStand and washed thrice with MACS buffer. The eluent was discarded, and MACS buffer (500 μL) was added. The cell suspension was passed through a cell strainer (100 μm) into the column.
Once the liquid descended, the column was removed from the magnetic field and MACS buffer (500 μL) was added to elute the F4/80-positive cells for further use. Macrophage-tunneling nanotubes were co-cultured with fibroblasts for the same duration. Fibroblasts were obtained through dissection and fine mincing of fetal skin, followed by culturing in Dulbecco's modified Eagle’s medium (DMEM) with low glucose (FUJIFILM Wako Pure Chemical Co., Ltd., Osaka, Japan) containing FBS (10%) and penicillin/streptomycin (P/S) (1%). After incubating overnight at 37 °C, the medium was added every three days. Fibroblasts were separated using trypsin–EDTA at 37 °C for 3 min and placed in a 10 cm culture dish. Dermal fibroblasts from E13 were derived from a pool of fibroblasts, and cells from passage 3 onwards were used for in vitro experiments. Macrophages and fibroblasts were co-cultured in RPMI supplemented with FBS (10%) and P/S (1%) (1:1 ratio) for 24 h.
The following steps were employed to prepare samples for scanning electron microscope (SEM) analysis. The samples were pre-fixed in a solution containing PFA (4%) and glutaraldehyde (2.5%) in 0.05 M cacodylate buffer (pH 7.4) at RT for 30 min. The samples were then rinsed five times in 0.05 M cacodylate buffer for 5 min each. Postfixation was performed by immersing the samples in a solution of OsO4o (1%) and cacodylate buffer (0.05 M) at RT for 2 h. Following fixation, the samples were washed thrice with distilled water. The samples were dehydrated using a series of ethanol solutions with increasing concentrations (25, 50, 75, 90, and 100%), with each step lasting 30 min. The samples were immersed in an equal-volume mixture of ethanol (100%) and isoamyl acetate for 30 min, followed by two 15-min immersions in isoamyl acetate at RT for substitution. Finally, the samples were dried using a critical point drying apparatus (EM CPD300, Leica Leica, Wetzlar, Germany). The dried samples were observed using SEM (SU6600-A, Hitachi, Tokyo, Japan). To observe live-cell interactions among primary macrophages, 50% of the macrophages were incubated with DiI (0.25%) dissolved in ethanol (1%) for 5 min or Cell mask Deep Red Actin Tracking Stain (Invitrogen, Thermo Fisher Scientific, MT, USA). Following centrifugation, the cells were resuspended in buffer. DiI-unstained macrophages were co-cultured with DiI-stained macrophages in a 1:1 ratio in RPMI containing FBS (10%) and P/S (1%). Live imaging was immediately performed using a confocal laser scanning microscope (FLUO-VIEW FV3000, Evident Co., Ltd., Japan), with differential interference contrast and Cy5.
Blocking tunneling nanotubes in wound healing
Dermal fibroblasts were treated with cytochalasin B (1 or 10 μg/mL) (FUJIFILM Wako Pure Chemical Co., Ltd., Osaka, Japan) in DMEM for 24 h, and immunostaining was performed using standard culture medium. The cells were initially fixed with PFA (4%) for 10 min, followed by three 5-min washes in PBS and a 5-min incubation in PBST (0.1%). After another three washes with PBS for 5 min each, the cells were blocked with BSA (1%) for 20 min, followed by another three 5-min PBS washes. Subsequently, the sections were incubated with Acti-stain™ 488 phalloidin (Cytoskeleton Inc., Denver, CO, USA; 1:400) and rabbit anti-αSMA antibody (Abcam, Cambridge, UK; 1:200) at 4 °C overnight. The cells were then washed thrice with PBS, followed by a 1-h incubation with secondary antibodies from the Alexa Fluor series (Invitrogen, Waltham, MA, USA; Alexa Fluor 555; 1:500) at RT. After three additional PBS washes, nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI) staining solution (1:500). The cells were then mounted on glass slides using ProLong™ Gold Antifade Mountant and observed under a confocal microscope at an in vivo administration concentration of 10 μg/mL.
Similar fetal surgical procedures were performed on Slc: ICR mice on the 13th and 15th days of pregnancy. Each fetus was subcutaneously injected with cytochalasin B (10 ng/mL) immediately before wounding, and wounds were created immediately post-injection. The wound sites were collected after 24 and 72 h. For E13 cells, 0.25% DiI in PBS was used for staining after 72 h. Tissues collected after 24 and 72 h were subjected to whole-mount staining following a procedure similar to that described previously.
Data availability
The data that support to this study are available from the corresponding author, K.K. Data is provided within the manuscript or supplementary information files.
References
Kishi, K., Okabe, K., Shimizu, R. & Kubota, Y. Fetal skin possesses the ability to regenerate completely: Complete regeneration of skin. Keio J. Med. 61, 101–108 (2012).
Hess, A. Reactions of mammalian fetal tissues to injury II. Skin. Anat. Rec. 119, 435–447 (1954).
Colwell, A. S., Longaker, M. T. & Lorenz, H. P. Mammalian fetal organ regeneration. Adv. Biochem. Eng. Biotechnol. 93, 83–100 (2005).
Longaker, M. T. et al. Studies in fetal wound healing, IV. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J. Pediatr. Surg. 25, 63–68 (1990).
Takaya, K. et al. Downregulation of Lhx2 markedly impairs wound healing in mouse fetus. Biomedicines 10, 2132 (2022).
Takaya, K. et al. Fibroblast growth factor 7 suppresses fibrosis and promotes epithelialization during wound healing in mouse fetuses. Int. J. Mol. Sci. 23, 7087 (2022).
Takaya, K., Aramaki-Hattori, N., Sakai, S., Okabe, K. & Kishi, K. Effect of sonic hedgehog on the regeneration of epidermal texture patterns. Biomedicines 10, 3099 (2022).
Takaya, K. et al. Actin cable formation and epidermis-dermis positional relationship during complete skin regeneration. Sci. Rep. 12, 15913 (2022).
Hase, K. et al. M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat. Cell Biol. 11, 1427–1432 (2009).
Watkins, S. C. & Salter, R. D. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity 23, 309–318 (2005).
Benya, P. D. & Padilla, S. R. Dihydrocytochalasin B enhances transforming growth factor-beta-induced reexpression of the differentiated chondrocyte phenotype without stimulation of collagen synthesis. Exp Cell Res. 2, 268–277 (1993).
Shen, A. et al. An integrative web-based software tool for multi-dimensional pathology whole-slide image analytics. Phys. Med. Biol. https://doi.org/10.1088/1361-6560/ac8fde (2022).
Gomez Perdiguero, E. et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518, 547–551 (2015).
Hoeffel, G. et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 42, 665–678 (2015).
Hoeffel, G. et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 209, 1167–1181 (2012).
Nobusue, H. et al. Regulation of MKL1 via actin cytoskeleton dynamics drives adipocyte differentiation. Nat. Commun. 5, 3368 (2014).
Ojima, K., Lin, Z. X., Andrade, I. R., Costa, M. L. & Mermelstein, C. Distinctive effects of cytochalasin B in chick primary myoblasts and fibroblasts. PLoS One 11, e0154109 (2016).
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Contributions
Y.N. designed the concept of this study, performed all experiments, and wrote the manuscript. S.O. supervised the technique for electron microscopy and 3D reconstruction. K.T. and K.O. investigated the analysis. S.S. discussed the results of macrophages. Y.S. advised movies. N.A.-H. helped to write the manuscript. S.O., Y.K. and K.K. supervised all of this study. R.M. advised for the revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video 1.
Supplementary Video 2.
Supplementary Video 3.
Supplementary Video 4.
Supplementary Video 5.
Supplementary Video 6.
Supplementary Video 7.
Supplementary Video 8.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nakajima, Y., Obata, S., Takaya, K. et al. Tunneling nanotube-driven complete regeneration of murine fetal skin. Sci Rep 14, 17215 (2024). https://doi.org/10.1038/s41598-024-68083-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68083-6